Abstract
Background
In 2013-14, we achieved 89% adult HIV testing coverage using a hybrid testing approach in 32 communities in Uganda and Kenya (SEARCH: NCT01864603). To inform scalability, we sought to determine: 1) overall cost and efficiency of this approach; and 2) costs associated with point-of-care (POC) CD4 testing, multi-disease services, and community mobilization.
Methods
We applied micro-costing methods to estimate costs of population-wide HIV testing in 12 SEARCH Trial communities. Main intervention components of the hybrid approach are census, multi-disease community health campaigns (CHC), and home-based testing (HBT) for CHC non-attendees. POC CD4 tests were provided for all HIV-infected participants. Data were extracted from expenditure records, activity registers, staff interviews, and time and motion logs.
Results
The mean cost per adult tested for HIV was $20.5 (range: $17.1 - $32.1) [2014 US$], including a POC CD4 test at $16 per HIV+ person identified. Cost per adult tested for HIV was $13.8 at CHC vs. $31.7 via HBT. The cost per HIV+ adult identified was $231 ($87 - $1,245), with variability due mainly to HIV prevalence among persons tested (i.e., HIV positivity rate). The marginal costs of multi-disease testing at CHCs were $1.16/person for hypertension and diabetes, and $0.90 for malaria. Community mobilization constituted 15.3% of total costs.
Conclusions
The hybrid testing approach achieved very high HIV testing coverage, with POC CD4, at costs similar to previously reported mobile, home-based, or venue-based HIV testing approaches in sub-Saharan Africa. By leveraging HIV infrastructure, multi-disease services were offered at low marginal costs.
Keywords: Community-based HIV testing and counseling, Cost, Efficiency, East Africa
Introduction
Sub-Saharan Africa has 25.8 million people living with HIV, but only 45% of them are aware of their status,1,2 making universal HIV diagnosis a critical first step in ending the AIDS epidemic and the largest breakpoint to meet the UNAIDS 90-90-90 target.3 To expand HIV testing while providing lifelong treatment to tens of millions of people living with HIV in an environment of limited funding, sub-Saharan African countries need to identify and select the most efficient and affordable HIV testing approaches for rapid scale-up.4
Compared with health facility-based HIV testing and counseling (HTC), community-based HTC (CBHTC) increases early diagnosis, may reach populations that rarely use health services, and is effective in linking HIV-infected people to clinical care in sub-Saharan Africa.5,6 However, CBHTC programs are delivered through different strategies, and their costs and efficiency in HIV diagnosis vary both between and within specific CBHTC strategies. Prior published estimates of the cost per person tested varied from US$9.6 to $34.7 for mobile CBHTC strategies,7-13 $5.5 to $18.7 for home-based CBHTC strategies, 11,12,14-18 and $8.3 to $42.0 for venue-based CBHTC strategies that offer HIV testing at convenient locations within the communities (2014 dollars).8,15,19,20 The costs per person testing HIV positive through CBHTC programs had a wider range from $60.2 to $1725.3,7-24 which is mainly due to the differences in HIV positivity rate (i.e. the HIV prevalence among persons tested) that ranged from 3.5% in a health campaign in Swaziland12 to 20% in venue-based CBHTC in Kenya and Tanzania.19 However, many prior published costing analyses have been restricted to single sites or conducted over a relatively short duration, and data on cost and efficiency of community-based testing approaches that achieve high rates of testing coverage across multiple sites and countries are needed.
Several studies have reported HIV testing uptake – the proportion of persons offered HIV testing that agree to test during CBHTC – as a way to assess the effectiveness of CBHTC programs, with uptake ranging from 64% to 98% among those reached.7,11,12,17,18,23 However, uptake measures do not provide an estimate of HIV testing coverage – the proportion of a target population tested – the key metric in determining the effectiveness of universal testing efforts. Among the few costing studies that have reported HIV testing coverage, only two included an individual-level census as part of home-based HTC, and achieved 91% testing coverage among all adults24 and 77% among adults aged 18-35.14 Other studies reported HIV testing coverage from 26% to 80% based on either estimated number of individuals living in the catchment area 7,10,17 or number of households.12,18 To reach the UNAIDS 90-90-90 goal, empirical data on the costs of achieving 90% population-level HIV testing coverage are urgently needed.
In addition, few costing estimates are available to evaluate the feasibility of integrating POC CD4 into CBHTC programs on a large scale.7,10,11,21,24-26 Further, broadening CBHTC to include services for other diseases could improve its cost-effectiveness27 and provide a means to address additional health priorities of communities. WHO has recommended integrated HIV and multi-disease campaigns to be routinely offered in generalized epidemic settings.28 However, very few costing studies have looked at such integrated multi-disease interventions,7,10,11 especially when implemented on a large scale. Finally, mobile, home-based, and venue-based CBHTC approaches have their own strengths and might be more effective in different contexts;28 while existing CBHTC programs fall into one of the three main categories, innovative program design that draws upon the strengths of different types of CBHTC strategies should be explored and evaluated to maximize the efficiency of CBHTC in improving HIV diagnosis in resource-limited settings.
In 2013-14, we achieved 89% adult HIV testing coverage using a hybrid mobile HIV testing approach of two-week multi-disease community health campaigns (CHC) followed by home-based testing (HBT) of CHC non-attendees in 32 communities in Uganda and Kenya during baseline testing for a community cluster-randomized controlled trial.29 To better understand the costs of this innovative CBHTC approach and to inform scalability, we sought to determine the overall cost and efficiency of the SEARCH hybrid mobile testing approach and the costs associated with including POC CD4 testing, multi-disease services, and community mobilization efforts – elements crucial to the success of our hybrid mobile testing approach, but absent in most previously reported CBHTC costing studies.
Methods
In 2013-14, population-wide HIV testing was offered in the SEARCH Trial through a hybrid mobile multi-disease testing approach that combined multi-disease CHCs and home-based HIV testing in 32 communities with a total of 146,906 stable adult residents in southwestern Uganda, eastern Uganda, and western Kenya.29 We applied standard micro-costing methods to estimate the overall costs of population-wide HIV testing as well as the costs associated with specific elements of the hybrid mobile testing approach.
Hybrid Mobile Multi-Disease Testing Approach
The hybrid mobile multi-disease testing approach has been previously described.29 In brief, it consisted of three main intervention components implemented sequentially in each community: door-to-door census enumeration, a multi-disease CHC, and HBT for CHC non-attendees. Census workers gathered demographic information, recorded household locations using handheld global positioning system (GPS) devices, and collected fingerprint biometric measurements. After the 2-4 week long census, a two-week mobile CHC was held at well-known public gathering sites and offered the following testing services: POC rapid HIV antibody testing for all persons in the community regardless of self-reported HIV status, POC CD4 for HIV-infected persons (PIMA, Inverness Medical), hypertension and diabetes screening, and malaria testing and treatment. Immediately after each CHC, individuals ≥15 years of age who did not attend the CHC were located using GPS and biometric identifiers and offered HIV antibody and POC CD4 testing through HBT, which lasted a month on average.29
Community mobilization was a critical aspect of the hybrid mobile testing approach and was incorporated in all intervention components. Mobilization activities included meetings with village leaders and key community representatives (e.g., boda boda [motorcycle taxi] drivers), enlisting community volunteers as campaign promoters, poster and leaflet advertising, radio advertising, information sharing during census, community events at CHC (e.g., soccer games and live music), and gender-specific raffle prizes at CHCs.29
Data Collection and Analysis
We adopted an ingredients-based approach30 to estimate the costs of population-wide HIV testing using the hybrid mobile multi-disease testing approach from the health system's perspective. Our goal was to characterize stable program functioning, as would be relied upon in a large-scale program. Thus, we started cost data collection in the latter half of the first study year when the intervention reached a stable operational state. In total, twelve of the 32 SEARCH Trial communities had their CHCs during this time frame and were included in the current study.
The costs of each intervention component (census, CHC, and HBT) were identified through structured extraction of cost information from expenditure records and study logs, supplemented by interviews with administrative staff and study teams that delivered the services. For the censuses, we conducted a limited number of time and motion exercises to estimate census workers' time spent on enumeration and community mobilization, and to exclude time used only for research purposes (e.g., questions in the census survey to collect social network data).
Standard data collection spreadsheets were used to record cost data from the three regions where independent study teams delivered the intervention under the same protocol. The costing methods emphasized resources utilized and economic costs, rather than financial costs: i.e., where expenditures did not reflect the full cost of the resources used (e.g., donated drugs), we adjusted the valuations by applying the market price instead of the actual expenses of the research project. We classified the resources under four main categories: personnel (including fringe benefits such as health insurance in addition to salary), recurring supplies and services, capital and equipment, and facility space. Costs of capital items were amortized on a straight-line basis over three years for lab equipment and five years for furniture, vehicles and computers, assuming no salvage value. In addition to assignment to these broad categories, we further allocated each resource by activity purpose to estimate the operating costs associated with intervention implementation as opposed to costs incurred only for research purposes (e.g., coordinators' time spent on regulatory activities). The activity purposes also include overhead and administrative costs, training, community mobilization, transportation, counseling, and laboratory testing costs. All costs were converted to U.S. dollars based on the exchange rate on February 17, 2014 at 2463 Ugandan shillings and 86 Kenyan shillings per U.S. dollar.31
Given the scope of the intervention and the challenges in cost data collection across multiple sites and regions, we adopted the “forest and trees” concept to guide our data collection efforts and focused on the “forest” items – i.e., resources or cost details most likely to substantially affect costing results (e.g., personnel or lab tests). By comparison, we spent minimal efforts to collect data for “trees” – resources of low economic value or cost details that would not significantly affect overall patterns (e.g., stationery supplies). After accounting for all resources used in at least one community per region, we examined the list of items that constituted 95% of the total costs and compiled a standard costing priority input list to guide the data collection efforts, which consisted of 81 items for CHC and 36 items for HBT.
For each community, we calculated the total costs of the hybrid mobile testing approach and utilized data on testing coverage to calculate the costs per adult tested and per adult testing HIV-positive (see Supplemental Digital Content 1 for a table listing the unit costs of key inputs). Per-person costs were also calculated for the CHC and HBT. We then calculated mean costs in the 12 study communities in which costing activities took place. Similarly, we calculated the costs of POC CD4 by dividing the total costs of testing supplies by the number of adults who received the test. To estimate the costs by disease, we assigned each input item to specific disease categories based on how they were used and calculated the disease-specific costs accordingly. To put the costing outcome into perspective, we compared the costs, testing coverage, and intervention elements of the hybrid mobile multi-disease testing approach with other CBHTC strategies in sub-Saharan Africa (studies from South Africa excluded due to its classification under upper-middle-income economics32). All previously published costs (in U.S. dollars) were adjusted to 2014 dollars based on the consumer price index.33
Results
The mean operating cost of providing HIV testing with POC CD4 using the hybrid mobile multi-disease testing approach was $92,403 (SD: $14,245) per community in Uganda and Kenya. This included $20,901 (22.6%) for the census, $50,189 (54.3%) for the CHC, and $21,313 (23.1%) for home-based testing.
Community mobilization and POC CD4 testing constituted 15.3% and 6.8% of total costs. On average, 4,505 (SD: 876) adults were tested per community, representing 89% of stable adult residents (i.e. living in the community for at least six months in the year prior to the census).
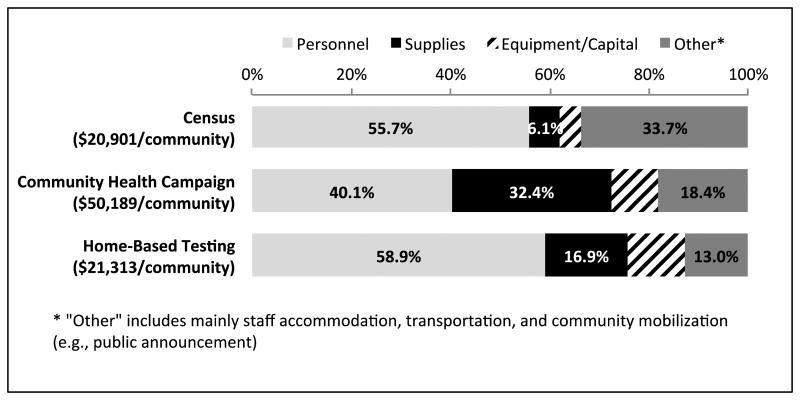
Personnel cost was the biggest input category for all intervention components, representing 56%, 40%, and 59% of the total costs of census, CHC, and HBT respectively. The breakdown of costs by input category is presented in Figure 1.
Figure 1.
Cost breakdown by input category for each intervention component in the hybrid mobile multi-disease testing approach among 12 communities in Uganda and Kenya
The mean cost per adult tested was $20.5 (range: $17.1 - $32.1 [SD: $3.8]) across the 12 communities, including the costs of POC CD4 at $16.0 (SD: $14.5) per HIV-positive individual tested (Table 1). The costs per adult tested were similar across communities, except for an island community in western Kenya where the main transport ferry broke down during the CHC, requiring hiring of a private ferry in addition to the costs associated with providing accommodation to CHC staff on the island. The cost per adult testing HIV-positive was $230.7 (range: $87 - $1,245 [SD: $336.1]), with the variability mainly due to differences in HIV positivity rate across study communities (e.g., adult HIV positivity rate of 3.4% in eastern Uganda and 17.3% in western Kenya).
Table 1. Costs overall and by intervention component using the SEARCH hybrid mobile testing approach.
| Variable | SEARCH Region | |||
|---|---|---|---|---|
|
|
||||
| Western Uganda | Eastern Uganda | Western Kenya | All Communities (SD) | |
| No. of communities | 2 | 5 | 5 | 12 |
| No. of adults tested per community | 5187 | 4935 | 3801 | 4505 (876) |
| - Community health campaign | 4417 | 4260 | 2969 | 3748 (839) |
| - Home-based testing | 771 | 675 | 832 | 756 (284) |
| HIV positivity rate | 6.6% | 3.4% | 17.3% | 8.9% (8.0%) |
| - Community health campaign | 6.5% | 3.6% | 17.5% | 8.8% (8.2%) |
| - Home-based testing | 7.4% | 2.1% | 16.4% | 9.6% (7.7%) |
|
| ||||
| Overall Costs | ||||
|
| ||||
| Per adult tested | $18.9 | $20.4 | $21.6 | $20.5 ($3.8) |
| Per adult tested HIV+ | $284.9 | $599.4 | $125.1 | $230.7 ($336.1) |
| Per POC CD4 test* | $16.9 | $27.8 | $12.7 | $16.0 ($14.5) |
| POC CD4 as % of total costs | 5.9% | 4.7% | 9.8% | 6.8% (2.8%) |
|
| ||||
| Costs by Intervention Component | ||||
|
| ||||
| Per adult enumerated in census | $4.2 | $4.4 | $3.4 | $4.0 ($0.79) |
| Per adult tested | ||||
| - Community health campaign† | $12.9 | $14.1 | $14.0 | $13.8 ($2.04) |
| - Home-based testing‡ | $28.0 | $31.9 | $32.8 | $31.7 ($12.8) |
| Per adult tested HIV+ | ||||
| - Community health campaign† | $191.6 | $378.6 | $78.3 | $153.3 ($209.0) |
| - Home-based testing‡ | $332.0 | $1,334.1 | $184.7 | $298.5 ($2,548.1) |
Point-of-care CD4 testing (PIMA, Inverness Medical) was performed for all individuals tested HIV positive. The costs reported here are incremental and excluded labor.
Costs of community health campaign included the proportion of census costs spent on community mobilization.
Costs of home-based testing included the proportion of census costs spent on obtaining GPS and biometric data of enumerated community members.
The cost per person by intervention component is presented in Table 1. Enumerating community members at the census cost $4.0 (SD: $0.8) per person, including obtaining GPS and fingerprint biometric measurements. The costs per adult tested in the CHC and with HBT were $13.8 (SD: $2.0) and $31.7 (SD: $12.8). The costs per adult testing HIV positive were $153.3 (SD: $209.0) and $298.5 (SD: $2,548.1) for the CHC and HBT, respectively. These costs include POC CD4 testing, and in addition CHC costs include community mobilization and HBT costs include GPS and fingerprint biometric data to assist in tracking CHC non-attendees. In one community in Eastern Uganda, only two adults were HIV-positive among the 315 adults tested by HBT (0.6%); by comparison, 114 out of the 563 adults tested by HBT were HIV-positive in a Kenyan community (20.2%).
Using the CHC platform, multi-disease services were offered at the added cost per person of $1.16 for hypertension and diabetes screening, and $0.90 for malaria screening. These marginal costs included staff time, supplies, and equipment associated with the screening tests, but excluded the overhead costs for setting up CHC, transportation, or community mobilization, which were considered part of HIV testing costs. Overall, overhead costs that were not specific to a given disease represented 53% of total CHC costs. The breakdown of the rest of the CHC costs were 38% for HIV, 4% for hypertension and diabetes, 4% for malaria, and 1% for diarrhea, worms, and TB.
The cost of HIV testing using our hybrid mobile multi-disease testing approach ($20.5 per person tested) is within the range of previously reported mobile, home-based, or venue-based HIV testing programs in sub-Saharan Africa (see Figure 2). The costs per person tested HIV positive were similar to costs reported by other mobile and home-based CBHTC interventions in communities with similar HIV positivity rate, and were on the low end in communities with high HIV positivity rate (e.g., western Kenya). A comparison of cost per person testing HIV positive and HIV positivity rate using different CBHTC strategies is presented in Figure 3.
Figure 2.
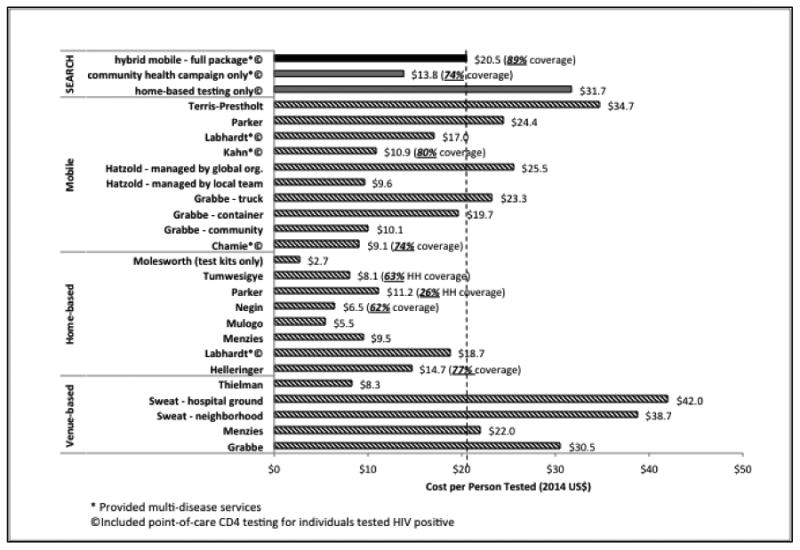
Comparison of cost per person tested and HIV testing coverage using community-based HIV testing and counseling strategies in sub-Saharan Africa (excluding South Africa). See discussion in text.
Figure 3.
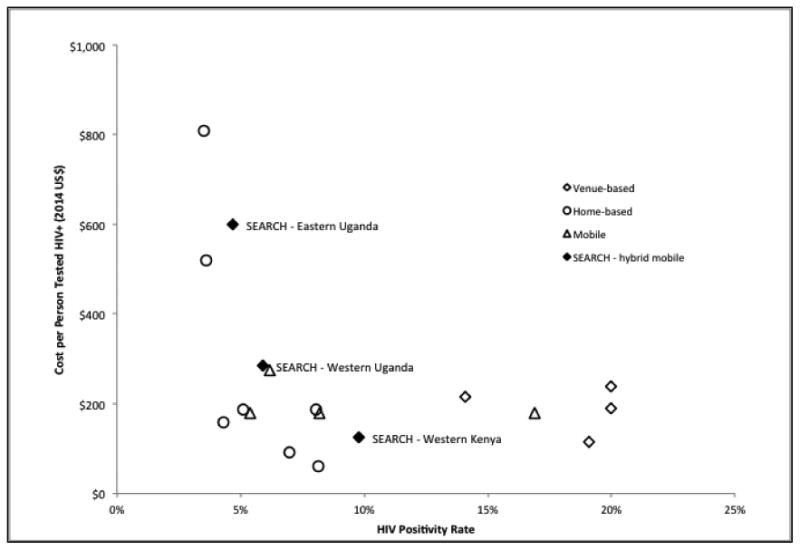
HIV positivity rate (i.e. HIV prevalence among persons tested) and cost per person testing HIV-positive using different community-based HIV testing strategies in sub-Saharan Africa
Discussion
Increasing HIV testing coverage is a major challenge to achieving universal access to HIV prevention, treatment, and care. To inform resource allocation and policy discussions, this study provides cost and efficiency estimates of achieving near-universal HIV testing coverage using an innovative community-based approach in rural communities in East Africa. Integrating mobile multi-disease community health campaigns and home-based HIV testing strategies, this hybrid approach achieved high testing coverage (89% of all stable adult residents of 32 study communities in Uganda and Kenya) at a cost of $20.5 per adult tested and $230.7 per adult testing HIV-positive. Even when including POC CD4 testing and substantial community mobilization efforts, the costs of this hybrid approach are competitive when compared to previously reported CBHTC strategies (further detail below), and offer the added advantage of multi-disease service delivery (using the CHC platform) at relatively low marginal cost.
The two-step design of the hybrid mobile testing approach provides a unique opportunity to assess the incremental cost required to raise HIV testing coverage to the level of the UNAIDS 90-90-90 targets. CHC alone would have achieved 74% HIV testing coverage at a cost of $13.8 per adult tested, before conducting additional testing through the more expensive HBT component ($31.7 per additional person tested after CHC). By comparison, the two-step hybrid mobile testing approach achieved 89% HIV coverage at an overall cost of $20.5 per adult tested. Thus, the difference of $6.7 per person could be considered as the added cost needed to raise HIV testing coverage by 15% to the high level SEARCH achieved. Although HBT appears to be more effective in identifying HIV-positive individuals,29 it remains substantially less efficient than CHC in terms of cost per HIV-positive individual found because the majority of adult residents were tested at CHCs (80% of all adults who tested)29. The cost difference across three regions also suggests that compared with CHC alone, the hybrid testing approach might be most efficient in high-prevalence settings: among CHC non-attendees, the costs per person tested HIV positive through HBT were $1,334 in communities with 3.4% HIV positivity rate (eastern Uganda) and $184.7 in communities with 17.3% HIV positivity rate (Kenya). Thus, future programs could adjust the composition of the hybrid mobile testing approach to maximize its efficiency based on the local contexts. However, it is important to acknowledge that we have not translated cost per person testing positive into cost per disability-adjusted life years (DALY) averted, which could be achieved by a formal cost-effectiveness analysis.
Our results are consistent with the few published estimates of the cost of integrating POC CD4 into community-based HIV testing in sub-Saharan Africa.7,10,11,21,24,34 A shorter five-day mobile testing campaign conducted in Uganda in 2011, when adjusted to scaled-up operation by amortizing equipment costs, indicates a lower cost of $9.1 per person tested, notably with no initial census conducted, no HBT for CHC non-attendees, and less intensive community mobilization (all costs below in 2014 U.S. dollars).7 In Kenya, a seven-day integrated prevention campaign in 2008 provided HIV testing at a cost of $11 per person, also without HBT or a precise population estimate.10 A multi-disease testing approach was also used in 2011 in Lesotho, with costs per person tested of $18.7 and $17.0 in home-based and mobile campaigns respectively.11 Additional costing estimates from South Africa have reported costs per person HIV tested of $30.2 using a mobile unit 21 and $25.8 using home-based testing, which could be further reduced to $13.5 with improved efficiency and task shifting.24 Looking at POC CD4 testing alone, the costs per test (excluding labor) were $17.8 in South Africa,34 $12.5 in Uganda,7 and $7.9 in South Africa,21 compared with our result of $16.0.
Among the few costing studies of HIV testing in the literature that report testing coverage for a given target population, most are home-based CBHTC programs. By design, door-to-door testing requires health workers to visit each household in the target population, making it easier to evaluate testing coverage compared to mobile or venue-based CBHTC strategies. The highest testing coverage to date was reported from a home-based CBHTC program that offered POC CD4 testing and facilitated referral in South Africa: 91% of all targeted adults were tested at a cost of $25.8 per person in a research model and $13.5 in an operational model.24 However, this program had a relatively small target population of 739 adults, was conducted in a geographically distinct area of contiguous households within walking distance to a health center, and lasted for a year.35 On the other hand, our hybrid mobile testing approach was able to achieve high HIV testing coverage at similar costs on a much larger scale in rural areas where dwellings were often very far apart and access to roads was limited. The results from our study provide further evidence for the feasibility of integrating POC CD4 diagnostics in population-level HIV testing programs.
As countries in sub-Saharan Africa work towards achieving the UNAIDS 90-90-90 targets, there is a need to adopt new strategies that produce positive outcomes without dramatically increasing costs. While providing an efficient and low-cost model to achieve universal HIV diagnosis, the hybrid testing approach also leverages HIV infrastructure to tackle other emerging health challenges. The integration of HIV and multi-disease services offers an opportunity for early detection of noncommunicable diseases (NCDs), and has the potential to lead to earlier treatment with subsequent reduction in NCD morbidity and mortality. The multi-disease approach may also provide a means to cope with the stigma associated with seeking HIV testing by offering broad health services in a single setting. Lastly, we suspect that community engagement and mobilization were a major factor in the high testing coverage achieved with the hybrid approach, and our data provide an estimate of the costs associated with mobilization efforts – a cost not routinely shared in prior published literature.
Our study has several limitations. First, the costing analysis only included 12 out of the 32 communities in the SEARCH trial and was conducted at a relatively stable stage of intervention implementation. Thus, we are not able to report start-up costs associated with this novel program, or fully assess the variability in costs across all SEARCH sites. Second, the present analysis does not provide cost estimates for linkage to care or viral suppression. As we collect data on treatment outcomes, subsequent analyses will estimate the cost-effectiveness of the hybrid mobile testing approach. Third, our hybrid mobile testing campaign provided HIV testing to all community members regardless of their testing history. Thus, the cost of identifying HIV positive individuals that are unaware of their status might be much higher than the costs we reported here. While this information would be informative for resource allocation to reach the first goal in the 90-90-90 target, we believe that the hybrid mobile testing approach implemented in similar settings in the future should still provide HIV and multi-disease services to all community members to minimize errors in self-reported HIV status, improve linkage to care, avoid stigmatization, and leverage the synergies of combining HIV and multi-disease services. Lastly, we did not conduct scenario analyses to model scale-up costs. Given the large scope of the SEARCH trial and the inherent differences in the communities located in the three regions in Uganda and Kenya, the hybrid mobile testing approach we costed resembles “scaled-up” versions of such an approach, and as such the costs reported here are relevant for future policy and program decisions.
In conclusion, the hybrid mobile testing approach achieved near-universal HIV testing coverage at a cost similar to previously reported community-based mobile, home-based, or venue-based HIV testing implementations in sub-Saharan Africa. It demonstrated POC CD4 was affordable in population-wide HIV testing and multi-disease services could be offered at low marginal costs by leveraging HIV testing infrastructure. Community mobilization was a significant component both in terms of costs and as a reason for the success of this community-based approach.
Supplementary Material
Acknowledgments
We acknowledge the SEARCH costing team members based in Uganda and Kenya for their extraordinary efforts in data collection, including Aine Ronald Mwesigye, Assurah Elly, Easter Olwanda, Imukeka Haawa, Kwizera Enos, and Mwebaza Betty.
Sources of Support: Research reported in this publication was supported by Division of AIDS, NIAID of the National Institutes of Health under award number U01AI099959 and in part by the President's Emergency Plan for AIDS Relief and Gilead Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, PEPFAR, or Gilead. The SEARCH project gratefully acknowledges the Ministries of Health of Uganda and Kenya, our research team, collaborators and advisory boards, and especially the 12 study communities and participants involved.
Footnotes
Meeting Presentation: CROI, Boston, February 24, 2016 (Poster Presentation)
Conflicts of Interest: None
References
- 1.UNAIDS. Fact sheet 2014. 2014 http://www.unaids.org/sites/default/files/media_asset/20150714_FS_MDG6_Report_en.pdf.
- 2.UNAIDS. Gap report. Geneva, Switzerland: UNAIDS; 2014. [Google Scholar]
- 3.Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A. Can the UNAIDS 90-90-90 target be achieved?. Analysis of 12 national level HIV treatment cascades; Paper presented at: 8th IAS Conference on HIV Pathogenesis Treatment and Prevention; 18-22 July 2015; Vancouver, Canada. 2015. [Google Scholar]
- 4.UNAIDS 90-90-90 - An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); Oct, 2014. 2014. [Google Scholar]
- 5.Suthar AB, Ford N, Bachanas PJ, et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10(8):e1001496. doi: 10.1371/journal.pmed.1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2013. p. 2013. [PubMed] [Google Scholar]
- 7.Chamie G, Kwarisiima D, Clark TD, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One. 2012;7(8):e43400. doi: 10.1371/journal.pone.0043400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabbe KL, Menzies N, Taegtmeyer M, et al. Increasing access to HIV counseling and testing through mobile services in Kenya: strategies, utilization, and cost-effectiveness. J Acquir Immune Defic Syndr. 2010;54(3):317–323. doi: 10.1097/QAI.0b013e3181ced126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatzold K, Czerwinski L, Joseph D, Rupanga M, Mushayi W, Dhlamini R. A comparison of four HIV testing and counseling service delivery models in Zimbabwe: where to best invest for reaching high-risk groups. AIDS 2008 - XVII International AIDS Conference. Vol Abstract TUPE04652008. [Google Scholar]
- 10.Kahn JG, Harris B, Mermin JH, et al. Cost of community integrated prevention campaign for malaria, HIV, and diarrhea in rural Kenya. BMC Health Serv Res. 2011;11:346. doi: 10.1186/1472-6963-11-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labhardt ND, Motlomelo M, Cerutti B, et al. Home-based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster-randomized trial. PLoS Med. 2014;11(12):e1001768. doi: 10.1371/journal.pmed.1001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker LA, Jobanputra K, Rusike L, et al. Feasibility and effectiveness of two community-based HIV testing models in rural Swaziland. Trop Med Int Health. 2015 doi: 10.1111/tmi.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terris-Prestholt F, Kumaranayake L, Foster S, et al. The role of community acceptance over time for costs of HIV and STI prevention interventions: analysis of the Masaka Intervention Trial, Uganda, 1996-1999. Sex Transm Dis. 2006;33(10 Suppl):S111–116. doi: 10.1097/01.olq.0000175389.10289.ba. [DOI] [PubMed] [Google Scholar]
- 14.Helleringer S, Mkandawire J, Reniers G, Kalilani-Phiri L, Kohler HP. Should home-based HIV testing and counseling services be offered periodically in programs of ARV treatment as prevention? A case study in Likoma (Malawi) AIDS Behav. 2013;17(6):2100–2108. doi: 10.1007/s10461-012-0365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menzies N, Abang B, Wanyenze R, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23(3):395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 16.Mulogo EM, Batwala V, Nuwaha F, Aden AS, Baine OS. Cost effectiveness of facility and home based HIV voluntary counseling and testing strategies in rural Uganda. Afr Health Sci. 2013;13(2):423–429. doi: 10.4314/ahs.v13i2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negin J, Wariero J, Mutuo P, Jan S, Pronyk P. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Trop Med Int Health. 2009;14(8):849–855. doi: 10.1111/j.1365-3156.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 18.Tumwesigye E, Wana G, Kasasa S, Muganzi E, Nuwaha F. High uptake of home-based, district-wide, HIV counseling and testing in Uganda. AIDS Patient Care STDS. 2010;24(11):735–741. doi: 10.1089/apc.2010.0096. [DOI] [PubMed] [Google Scholar]
- 19.Sweat M, Gregorich S, Sangiwa G, et al. Cost-effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. Lancet. 2000;356(9224):113–121. doi: 10.1016/S0140-6736(00)02447-8. [DOI] [PubMed] [Google Scholar]
- 20.Thielman NM, Chu HY, Ostermann J, et al. Cost-effectiveness of free HIV voluntary counseling and testing through a community-based AIDS service organization in Northern Tanzania. Am J Public Health. 2006;96(1):114–119. doi: 10.2105/AJPH.2004.056796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassett IV, Govindasamy D, Erlwanger AS, et al. Mobile HIV screening in Cape Town, South Africa: clinical impact, cost and cost-effectiveness. PLoS One. 2014;9(1):e85197. doi: 10.1371/journal.pone.0085197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marseille E, Dandona L, Marshall N, et al. HIV prevention costs and program scale: data from the PANCEA project in five low and middle-income countries. BMC Health Serv Res. 2007;7:108. doi: 10.1186/1472-6963-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molesworth AM, Ndhlovu R, Banda E, et al. High accuracy of home-based community rapid HIV testing in rural Malawi. J Acquir Immune Defic Syndr. 2010;55(5):625–630. doi: 10.1097/QAI.0b013e3181f98628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith JA, Sharma M, Levin C, et al. Cost-effectiveness of community-based strategies to strengthen the continuum of HIV care in rural South Africa: a health economic modelling analysis. Lancet HIV. 2015;2(4):e159–e168. doi: 10.1016/S2352-3018(15)00016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson BA, Schnippel K, Ndibongo B, et al. Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: an evaluation of a pilot program in South Africa. J Acquir Immune Defic Syndr. 2012;61(2):e13–17. doi: 10.1097/QAI.0b013e31825eec60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wynberg E, Cooke G, Shroufi A, Reid SD, Ford N. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc. 2014;17:18809. doi: 10.7448/IAS.17.1.18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn JG, Muraguri N, Harris B, et al. Integrated HIV testing, malaria, and diarrhea prevention campaign in Kenya: modeled health impact and cost-effectiveness. PLoS One. 2012;7(2):e31316. doi: 10.1371/journal.pone.0031316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Consolidated guidelines on HIV testing services 2015. Genera, Switzerland: WHO; Jul, 2015. p. 2015. [Google Scholar]
- 29.Chamie G, Clark T, Kabami J, et al. A hybrid mobile HIV testing approach for population-wide HIV testing in rural East Africa: An observational study. The Lancet HIV. 2016 doi: 10.1016/S2352-3018(15)00251-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drummond M, O'Brien B, Stoddart G, Torrance G. Methods for the economic evaluation of health care programmes. Oxford: Oxford Medical Publications; 1997. [Google Scholar]
- 31. [February 17, 2014];XE Currency Charts. 2015 2015 http://www.xe.com/currencycharts/
- 32.World Bank. Country and lending groups. 2015:2015. http://data.worldbank.org/about/country-and-lending-groups.
- 33.Bureau of Labor Statistics U.S. Department of Labor. Consumer Price Index. 2015:2015. http://www.bls.gov/cpi/
- 34.Larson B, Schnippel K, Ndibongo B, Long L, Fox MP, Rosen S. How to estimate the cost of point-of-care CD4 testing in program settings: an example using the Alere Pima Analyzer in South Africa. PLoS One. 2012;7(4):e35444. doi: 10.1371/journal.pone.0035444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Rooyen H, Barnabas RV, Baeten JM, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64(1):e1–8. doi: 10.1097/QAI.0b013e31829b567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.