Abstract
Purpose of review
To present recent findings and perspectives on the relationship between early life respiratory infections and asthma inception and to discuss emerging concepts on strategies that target these infectious agents for asthma prevention.
Recent findings
Cumulative evidence supports the role of early life viral infections, especially respiratory syncytial virus and human rhinovirus, as major antecedents of childhood asthma. These viruses may have different mechanistic roles in the pathogenesis of asthma. The airway microbiome and virus-bacteria interactions in early life have emerged as additional determinants of childhood asthma. Innovative strategies for the prevention of these early life infections, or for attenuation of acute infection severity, are being investigated and may identify effective strategies for the primary and secondary prevention of childhood asthma.
Summary
Early life infections are major determinants of asthma development. The pathway from early life infections to asthma is the result of complex interactions between the specific type of the virus, genetic and environmental factors. Novel intervention strategies that target these infectious agents have been investigated in proof-of-concepts trials, and further study is necessary to determine their capacity for asthma prevention.
Keywords: asthma, infections, microbiome, Respiratory syncytial virus, Human rhinovirus
INTRODUCTION
Infection-related wheezing in early childhood is common and may be the first presentation of asthma. Much research has focused on the role of early life respiratory syncytial virus (RSV) and human rhinovirus (HRV) infections in asthma inception. In addition, emerging data suggest that the composition of the airway microbiome in early life and virus-bacteria interactions are important determinants of future asthma. This review summarizes recent findings regarding the role of early life infections in asthma inception, and how interventions directed against these infectious agents may allow for asthma prevention.
THE ROLE OF EARLY LIFE RESPIRATORY VIRAL INFECTIONS IN ASTHMA INCEPTION
Respiratory syncytial virus
Early life severe RSV bronchiolitis confers substantial risk for subsequent wheezing and asthma development1–5. For the purpose of this review, we will use the term “severe RSV bronchiolitis” to refer to an episode of bronchiolitis requiring hospitalization. The highest rates of asthma development following severe RSV bronchiolitis has been reported in the RSV Bronchiolitis in Early Life (RBEL) study, a prospective cohort of infants hospitalized for RSV bronchiolitis. By their 7th birthday, almost half of these infants had a physician diagnosis of asthma5. A more recent prospective European study6 has also demonstrated the high prevalence of asthma following severe RSV bronchiolitis, with 21% of infants hospitalized for RSV bronchiolitis having asthma at age 6 years compared to 5% of a control cohort. Early life hospitalization for RSV bronchiolitis was also associated with the development of obstructive pattern in pulmonary function testing and elevated FeNO levels6.
There is direct relationship between the risk of future asthma and the severity of the initial RSV lower respiratory tract infection (LRTI), with the greatest risk of asthma following episodes that required hospitalization7, 8. This severity-outcome association may explain the observation that increased asthma risk persisted into early adulthood in a cohort of children hospitalized in infancy for RSV LRTI 9; while among children who had less severe RSV bronchiolitis, with predominantly outpatient episodes, this association diminished with age and became non-significant by the age of 13 years10. Environmental factors may augment asthma risk, even among children who had less severe disease not requiring hospitalization: young adults who experienced generally mild RSV LRTI in early life were more likely to have current asthma if they became active smokers11. The authors suggested that airway abnormalities preexisting RSV LRTI and/or airway damage caused by RSV may increase susceptibility to active cigarette smoking, which results in asthma11.
A detailed description of mechanisms that may mediate the progression from severe RSV LRTI to asthma is beyond the scope of this review and are discussed elsewhere12–14.
Human rhinovirus
Advances in virologic detection techniques have led to the identification of early life HRV LRTI (especially HRV-C) as a very important determinant of future asthma. Insightful data on the role of HRV in the inception of asthma have originated form Childhood Origin of Asthma (COAST) study, a birth cohort of children at high risk for allergic diseases and/or asthma15. Early life HRV-associated wheezing LRTIs (mostly outpatient episodes) were a significant predictor of asthma at age six years16 and of an obstructive lung function pattern at age 5–8 years17. Asthma risk was much higher after HRV LRTI compared to the risk following RSV LRTI16. Similar findings were obtained from the Australian Childhood Asthma Study (CAS), a birth cohort infants at high risk of atopy18. However, it should be noted that these 2 cohorts were comprised of children at high risk to develop asthma; therefore, these findings may not necessarily be generalizable to other populations. Nevertheless, these findings highlight the importance of early life HRV LRTI in asthma pathogenesis in these populations. Furthermore, the highest risk for future asthma was detected among children with genetic variants in an asthma-associated gene locus on chromosome 17 (17q21) who experienced early life HRV wheezing illnesses, highlighting the importance of interactions between the child’s genetic background and the viral infection 19.
RSV AND HRV MAY HAVE DIFFERENT ROLES IN ASTHMA PATHOGENESIS: IMPLICATIONS FOR PREVENTION STRATEGIES
Despite intensive investigation, it remains uncertain if severe early life viral LRTI causes future asthma by causing airway epithelial injury and/or creating the appropriate pro-inflammatory allergenic milieu that with a subsequent allergen exposure, in the appropriate time-frame, could result in allergic airway inflammation and asthma; or whether the wheezing viral LRTI serves as a marker for asthma susceptibility among children with the appropriate genetic background20,21.
Children who have their initial wheeze during HRV illnesses differ from those who experience their initial wheeze during RSV LRTI: they tend to have personal and family history of asthma, and are usually older20, 22. These differences may be related, at lest in part, to different study designs, but may also suggest that the initial wheezing HRV LRTI may serve as a marker for asthma tendency, while early life RSV bronchiolitis (especially the severe episodes) may have a causative role in asthma inception. The potential causal role of RSV infection in asthma inception is supported by the finding that treatment of late preterm infants, during the RSV season, with the anti-RSV monoclonal antibody palivizumab, resulted in reductions in the occurrence of RSV bronchiolitis and the number of wheezing days during the first year of life even following the treatment period23. These results suggest that primary prevention of RSV bronchiolitis may be an effective strategy to prevent post-RSV recurrent wheeze and potentially asthma 23.
The presence of atopy (personal or family) has a role in the development of post-RSV asthma, as maternal asthma was a risk factor for a more severe RSV bronchiolitis24 and for the development of post-RSV asthma5. Atopic predisposition is also a very significant risk factor for the development of asthma following HRV LRTI. This concept was shown in two reports demonstrating that atopy among children who wheeze with HRV may modify their asthma risk. Having a mother with atopic asthma significantly increased the infant’s risk of having HRV LRTI compared to RSV LRTI; and having a mother with asthma increased the severity of infant HRV but not RSV LRTI25. Moreover, the sequence of acquisition of allergic sensitization and experiencing HRV wheezing is non-random, as sensitization to aeroallergens preceded, and was a significant risk factor for, HRV wheezing illness26, whereas having an HRV wheezing illness was not a risk factor for the development of allergic sensitization. This sequence of events was confirmed in a report from the CAS high risk birth cohort, where LRTI associated-wheeze was associated with the presence of HRV (mainly HRV-C), but only in high-risk children who developed allergic sensitization during infancy 27. Collectively, these results suggest that HRV wheezing illness followed, rather than predisposed to, the development of atopy, at least children who are at high-risk for asthma/atopy development.
These potential differences in the contributions of RSV vs. HRV LRTI to subsequent asthma risk suggest the need for potentially virus-specific strategies for asthma prevention. As evidence suggest RSV’s probably has a causal role in the development of asthma, prevention of RSV infection and/or attenuation of RSV illness severity and/or the associated inflammatory response may reduce the risk of subsequent asthma. On the other hand, as allergic sensitization precedes HRV-LRTI, prevention of the development of allergic sensitization among young children may prevent HRV-LRTI, and thus may also reduce the likelihood of asthma.
Finally, although studies to date have focused on the role of specific viruses (i.e., RSV and HRV) causing the initial wheezing illness and in asthma inception, it is likely that these 2 pathways are not independent. Given the necessity for multiple wheezing episodes, most of which occur in the setting of viral infections, to precede an asthma diagnosis, the outcome of childhood asthma is likely to follow occurrence of multiple viral-associated wheezing episodes due to multiple different viruses. More research on this topic is needed, but results of a recent study support this concept by reporting that the number of RTIs in early life, but not the specific viral trigger, was associated with development of asthma at school age28.
THE ROLE OF THE AIRWAY MICROBIOME IN ASTHMA INCEPTION
Airway bacterial presence and asthma inception
In addition to the long-established association between early-life viral respiratory infections and subsequent asthma20, 21, bacterial microbes are detectable in the airway and comprise the airway microbiome have recently emerged as significant contributors to asthma inception and exacerbation29. The contribution of the airway microbiome to asthma inception should be viewed in the broader context of the effects of the environmental and enteral microbiomes on the immune system, as exposure to a wide range of microbes has found to be protective against asthma development. These topics are reviewed by Drs. Boushey and Lynch in this issue of the journal(Please insert a citation to Dr. Lynch’s manuscript published in this issue of the journal (ACI160215)).
Asymptomatic bacterial colonization of the hypopharynx at the age of one month was associated with higher risk of developing persistent wheezing and asthma30. Moreover, bacteria, either as an isolated pathogen or together with a virus, were identified in 86% of wheezing episodes during the first 3 years of life31. The authors suggested that the commonly used term “viral wheeze” is inappropriate as it underestimates the role of bacteria in the pathophysiology of these wheezing episodes31.
A subsequent nested-case-control study from this group provides additional mechanistic rationale to explain the role of airway bacteria in asthma inception. The investigators compared the immune responses to H. influenzae, M. catarrhalis, and S. pneumoniae, in PBMCs obtained at the age of 6 months, between children who were subsequently diagnosed with asthma at the age of 7 years and children who did not develop asthma32. Children who eventually developed asthma had an aberrant early life immune response evident by increased IL-5, IL-13, IL-17, and IL-10 production. The investigators hypothesized that aberrant immune response to pathogenic bacteria in the airways may predispose to persistent airway colonization of the bacteria, which in turn may result in Th-2 chronic airway inflammation, and eventually asthma32. However, the exact direction of the association between early life airway colonization with bacteria and the aberrant immune response is yet to be determined.
Bacteria-virus interactions and the inception of asthma
The ability to characterize airway bacterial communities using genetic based techniques has highlighted the importance of bacteria and virus interplay in asthma inception and exacerbations. Kloepfer et al33 performed viral studies and PCR for common airway bacteria in upper-airway samples obtained weekly from school aged children with and without asthma during the peak HRV season. HRV infection predisposed to subsequent airway bacterial infection and/or colonization. Furthermore, the presence of M catarrhalis and S pneumoniae in the airway contributed to the severity of respiratory tract illnesses, including asthma exacerbations33. The investigators proposed that the secondary bacterial infections, potentially caused by disruption of the mucosal barrier by the virus, may augment the ongoing inflammatory response and could result in asthma exacerbation.
Another recent report provided valuable information on the composition of the bacterial and viral airway microbiomes during health and disease periods, during the first year of life, and their relationship with the development of chronic wheeze27. The investigators performed 16S sequencing and screened for the presence of common respiratory viruses in nasopharyngeal samples collected during the first year of life at well visits and during acute respiratory infections (ARI) among participants of the Childhood Asthma Study (CAS)18. The upper airway microbiome in infancy was found to have a simple structure dominated by only six genera, and each genus was dominated by a single species. Staphylococcus, Corynebacterium, and Alloiococcus dominated the samples taken during well visits, and could be viewed as the component of a “healthy” airway microbiome; while Streptococcus, Moraxella, and Haemophilus became more dominant during viral ARIs27. These findings are similar to previous report on this topic34. Environmental factors shaped the microbiome structure, as antibiotic use and day-care attendance significantly selected for more “pathogenic” bacteria in “well samples”.
Furthermore, the airway microbiome composition during viral infections affected the severity of ARI and the likelihood of progression to lower respiratory symptoms27. Specifically, the presence of Streptococcus, Moraxella, and Haemophilus (collectively or individually) during RSV (but not HRV) infection was associated with progression from URI to LRTI. A specific interaction was noted between Moraxella and RSV, as the presence of Moraxella during RSV-LRTI was associated with a more severe RSV infection evidenced by concomitant fever. The following risk factors were identified for chronic wheeze at age of 5 years: 1) asymptomatic high-abundance colonization with Streptococcus before the age of 2 months, 2) febrile LRTI, 3) HRV-wheeze, but only among infants who developed early life sensitization, and 4) young age at the time of first febrile LRTI27. Finally, a potential causal pathway linking antibiotic use to later asthma was proposed: antibiotic use in infants may select for illness-associated bacteria in the airway microbiome, leading to increased risk of febrile LRTI, which in-turn is associated with asthma development.
INFECTIOUS AGENTS: TARGETS FOR ASTHMA PREVENTION
Given the strong associations between early life respiratory infection and subsequent asthma, strategies which either prevent the development of early life respiratory infections or attenuate the severity of infection and the consequences of the immune response to the pathogen may lead to reductions in asthma risk. HRV therapeutics are currently not available mainly due to objective obstacles related to the complex structure of this virus22. However, numerous RSV vaccines and antiviral are currently in development 35. This section highlights some of the recent advancements in this field.
Active and passive RSV vaccines
As noted above, the prevention of severe RSV-LRTI utilizing palivizumab resulted reduction in recurrent wheeze during the 1st year of life23. These results support the potential causative role of RSV infection in asthma inception, and provide encouragement that post-RSV asthma may be preventable, which may result in significant reduction in new asthma cases36. However, the substantial expense of palivizumab, the need to treat a broad population early in life and prior to acquisition of RSV infection, and the need for parenteral administration limit its widespread use. Therefore, there is a need for additional RSV therapeutics that overcome these limitations.
Primary prevention of RSV infection via early life active immunization
Efforts to develop an effective RSV vaccine have continued since the 1960s but, until recently, were generally unsuccessful or even harmful37. A major recent advancement in this field includes the utilization of a structure-based approach to develop an innovative RSV vaccine directed against a specific antigenic site of the RSV fusion (F) glycoprotein, a target of RSV-neutralizing antibodies38. This vaccine has shown promising results in mice studies38. Moreover, a genetically engineered live-attenuated vaccine was effective in a phase 1 study in children and adults in producing neutralizing antibodies and to preventing illnesses39.
RSV antiviral therapies
Currently, ribavirin is the only anti-viral therapy available for RSV; however, its utilization is limited by modest efficacy and side effects. Development of new anti-viral medications is ongoing and recent studies have reported that the use of an oral RSV-cell-entry inhibitor40 and an oral nucleoside analogue41 among healthy adults resulted in faster reduction of RSV load accompanied with a reduction in disease severity. Given the association between the severity of the acute RSV infection and post-RSV asthma, the attenuation of disease severity by such RSV therapeutics could reduce in the risk of post-RSV asthma.
Attenuation of the inflammatory response during the acute RSV LRTI
As there is no cost effective, broadly available, simple therapeutic option capable of preventing severe RSV bronchiolitis, there is an ongoing need for an intervention capable of modifying the outcomes of post-RSV asthma. Based on the anti-inflammatory properties of azithromycin in other inflammatory airway diseases42 and our previous findings in a murine model of viral bronchiolitis43, we performed a proof-of-concept, double-blinded, randomized trial in 40 infants hospitalized with RSV bronchiolitis investigating the utility of adding 2 weeks of azithromycin therapy to routine bronchiolitis care. Infants who received azithromycin had a significant lower likelihood if developing recurrent wheeze and had fewer days with respiratory symptoms over the subsequent year44. Anti-inflammatory effects may have mediated some of the beneficial effects of azithromycin, as azithromycin reduced levels of upper-airway IL-8, a major neutrophil chemoattractant44. However, and in contrast to previous in vitro reports45, 46, we did not detect a reduction in viral load among children treated with azithromycin47.
Prevention of post-HRV wheezing
A recent trial suggested that systemic corticosteroids may prevent the respiratory sequelae of post-RSV wheezing in a subset of children presenting with high HRV loads in the airway. Toddlers presenting in the first HRV-wheeze episode were randomized to receive oral prednisolone or placebo for 4 day48. Overall, there was no difference between the groups in the primary outcomes: wheezing occurrences and initiation of asthma controller medication within 12 months48. However, a stratified analysis showed that children with high HRV loads who were treated with prednisolone had longer time to the next wheezing episode over the following 12 months. The investigators hypothesized that high HRV load is a marker for significant airway inflammation, which identifies children who may benefit from oral corticosteroids therapy given as a modifier of the short-term respiratory sequel of post-HRV wheeze48. It is still unknown if this intervention provides long-term impact, and studies with longer follow-up periods are needed to address this question.
CONCLUSIONS
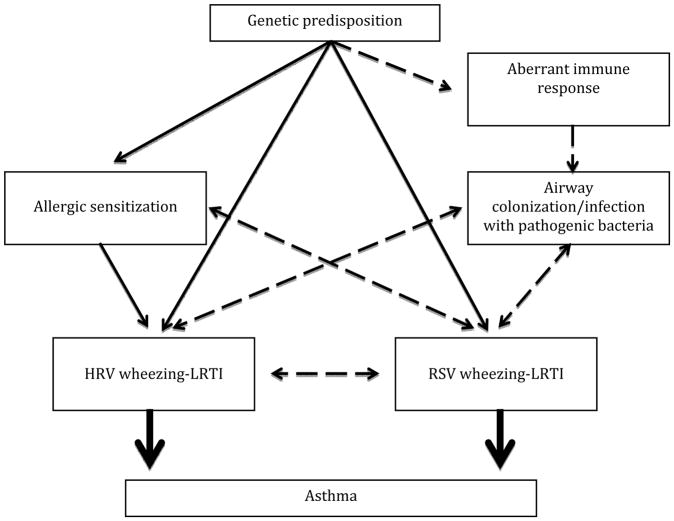
Current evidence supports the major roles of early life RSV and HRV infections in asthma inception. Asthma prevention approaches targeting these viral triggers may need to differ according to the specific causative virus leading to the initial wheezing LRTI episode. The airway microbiome composition in early life and bacteria-virus interactions have emerged as relevant determinants of childhood asthma (Figure 1). Emerging data have shown that species of the Streptococcus, Moraxella, and Haemophilus genera are associated with respiratory illnesses and with asthma development; hence, airway microbiome modifications, which specifically target these bacteria, should be pursued as potential asthma prevention strategies. Finally, interventions aimed at preventing early life respiratory infections and/or attenuating the inflammatory response during the acute illness are currently under intense research and development, and may allow us to achieve the ultimate goal of prevention of childhood asthma.
Figure 1.
potential pathways illustrating the contribution of airway infections on the inception of childhood asthma
KEY POINTS.
Early life lower respiratory tract infections caused by respiratory syncytial virus and human rhinovirus are significant risk factors for childhood asthma.
Severe respiratory syncytial virus infections may have a casual role in asthma inception.
Emerging data identify the airway microbiome and virus-bacteria interactions as important determinants of childhood asthma.
Prevention of early life infections, and/or attenuation of the acute infection severity, may serve as approaches for the prevention of childhood asthma.
Acknowledgments
Funding information:
Acknowledgements: none.
Financial support or sponsorship: This publication was supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences, sub award KL2 TR000450.
conflicts of interest: none.
ABBREVIATIONS
- ARI
Acute respiratory infection
- CAS
Australian Childhood Asthma Study
- COAST
Childhood Origin of Asthma
- FeNO
Fractional Exhaled Nitric Oxide Levels
- HRV
Human rhinovirus
- LRTI
Lower respiratory tract infection
- RBEL
RSV Bronchiolitis in Early Life
- RSV
Respiratory syncytial virus
References
- 1.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–5. [PubMed] [Google Scholar]
- 2.Sigurs N. Epidemiologic and clinical evidence of a respiratory syncytial virus-reactive airway disease link. Am J Respir Crit Care Med. 2001;163:S2–6. doi: 10.1164/ajrccm.163.supplement_1.2011109. [DOI] [PubMed] [Google Scholar]
- 3.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 4.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 5.Bacharier LB, Cohen R, Schweiger T, Yin-Declue H, Christie C, Zheng J, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130:91–100. e3. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zomer-Kooijker K, van der Ent CK, Ermers MJ, Uiterwaal CS, Rovers MM, Bont LJ, et al. Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLoS One. 2014;9:e87162. doi: 10.1371/journal.pone.0087162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–61. 61 e1. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escobar GJ, Masaquel AS, Li SX, Walsh EM, Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97. doi: 10.1186/1471-2431-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–52. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 10.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 11*.Voraphani N, Stern DA, Wright AL, Guerra S, Morgan WJ, Martinez FD. Risk of current asthma among adult smokers with respiratory syncytial virus illnesses in early life. Am J Respir Crit Care Med. 2014;190:392–8. doi: 10.1164/rccm.201311-2095OC. a report suggesting that active smoking may increase the risk of developing post-RSV asthma even after mild RSV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Holt PG. The mechanism or mechanisms driving atopic asthma initiation: The infant respiratory microbiome moves to center stage. J Allergy Clin Immunol. 2015;136:15–22. doi: 10.1016/j.jaci.2015.05.011. A comprehensive review on the mechanisms by which infectious again may lead to childhood asthma. [DOI] [PubMed] [Google Scholar]
- 13.Saglani S. Viral infections and the development of asthma in children. Ther Adv Infect Dis. 2013;1:139–50. doi: 10.1177/2049936113497202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Geerdink RJ, Pillay J, Meyaard L, Bont L. Neutrophils in respiratory syncytial virus infection: A target for asthma prevention. J Allergy Clin Immunol. 2015;136:838–47. doi: 10.1016/j.jaci.2015.06.034. A comprehensive review on the role of neutrophilic inflammation during RSV infection and how it may lead to asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13(Suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 16.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton R, et al. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. The Journal of allergy and clinical immunology. 2011;128:532–8. e1–10. doi: 10.1016/j.jaci.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. The Journal of allergy and clinical immunology. 2007;119:1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caliskan M, Bochkov YA, Kreiner-Moller E, Bonnelykke K, Stein MM, Du G, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191:34–44. doi: 10.1164/rccm.201405-0901PP. A comprehensive review summarizing data on the role of RSV and HRV in asthma inceptions and identifying potential pathways for asthma prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigelman A, Bacharier LB. The role of early life viral bronchiolitis in the inception of asthma. Curr Opin Allergy Clin Immunol. 2013;13:211–6. doi: 10.1097/ACI.0b013e32835eb6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson DJ. Early-life viral infections and the development of asthma: a target for asthma prevention? Curr Opin Allergy Clin Immunol. 2014;14:131–6. doi: 10.1097/ACI.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–9. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 24.Bradley JP, Bacharier LB, Bonfiglio J, Schechtman KB, Strunk R, Storch G, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115:e7–14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]
- 25.Carroll KN, Gebretsadik T, Minton P, Woodward K, Liu Z, Miller EK, et al. Influence of maternal asthma on the cause and severity of infant acute respiratory tract infections. The Journal of allergy and clinical immunology. 2012;129:1236–42. doi: 10.1016/j.jaci.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–5. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–15. doi: 10.1016/j.chom.2015.03.008. A manuscript that provides comprehensive data on the composition of the bacterial and viral airway microbiomes during health and disease periods in the first year of life, and their relationship with the development future chronic wheeze. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnelykke K, Vissing NH, Sevelsted A, Johnston SL, Bisgaard H. Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015;136:81–6. e4. doi: 10.1016/j.jaci.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beigelman A, Weinstock GM, Bacharier LB. The relationships between environmental bacterial exposure, airway bacterial colonization, and asthma. Curr Opin Allergy Clin Immunol. 2014;14:137–42. doi: 10.1097/ACI.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–95. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 31*.Carlsson CJ, Vissing NH, Sevelsted A, Johnston SL, Bonnelykke K, Bisgaard H. Duration of wheezy episodes in early childhood is independent of the microbial trigger. J Allergy Clin Immunol. 2015;136:1208–14. e5. doi: 10.1016/j.jaci.2015.05.003. A report highlighting the major role of bacteria in the pathogenesis of wheezing exacerbations among preschool children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Larsen JM, Brix S, Thysen AH, Birch S, Rasmussen MA, Bisgaard H. Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J Allergy Clin Immunol. 2014;133:1008–13. doi: 10.1016/j.jaci.2014.01.010. A report that provides a mechanistic rationale to explain the role of airway bacteria in asthma inception. [DOI] [PubMed] [Google Scholar]
- 33.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–7. 7 e1–3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biesbroek G, Tsivtsivadze E, Sanders EA, Montijn R, Veenhoven RH, Keijser BJ, et al. Early Respiratory Microbiota Composition Determines Bacterial Succession Patterns and Respiratory Health in Children. Am J Respir Crit Care Med. 2014 doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 35*.Mazur NI, Martinon-Torres F, Baraldi E, Fauroux B, Greenough A, Heikkinen T, et al. Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med. 2015;3:888–900. doi: 10.1016/S2213-2600(15)00255-6. A comprehensive review on current and in-development RSV therapeutics. [DOI] [PubMed] [Google Scholar]
- 36.James KM, Gebretsadik T, Escobar GJ, Wu P, Carroll KN, Li SX, et al. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J Allergy Clin Immunol. 2013;132:227–9. doi: 10.1016/j.jaci.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luoto R, Ruuskanen O, Waris M, Kalliomaki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014;133:405–13. doi: 10.1016/j.jaci.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–7. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Karron RA, Luongo C, Thumar B, Loehr KM, Englund JA, Collins PL, et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med. 2015;7:312ra175. doi: 10.1126/scitranslmed.aac8463. A genetically engineered live-attenuated vaccine, have shown promising results in phase 1 study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeVincenzo JP, Whitley RJ, Mackman RL, Scaglioni-Weinlich C, Harrison L, Farrell E, et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371:711–22. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 41.DeVincenzo JP, McClure MW, Symons JA, Fathi H, Westland C, Chanda S, et al. Activity of Oral ALS-008176 in a Respiratory Syncytial Virus Challenge Study. N Engl J Med. 2015;373:2048–58. doi: 10.1056/NEJMoa1413275. [DOI] [PubMed] [Google Scholar]
- 42.Friedlander AL, Albert RK. Chronic macrolide therapy in inflammatory airways diseases. Chest. 2010;138:1202–12. doi: 10.1378/chest.10-0196. [DOI] [PubMed] [Google Scholar]
- 43.Beigelman A, Mikols CL, Gunsten SP, Cannon CL, Brody SL, Walter MJ. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir Res. 2010;11:90. doi: 10.1186/1465-9921-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Beigelman A, Isaacson-Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135:1171–8. e1. doi: 10.1016/j.jaci.2014.10.001. In a proof-of-concept trial, adding azithromycin therapy to routine bronchiolitis care resulted in significantly lower likelihood to develop recurrent wheeze over the subsequent year. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36:646–54. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 46.Asada M, Yoshida M, Suzuki T, Hatachi Y, Sasaki T, Yasuda H, et al. Macrolide antibiotics inhibit respiratory syncytial virus infection in human airway epithelial cells. Antiviral Res. 2009;83:191–200. doi: 10.1016/j.antiviral.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Beigelman A, Bacharier LB, Baty J, Buller R, Mason S, Schechtman KB, et al. Does azithromycin modify viral load during severe respiratory syncytial virus bronchiolitis? J Allergy Clin Immunol. 2015;136:1129–31. doi: 10.1016/j.jaci.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Jartti T, Nieminen R, Vuorinen T, Lehtinen P, Vahlberg T, Gern J, et al. Short- and long-term efficacy of prednisolone for first acute rhinovirus-induced wheezing episode. J Allergy Clin Immunol. 2015;135:691–8. e9. doi: 10.1016/j.jaci.2014.07.001. A report suggesting that oral corticosteroids may reduce the occurrence of post- HRV wheeze in subgroup of patients presenting with high airway HRV load. [DOI] [PMC free article] [PubMed] [Google Scholar]