Abstract
Introduction
This study evaluates the modeling of gastric electrophysiology tracings during long-term gastric electrical stimulation for gastroparesis. We hypothesized that serosal electrogastrogram may change over time representing gastric remodeling from gastric stimulation.
Patients
Sixty-five patients with gastroparesis underwent placement of gastric stimulator for refractory symptoms. Mean age at initial stimulator placement was 44 years (range, 8–76), current mean age was 49, and the majority of the subjects were female (n = 51, 78 %). Only a minority had diabetes-induced gastroparesis (n = 16, 25 %); the remainder were either idiopathic or postsurgical.
Methods
At the time of stimulator placement, electrogastrogram was performed after the gastric leads were placed but before stimulation was begun. Patients underwent continuous stimulation until pacer batteries depleted. At the time of replacement, before the new pacemaker was attached, electrogastrogram was again performed.
Results
After a mean of 3.9 years of stimulation therapy, the mean of baseline frequency before stimulation therapy was 5.06 cycles/min and declined to 3.66 after replacement (p = 0.0000002). The mean amplitude was 0.33 mV before stimulation therapy and decreased to 0.31 mV (p = 0.73). The frequency/amplitude ratio was 38.4 before stimulation therapy and decreased to 21.9 (p = 0.001).
Conclusion
Long-term gastric electrical stimulation causes improvement in basal unstimulated gastric frequency to near normal.
Keywords: Electrogastrogram, Gastric electrical stimulation, Gastroparesis, Gastric motility
Introduction
Gastric electrical stimulation (GES) has been shown to effectively reduce symptoms of nausea and vomiting in patients with drug refractory gastroparesis, a gastric motility disorder characterized by the symptoms of the above, as well as early satiety, bloating, anorexia, and abdominal pain.1–9 In patients with this disorder, GES therapy with the Enterra system has been shown to: (1) reduce vomiting, (2) improve nausea better than placebo, (3) reduce total symptom severity scores from severe to mild–moderate ranges, (4) increase quality of life, (5) increase weight, (6) decrease use of medications, (7) decrease the use of J-tubes, and (8) decrease medical billing costs compared to medical therapy alone.6,10–16 Several novel GES methods to improve gastric motility have been introduced over the last 20 years, such as multichannel GES, dual-pulse GES, and synchronized GES.17–26 The term “gastric neuromodulation” has been used as a term to describe the antiemetic effect of GES therapy, as the symptom improvement is believed to be mediated through neurons and neuronal connectivity. A number of GES studies have demonstrated that gastric slow-wave pacing has entrained these slow waves with the pulse rates of the GES device.17,18,20,27–29 Not much is known about the long-term changes of gastric neurophysiology after chronic GES therapy. It has been shown that GES therapy is not effective in improvement of gastric dysrhythmia.30,31 What chronic GES therapy is effective at improving or changing in terms of baseline neurophysiology, in particular baseline frequency and amplitude of gastric slow waves, has not been documented. This study hypothesized that the serosal electrogastrogram (EGG) recordings may instead change over time, which may represent gastric remodeling due to GES.
Material and Methods
Data were retrospectively collected on all patients who had a permanent gastric pacemaker in which serosal EGG data of the initial GES device implantation could be found. Mean age at initial GES placement was 44 years (range 8–76); current mean age was 49; the majority of the subjects were female (n = 51, 78 %). Only a minority had diabetes-induced gastroparesis (n = 16, 25 %); the remainder were either idiopathic (n = 48, 74 %) or postsurgical (n = 1, 1 %).
Permanent Placement
Sixty-five patients with gastroparesis underwent placement of gastric stimulator for refractory symptoms. GES electrodes were inserted via laparotomy and fixed to the gastric serosa. All patients were treated with the identical Enterra system, which is the only device approved in the USA (or anywhere) for the treatment of gastroparesis. This utilizes high-frequency, low-energy stimulation, and all patients initially received identical settings. Serosal EGG was performed after the gastric leads were placed but before stimulation was begun. Patients underwent continuous stimulation until pacer batteries depleted, for a mean of 3.9 years (46 months); the stimulator was then replaced. At the time of stimulator replacement, after the stimulator was removed, but before the new pacemaker was attached, serosal EGG was again performed using the gastric leads. Gastric emptying tests were not part of this analysis. However, a published meta-analysis has shown that there may be improvement in gastric emptying with GES as delivered here.32
Data Collection and Analysis
EGG tracings from the serosal recordings of the initial GES placement of each patient were obtained. It was imperative that the recordings were from the initial operation, in order to obtain an accurate baseline of gastric electrical activity before any GES-induced neuromodulation could take place. EGG tracings from the most recent replacement operation were also obtained. These values were analyzed using a paired t test with each patient as his or her own control. The graphs were analyzed using a Gaussian GEE regression analysis.
Results
See Table 1. After a mean of 3.9 years (46 months) of GES therapy, the mean unstimulated baseline frequency for gastroparesis patients before initial GES therapy was 5.06 cycles/min (SD±1.39) and declined to 3.66 (SD± 1.43) after replacement (p = 0.0000002). The mean amplitude was 0.33 mV (SD±0.39) before initial GES therapy and decreased to 0.31 (SD±0.34) afterward (p = 0.73). The frequency/amplitude ratio was 38.4 (SD±38.7) before initial GES therapy and decreased favorably to 21.9 (SD±18.7) afterward (p = 0.001). Both diabetic gastroparesis subjects and idiopathic gastroparesis subjects saw a significant decrease in frequency only, when analyzed separately (p = 0.002 and p = 0.00004, respectively).
Table 1.
Comparison of mean serosal EGG values of primary vs. replacement GES in gastroparesis patients
| Frequency | Amplitude | Frequency/amplitude | |
|---|---|---|---|
| Primary serosal EGG | 5.06 | 0.33 | 38 |
| Replacement serosal EGG | 3.66 | 0.31 | 21 |
| p value | 0.0000002 | 0.73 | 0.001 |
| Normal EGG values | 2.7±3.3 | 0.50 | <10 |
Primary, at initial GES implantation. Replacement, at battery replacement; mean of 3.9 years later
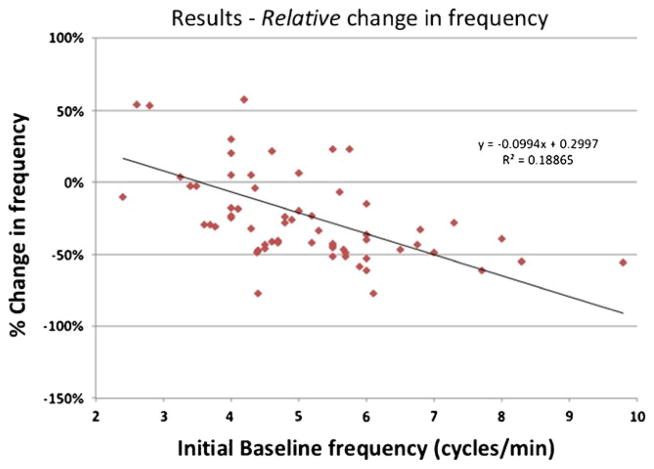
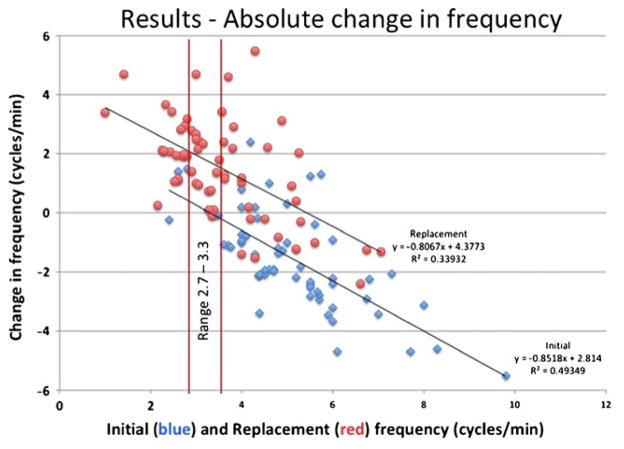
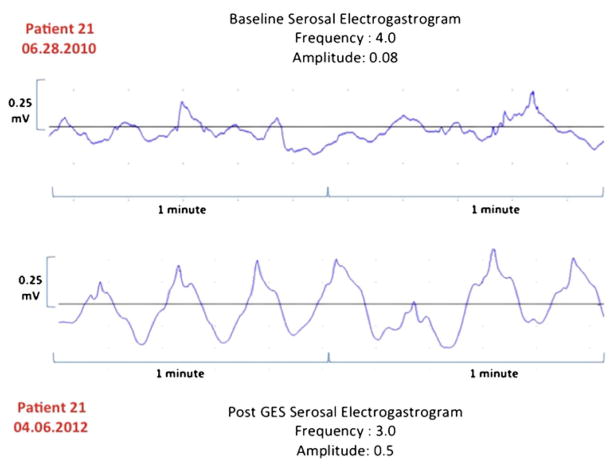
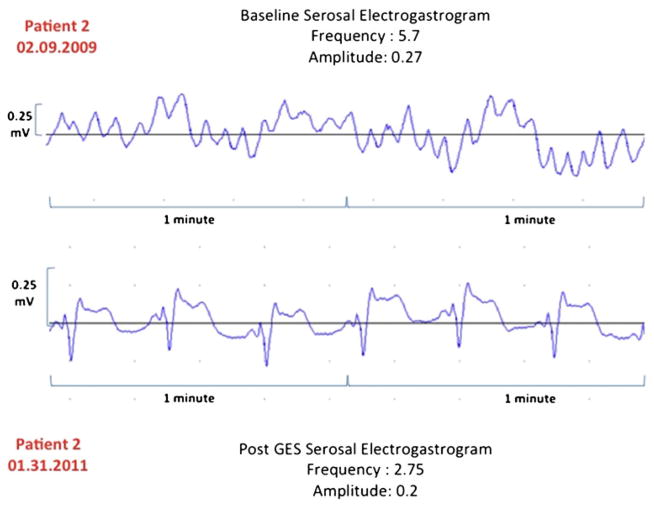
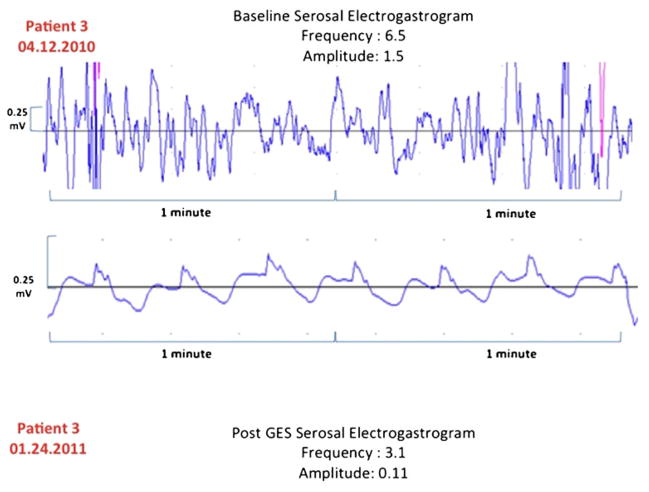
In a regression analysis, both the percent change in serosal EGG frequency (Fig. 1) and the absolute change in EGG frequency have decreased (Fig. 2) relative to the initial and replacement frequency increase as the initial frequency increased (R2 = 0.19, 0.49, and 0.34, respectively). Our patient cases also showed improvement in serosal EGG amplitude after the long-term gastric stimulation (Figs. 3, 4, and 5). Our preliminary analysis of gastroparesis symptoms change from time of primary GES placement to time of most recent GES replacement and serosal EGG change in frequency and amplitude has showed no correlation.
Fig. 1.
Relative change in EGG frequency. Percent change in EGG frequency between the initial baseline frequency and the pre-replacement frequency, which illustrates that the higher the baseline frequency, the more likely the frequency is to improve (decrease to normal)
Fig. 2.
Absolute change in EGG frequency. Change in baseline frequency with respect to the initial baseline frequency and with respect to the replacement frequency. Mean of initial frequency is 5.06 cycles/min; mean of replacement frequency is 3.66 cycles/min. Notice how many of the replacement points now fall within the normal range of frequency
Fig. 3.
Patient 21. Change in EGG frequency
Fig. 4.
Patient 2. Change in EGG frequency
Fig. 5.
Patient 3. Change in EGG frequency
Discussion
Previous studies have discussed the prokinetic abilities of GES therapy on gastric electrical physiology, namely in improvement of 4-h gastric emptying.10 This is the first study that has demonstrated quantitative improvement in baseline frequency of gastric slow waves after chronic GES therapy, on the order of several years rather than several weeks or months. A number of possible mechanisms of how this neuromodulation is occurring have been discussed. One such mechanism concerns the interstitial cells of Cajal (ICC). In one study, with a cohort of 41 gastroparesis patients, 36 % had nearly no ICC, while the remainder had adequate amount of ICC. Relevant to our finding, the group without ICC also had significantly more tachygastria than the adequate ICC group, 32 vs. 11 %, respectively. This ICC depletion did not correlate with the severity of disease, but it did correlate with the likelihood of response to GES therapy.33 Our tachygastric patients were far more likely to have a significant improvement in frequency, and the probable lack of ICC in our more tachygastric patients may account for the dramatic improvement.
Another such mechanism is gastric accommodation, a term which describes the reflexive postprandial expansion of the stomach. This is frequently impairment in gastoparetic patients and has been shown to improve following GES therapy.15,34–37 However, this phenomenon is likely only an explanation for symptomatic reduction and probably does not describe the improvement in baseline gastric frequency.
Further causes of the observed effect of neuromodulation after initiation of GES therapy have been investigated, from the observations of: (1) increased activation of vagal afferent and efferent pathways; (2) increased radioactive counts via positron emission tomography in the thalamic and caudate nuclei15; (3) activation of neurons in the tract of the solitary nucleus38; (4) in rats, inhibited action potentials of neurons in the paraventricular hypothalamic nucleus39; and (4) most recently, in rats, an increase in ghrelin activity, in the form of more ghrelin mRNA, higher quantities of ghrelin-positive cells, and increased plasma ghrelin levels.40 Having mentioned all of these possible mechanisms, it is indeed possible that not only one but many of these explanations are evolving simultaneously, accounting for the documented change in gastric electrical physiology.
One weakness of our study is the omission of symptom data. This study did not target symptoms, which have been shown in all published studies to improve. The main observation supports the concept of remodeling. Remodeling, at least in the cardiac world, and perhaps in the gut, does not always correlate with clinical status. This, however, does not diminish its potential importance.
Conclusion
More and higher quality studies with control arms are needed for gastroenterological acceptance of this treatment. We know that GES therapy improves symptoms. We also know that GES improves gastric motility function, but we do not know how this happens. Further studies such as gastroparesis onset and neural changes at the cellular level may help us gain a further understanding of how GES can provide symptom relief and, in particular, how GES improves gastric motility function. More studies with placebo arms are especially needed, as one meta-analysis found that only one of 13 studies concerning GES therapy for gastroparesis contained controls.10
Contributor Information
Patrick A. Williams, Email: pawilliams2@umc.edu, Division of Digestive Diseases, Department of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216, USA
Yana Nikitina, Division of Digestive Diseases, Department of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216, USA.
Archana Kedar, Division of Digestive Diseases, Department of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216, USA.
Christopher J. Lahr, Department of Surgery, University of Mississippi Medical Center, Jackson, MS, USA
Thomas S. Helling, Department of Surgery, University of Mississippi Medical Center, Jackson, MS, USA
Thomas L. Abell, Email: thomas.abell@louisville.edu, Division of Digestive Diseases, Department of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216, USA
References
- 1.Yin J, Abell TL, McCallum RW, Chen JDZ. Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation. 2012 doi: 10.1111/j.1525-1403.2012.00429.x. [DOI] [PubMed] [Google Scholar]
- 2.Hocking MP, Vogel SB, Sninsky CA. Human gastric myoelectric activity and gastric emptying following gastric surgery and with pacing. Gastroenterology. 1992;103:1811–1816. doi: 10.1016/0016-5085(92)91439-b. [DOI] [PubMed] [Google Scholar]
- 3.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 4.Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. American Journal of Physiology. 1998;274:G186–G191. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- 5.Maranki JL, Lytes V, Meilahn JE, Harbison S, Friedenberg FK, Fisher RS, Parkman HP. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Digestive Diseases and Sciences. 2008;53:2072–2078. doi: 10.1007/s10620-007-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCallum RW, Lin Z, Forster J, Roeser K, Hou Q, Sarosiek I. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clinical Gastroenteroly and Hepatology. 2011;9:314–319. doi: 10.1016/j.cgh.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Runnels JEW, Johnson W, Abell TL. Long-term follow up double masked temporary GES study: the importance of baseline physiologic measures. Neurogastroenterology and Motility. 2008;20:A30. [Google Scholar]
- 8.Thompson JJW, Minocha A, Abell TL. Double blinded randomized study of temporary gastric electrical stimulation (GES): preliminary results of the Endostim study (Endoscopic Stimulation Temporarily Implanted Mucosally) Gastroenterology. 2007;132(Suppl 2):780. [Google Scholar]
- 9.McCallum RW, Snape W, Brody F, Wo J, Parkman HP, Nowak T. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clinical Gastroenterology and Hepatology. 2010 Nov;8(11):947–954. doi: 10.1016/j.cgh.2010.05.020. quiz e116. [DOI] [PubMed] [Google Scholar]
- 10.O’Grady G, Egbuji JU, Du P, Cheng LK, Pullan AJ, Windsor JA. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World Journal of Surgery. 2009;33:1693–1701. doi: 10.1007/s00268-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bortolotti M. Gastric electrical stimulation for gastroparesis: a goal greatly pursued, but not yet attained. World Journal of Gastroenterology. 2011;17:273–282. doi: 10.3748/wjg.v17.i3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasler WL. Methods of gastric electrical stimulation and pacing: a review of their benefits and mechanisms of action in gastroparesis and obesity. Neurogastroenterology and Motility. 2009;21:229–243. doi: 10.1111/j.1365-2982.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- 13.Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, Tougas G, Starkebaum W. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 14.Stanghellini V. Unfulfilled wishes by gastric electrical stimulation. Clinical Gastroenterology and Hepatology. 2011;9:447–448. doi: 10.1016/j.cgh.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 15.McCallum RW, Dusing RW, Sarosiek I, Cocjin J, Forster J, Lin Z. Mechanisms of symptomatic improvement after gastric electrical stimulation in gastroparetic patients. Neurogastroenterology and Motility. 2010;22:161–167. e150–161. doi: 10.1111/j.1365-2982.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- 16.Cutts TF, Luo J, Starkebaum W, Rashed H, Abell TL. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterology and Motility. 2005;17:35–43. doi: 10.1111/j.1365-2982.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen JD, Xu X, Zhang J, Abo M, Lin X, McCallum RW, Ross B. Efficiency and efficacy of multi-channel gastric electrical stimulation. Neurogastroenterology and Motility. 2005;17:878–882. doi: 10.1111/j.1365-2982.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 18.Song G, Hou X, Yang B, Liu J, Qian W, Chen JD. Two-channel gastric electrical stimulation accelerates delayed gastric emptying induced by vasopressin. Digestive Diseases and Sciences. 2005;50:662–668. doi: 10.1007/s10620-005-2553-5. [DOI] [PubMed] [Google Scholar]
- 19.Mintchev MP, Sanmiguel CP, Amaris M, Bowes KL. Microprocessor-controlled movement of solid gastric content using sequential neural electrical stimulation. Gastroenterology. 2000;118:258–263. doi: 10.1016/s0016-5085(00)70207-1. [DOI] [PubMed] [Google Scholar]
- 20.Mintchev MP, Sanmiguel CP, Otto SJ, Bowes KL. Microprocessor controlled movement of liquid gastric content using sequential neural electrical stimulation. Gut. 1998;43:607–611. doi: 10.1136/gut.43.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei Y, Chen JD. Effects of dual pulse gastric electrical stimulation on gastric tone and compliance in dogs. Digestive and Liver Diseases. 2009;41:277–282. doi: 10.1016/j.dld.2008.07.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song GQ, Hou X, Yang B, Sun Y, Qian W, Chen JD. A novel method of 2-channel dual-pulse gastric electrical stimulation improves solid gastric emptying in dogs. Surgery. 2008;143:72–78. doi: 10.1016/j.surg.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Qiao X, Chen JD. Therapeutic potentials of a novel method of dual-pulse gastric electrical stimulation for gastric dysrhythmia and symptoms of nausea and vomiting. American Journal of Surgery. 2006;191:255–261. doi: 10.1016/j.amjsurg.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Koothan T, Chen JD. Synchronized gastric electrical stimulation improves vagotomy-induced impairment in gastric accommodation via the nitrergic pathway in dogs. American Journal of Physiology and Gastrointestinal Liver Physiology. 2009;296:G310–G318. doi: 10.1152/ajpgi.90525.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song GQ, Chen JD. Synchronized gastric electrical stimulation improves delayed gastric emptying in nonobese mice with diabetic gastroparesis. Journal of Applied Physiology. 2007;103:1560–1564. doi: 10.1152/japplphysiol.00319.2007. [DOI] [PubMed] [Google Scholar]
- 26.Zhu H, Sallam H, Chen DD, Chen JD. Therapeutic potential of synchronized gastric electrical stimulation for gastroparesis: enhanced gastric motility in dogs. American Journal of Physiology Regulatory, Integrative, and Comparative Physiology. 2007;293:R1875–R1881. doi: 10.1152/ajpregu.00821.2006. [DOI] [PubMed] [Google Scholar]
- 27.Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of gastro-paresis with electrical stimulation. Digestive Diseases and Sciences. 2003;48:837–848. doi: 10.1023/a:1023099206939. [DOI] [PubMed] [Google Scholar]
- 28.Eagon JC, Soper NJ. Gastrointestinal pacing. The Surgical Clinics of North America. 1993;73:1161–1172.28.45. doi: 10.1016/s0039-6109(16)46185-2. [DOI] [PubMed] [Google Scholar]
- 29.Bortolotti M. The “electrical way” to cure gastroparesis. American Journal of Gastroenterology. 2002;97:1874–1883. doi: 10.1111/j.1572-0241.2002.05898.x. [DOI] [PubMed] [Google Scholar]
- 30.Lin Z, Forster J, Sarosiek I, McCallum RW. Effect of high-frequency gastric electrical stimulation on gastric myoelectric activity in gastoparetic patients. Neurogastroenterology and Motility. 2004;16:205–212. doi: 10.1111/j.1365-2982.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–409. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- 32.O’Grady G, Egbuji JU, Du P, Cheng LK, Pullan AJ, Windsor JA. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg. 2009 Aug;33(8):1693–701. doi: 10.1007/s00268-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Z, Sarosiek I, Forster J, Damjanov I, Hou Q, McCallum RW. Association of the status of interstitial cells of Cajal and electro-gastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterology and Motility. 2010;22:56–61. e10. doi: 10.1111/j.1365-2982.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 34.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 35.Undeland KA, Hausken T, Aanderud S, Berstad A. Lower post-prandial gastric volume response in diabetic patients with vagal neuropathy. Neurogastroenterology and Motility. 1997;9:19–24. doi: 10.1046/j.1365-2982.1997.d01-3.x. [DOI] [PubMed] [Google Scholar]
- 36.Samsom M, Roelofs JM, Akkermans LM, van Berge Henegouwen GP, Smout AJ. Proximal gastric motor activity in response to a liquid meal in types I diabetes mellitus with autonomic neuropathy. Digestive Diseases and Sciences. 1998;43:491–496. doi: 10.1023/a:1018894520557. [DOI] [PubMed] [Google Scholar]
- 37.Xing JH, Chen JD. Gastric electrical stimulation with parameters for gastroparesis enhances gastric accommodation and alleviates distention-induced symptoms in dogs. Digestive Diseases and Sciences. 2006;51:2160–2164. doi: 10.1007/s10620-006-9212-3. [DOI] [PubMed] [Google Scholar]
- 38.Qin C, Sun Y, Chen JD, Foreman RD. Gastric electrical stimulation modulates neuronal activity in nucleus tractus solitarii in rats. The Autonomic Neuroscience. 2005;119:1–8. doi: 10.1016/j.autneu.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Tang M, Zhang J, Chen JD. Central mechanisms of gastric electrical stimulation involving neurons in the paraventricular nucleus of the hypothalamus in rats. Obesity Surgery. 2006;16:344–352. doi: 10.1381/096089206776116372. [DOI] [PubMed] [Google Scholar]
- 40.Gallas S, Sinno MH, Boukhettala N, Coëffier M, Dourmap N, Gourcerol G, Ducrotté P, Déchelotte P, Leroi AM, Fetissov SO. Gastric electrical stimulation increases ghrelin production and inhibits catecholaminergic brainstem neurons in rats. The Europe-an Journal of Neuroscience. 2011;33:276–284. doi: 10.1111/j.1460-9568.2010.07474.x. [DOI] [PubMed] [Google Scholar]