Abstract
The development of a gold(I)-catalyzed sulfination of aryl boronic acids is described. This transformation proceeds through an unprecedented mechanism which exploits the reactivity of gold(I)–heteroatom bonds to form sulfinate anions. Further in situ elaboration of the sulfinate intermediates leads to the corresponding sulfones and sulfonamides, two pharmacophores routinely encountered in drug discovery.
Keywords: gold, heterocycles, structure elucidation, sulfonamides, synthetic methods
Gold complexes, often associated with the activation of π-bonds toward nucleophiles,[1] have rarely been employed in reactions with traditional coupling reagents such as boronic acids.[2] We envisioned that incorporation of a boronic acid into an X-type heteroatom ligand[3] for gold(I) might provide an alternative to the oxidative addition/transmetalation/reductive elimination pathway often associated with this class of reagents. In this context, we were inspired by the formation of gold–SO2 bonds from gold(I) aryl complexes, which have largely been unexplored since the seminal works of Johnson and Puddephatt,[4] and Aresta and Vasapollo.[5,6] Based on the literature precedent for SO2 insertion into gold–carbon bonds, we posited that this elementary step could be coupled with the more recent discovery of gold alkoxides and hydroxides and their ability to transmetalate with boronic acids[3b] to establish a redox neutral catalytic cycle for the synthesis of sulfinate salts [Eq. (1)]. Given the rapid development over the last decade of palladium- and copper-catalyzed methods[7] to access sulfonyl motifs, and the importance of such pharmacophores in drug discovery, a gold-mediated sulfination would be of great interest from both a fundamental organometallic and synthetic organic chemistry standpoint. Herein we report the first gold(I)-catalyzed synthesis of sulfinate salts from aryl and heteroaryl boronic acid derivatives.[8]
| (1) |
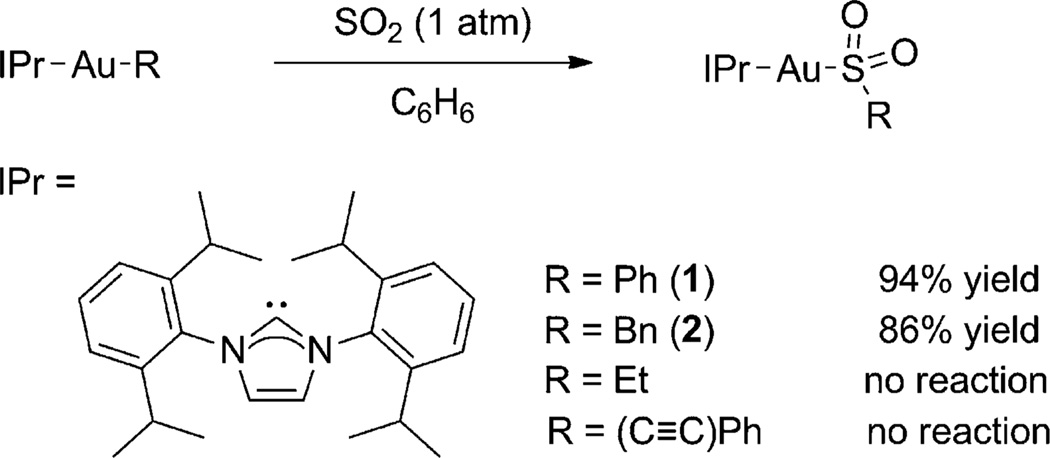
We initially explored sulfur dioxide insertion into a number of gold–carbon bonds with the metal center supported by the robust N-heterocyclic carbene (NHC) 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene (IPr; Figure 1).[9] Upon treatment with SO2 (1 atm), the NHC-supported gold sulfinates were formed in high yield when the starting material possessed a Au–C bond with an sp2-hybridized carbon atom (1) or activated benzylic carbon atom (2). The complexes 1 and 2 were characterized crystallographically and the sulfinate ligand was found to be sulfur-bound to the metal center (Figure 2).[10] No reaction was seen with the analogous phenylacetylide and ethyl gold complexes, and thus parallels the established reactivity of Au–C bonds to electrophilic attack.[10, 11]
Figure 1.
Synthesis of gold(I) sulfinate complexes.
Figure 2.
Solid-state structures of the sulfinate complexes 1 (left) and 2 (right). Thermal ellipsoids shown at 50% probability. Hydrogen atoms and solvent molecules have been omitted for clarity.
With these promising initial results, we began to construct a catalytic cycle which would incorporate the elementary steps necessary to synthesize sulfinates (Scheme 1). Thus, treatment of the model gold(I) phenyl sulfinate (1) with NaOtBu led to precipitation of NaSO2Ph and formation of the gold alkoxide IPrAuOtBu,[3c] which regenerated IPrAuPh when treated with phenyl boronic acid.
Scheme 1.
Stoichiometric reactions in a plausible catalytic sulfinate synthesis. [a] Yield of isolated product. [b] Yields were determined by 1H NMR spectroscopy with 1,3,5-trimethoxybenzene as an internal standard.
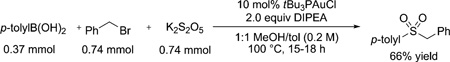
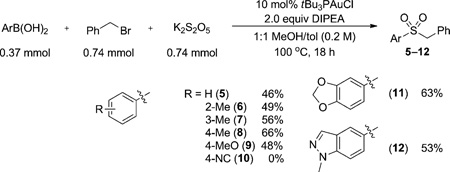
Establishment of a closed synthetic cycle in a stoichiometric fashion provided the impetus for rendering the net transformation truly catalytic. Potassium metabisulfite (K2S2O5) was chosen as the SO2 surrogate because of its ease of handling and accessibility.[7c] Benzyl bromide was employed as an electrophile to trap the sulfinate intermediate as the corresponding sulfone. Screening of solvents and bases revealed that a 1:1 MeOH/toluene mixture with 2 equivalents N,N-diisopropylethyl amine (DIPEA) provided the highest yields. Initial use of IPrAuCl as the precatalyst provided some product, albeit in low yield (21%). It was determined that bulky electron-rich phosphines were superior supporting ligands for the desired transformation, with tBu3PAuCl being the best [66%, Eq. (2)].[13]
 |
(2) |
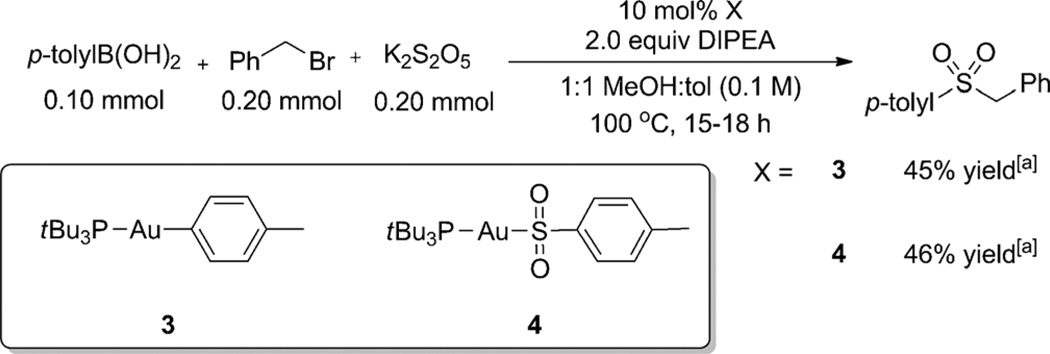
To determine the viability of our proposed catalytic cycle, we synthesized the putative gold aryl 3 and sulfinate 4 intermediates (Scheme 2).[14] These complexes were then subjected to standard reaction conditions as substitutes for tBu3PAuCl. The results showed comparable yields for the catalytic reaction regardless of the starting gold source. These data suggest that gold aryl and sulfinate complexes may be reasonably proposed as catalytic intermediates.
Scheme 2.
Subjection of proposed intermediates to reaction conditions. [a] Yield determined by NMR spectroscopy versus 1,3,5-trimethoxybenzene as an internal standard.
We next surveyed reaction conditions to determine the role of the reagents involved in the transformation. No reaction was observed without the catalyst (Table 1, entry 2) and other catalysts able to effect sulfonylation proved inferior to gold (entries 3–7). A bulky amine base was required for the reaction to proceed (entries 8 and 9). Though no special precautions are needed for the exclusion of air or moisture from the reaction mixture, large quantities of water did shut down catalysis (entry 10). The yield decreased significantly when the reaction was conducted under an atmosphere of SO2 (entry 11), which we attribute to the removal of DIPEA from the catalytic cycle through formation of an SO2–amine adduct.[15]
Table 1.
Variation from optimized reaction conditions.
 | ||
|---|---|---|
| Entry | Variation from standard conditions | Yield [%][a] |
| 1 | none | 51 |
| 2 | no catalyst | 0 |
| 3 | 10 mol% Pd(OAc)2 as catalyst | trace |
| 4 | 10 mol% Pd(OAc)2/[HPtBu3][BF4] as catalyst | 9 |
| 5 | 10 mol% Pd(PPh3)4 as catalyst | trace |
| 6 | 10 mol% Cu2O as catalyst | 10 |
| 7 | 10 mol% CuCl as catalyst | 3 |
| 8 | Et3N in place of DIPEA | 7 |
| 9 | no base | trace |
| 10 | addition of 10%-by-volume H2O | 4 |
| 11 | under SO2(g) (1 atm) in place of air | 9 |
Yield determined versus 1,3,5-trimethoxybenzene as an internal standard.
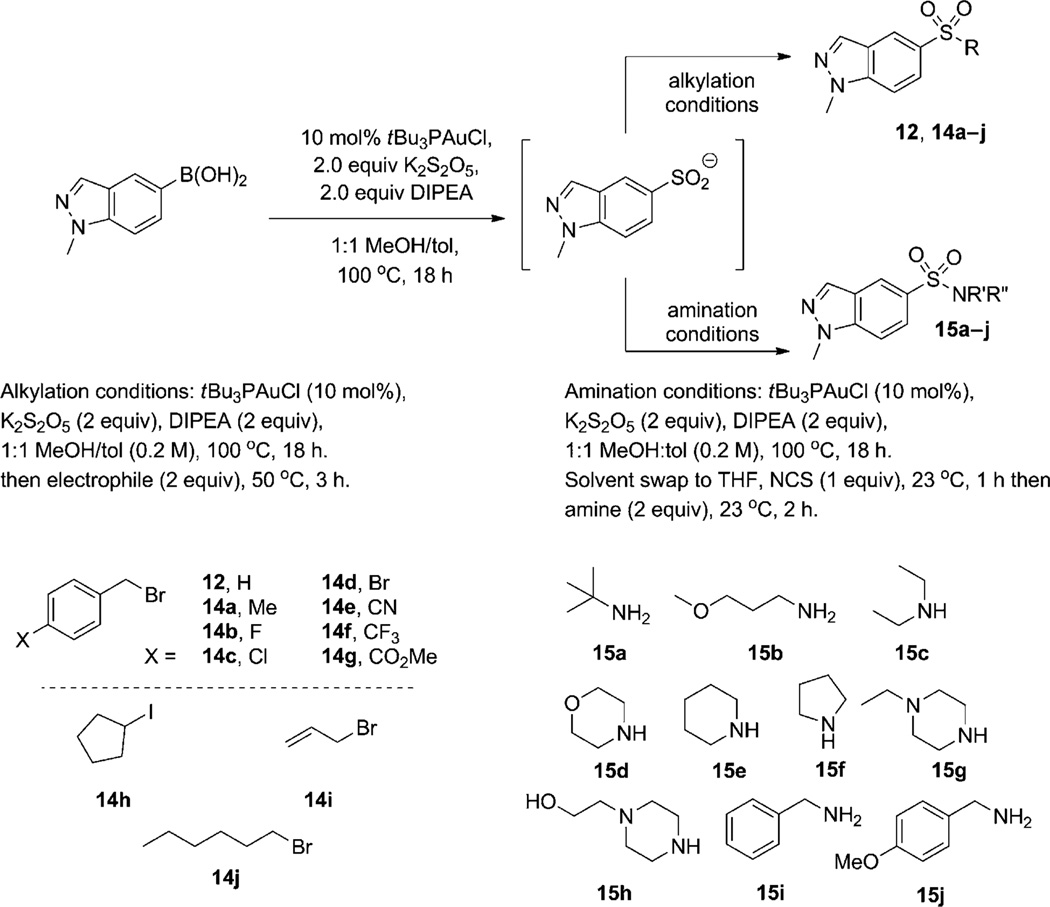
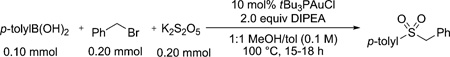
A brief survey of boronic acids supports our mechanistic hypothesis [Eq. (3)]. Boronic acids that are electronically unbiased or electron-rich (5–9, 11) provide yields comparable to those observed in the model reaction, whereas electronpoor boronic acids show no reactivity (10). The lack of reactivity of this latter boronic acid may be attributed to the electron-deficient intermediate gold(I) aryl complex being less susceptible to electrophilic attack by SO2. Of importance for potential applications in the context of drug discovery, nitrogen-containing aromatic heterocycles, such as indazole, appeared compatible with the reaction conditions (12).
 |
(3) |
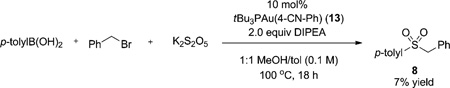
The lack of reactivity of 4-cyanophenyl boronic acid motivated us to examine this limitation. The organometallic intermediate for reaction with this boronic acid, 13, was prepared independently and subjected to catalytic conditions with the well-established coupling partner p-tolylboronic acid [Eq. (4)]. The sulfone 8 was observed in only 7% yield, less than a single turnover. Additionally, treatment of 13 with SO2(g) at 100 °C led to no reaction, unlike the facile synthesis of gold sulfinate 4.[16] This result suggests that electron-deficient boronic acids may be capable of the transmetalation step of the catalytic cycle, but the subsequent intermediate shows little reaction toward SO2, thus precluding the use of these coupling partners in catalysis.
 |
(4) |
The final aspect of the sulfinate functionalization, liberation of the sulfinate salt and its trapping, was confirmed to occur independently of the gold catalyst by the simple alkylation of p-tolylsulfinate under standard reaction conditions, but without catalyst (see the Supporting Information). This late addition allows introduction of diversity and provides a powerful tool for chemical space exploration in the context of drug discovery. Indeed, short analoguing sequences from a common modular intermediate, the use of readily available classes of monomers or reagents (such as boronic acids, amines, alkylating agents), and robust experimental conditions are hallmarks of a reaction suitable for rapid structure–activity relationship studies. These criteria are demonstrated in the construction of two targeted libraries consisting of 24 sulfones and sulfonamides based on the medicinally relevant indazole framework (Scheme 3).[17, 18] This latter set of experiments not only reaffirmed the fate of our catalytic cycle’s product but demonstrated the utility of this reaction in making divergent products, sulfones and sulfonamides, from a common versatile sulfinate intermediate.
Scheme 3.
Divergent synthesis of sulfonyl compounds.
In summary, we have advanced our understanding of the fundamental reactivity of gold(I)–heteroatom bonds and used that information to construct a catalytic cycle for the synthesis of sulfinates from boronic acids. Preparation of new sulfinato complexes, stoichiometric reactions representing elementary steps, and subjection of proposed intermediates to reaction conditions informed the development of this system. Additionally, this method is complementary to established methods in that it forms sulfinates directly, which in turn can be elaborated in situ into more complex sulfonyl compounds of broad interest, such as sulfones and sulfonamides.
Experimental Section
A 2-dram vial with a stir bar was charged with 4-methylphenyl boronic acid (50 mg, 0.37 mmol), K2S2O5 (167 mg, 0.74 mmol), and tBu3P-AuCl (16 mg, 0.037). The reagents were suspended in 1:1 PhCH3/MeOH (2 mL) and treated with diisopropylethylamine (128 µL, 0.74 mmol) and benzyl bromide (88 µL, 0.74 mmol). The vial was sealed with a septum-lined cap and heated in an aluminum block at 100°C for 18 h. The reaction was cooled to room temperature and the volatiles were removed in vacuo. The resultant solids were partitioned between EtOAc (30 mL) and water (30 mL) treated with sat. aq. NH4Cl (3 mL). The organic phase was dried over Na2SO4, filtered, and concentrated. The resultant crude product was purified by flash chromatography (0–60% EtOAc/heptanes gradient, 4 g silica gel) to yield 60 mg (66% yield) of the desired product 1-(benzylsulfonyl)-4-methylbenzene (8).
Supplementary Material
Acknowledgments
This research was supported in part by a grant from the NIHGMS (RO1 GM073932). M.W.J. is grateful to the National Science Foundation (no. DGE1106400) and UC Berkeley for pre-doctoral research fellowships. N.P.M. acknowledges the National Institute of Health for a Kirchstein-NRSA postdoctoral fellowship. S.W.B. thanks Andrei Shavyna, Aaron Smith, and Kevin Hesp for helpful discussions. William J. Wolf (UC Berkeley) and Brian Samas (Pfizer) are thanked for assistance with X-ray crystallographic studies.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201400037.
Contributor Information
Miles W. Johnson, Department of Chemistry, University of California, Berkeley Berkeley, CA 94720 (USA)
Scott W. Bagley, Pfizer Worldwide Medicinal Chemistry Groton, CT 06340 (USA)
Prof. Neal P. Mankad, Department of Chemistry, University of Illinois at Chicago (USA)
Prof. Robert G. Bergman, Department of Chemistry, University of California, Berkeley Berkeley, CA 94720 (USA)
Dr. Vincent Mascitti, Email: vincent.mascitti@pfizer.com, Pfizer Worldwide Medicinal Chemistry Groton, CT 06340 (USA).
Prof. F. Dean Toste, Email: fdtoste@berkeley.edu, Department of Chemistry, University of California, Berkeley Berkeley, CA 94720 (USA).
References
- 1.For recent reviews, see: Corma A, Leyva-Pérez A, Sabater MJ. Chem. Rev. 2011;111:1657–1712. doi: 10.1021/cr100414u. Shapiro ND, Toste FD. Synlett. 2010:675–691. doi: 10.1055/s-0029-1219369.
- 2.a) Zhang G, Cui L, Wang Y, Zhang L. J. Am. Chem. Soc. 2010;132:1474–1475. doi: 10.1021/ja909555d. [DOI] [PubMed] [Google Scholar]; b) Brenzovich WE, Benitez D, Lackner AD, Shunantona HP, Tkatchouk E, Goddard WA, Toste FD. Angew. Chem. 2010;122:5651–5654. doi: 10.1002/anie.201002739. Angew. Chem. Int. Ed.2010, 49, 5519 – 5522; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Melhado AD, Brenzovich WE, Lackner AD, Toste FD. J. Am. Chem. Soc. 2010;132:8885–8887. doi: 10.1021/ja1034123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Laitar DS, Müller P, Gray TJ, Sadighi JP. Organometallics. 2005;24:4503–4505. [Google Scholar]; b) Dupuy S, Crawford L, Bühl M, Slawin AMZ, Nolan SP. Adv. Synth. Catal. 2012;354:2380–2386. [Google Scholar]; c) Tsui EY, Müller P, Sadighi JP. Angew. Chem. 2008;120:9069–9072. doi: 10.1002/anie.200803842. Angew. Chem. Int. Ed.2008, 47, 8937 – 8940; [DOI] [PubMed] [Google Scholar]; d) Johnson MW, Shevick SL, Toste FD, Bergman RG. Chem. Sci. 2013;4:1023–1027. [Google Scholar]
- 4.a) Johnson A, Puddephatt RJ, Quirk JL. J. Chem. Soc. Chem. Commun. 1972:938–939. [Google Scholar]; b) Johnson A, Puddephatt RJ. J. Chem. Soc. Dalton Trans. 1977:1384–1388. [Google Scholar]
- 5.Aresta M, Vasapollo G. J. Organomet. Chem. 1971;26–33:C51–C53. [Google Scholar]
- 6.For the synthesis of gold(I) organosulfinates and organosulfonates via salt metathesis, see: Römbke P, Schier A, Schmidbaur H. J. Chem. Soc. Dalton Trans. 2001:2482–2486.
- 7.a) Nguyen B, Emmett EJ, Willis MC. J. Am. Chem. Soc. 2010;132:16372–16373. doi: 10.1021/ja1081124. [DOI] [PubMed] [Google Scholar]; b) Yang H, Li Y, Jiang M, Wang J, Fu H. Chem. Eur. J. 2011;17:5652–5660. doi: 10.1002/chem.201003711. [DOI] [PubMed] [Google Scholar]; c) Ye S, Wu J. Chem. Commun. 2012;48:7753–7755. doi: 10.1039/c2cc33725h. [DOI] [PubMed] [Google Scholar]; d) DeBergh JR, Niljianskul N, Buchwald SL. J. Am. Chem. Soc. 2013;135:10638–10641. doi: 10.1021/ja405949a. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Tang X, Huang L, Qi C, Wu X, Wu W, Jiang H. Chem. Commun. 2013;49:6102–6104. doi: 10.1039/c3cc41249k. [DOI] [PubMed] [Google Scholar]; f) Emmett EJ, Hayter BR, Willis MC. Angew. Chem. 2013;125:12911–12915. Angew. Chem. Int. Ed.2013, 52, 12679 – 12683; [Google Scholar]; g) Richards-Taylor CS, Blakemore DC, Willis MC. Chem. Sci. 2014;5:222–228. [Google Scholar]; h) Shavnya A, Coffey SB, Smith AC, Mascitti V. Org. Lett. 2013;15:6226–6229. doi: 10.1021/ol403072r. [DOI] [PubMed] [Google Scholar]
- 8.For other methods to synthesize sulfones and sulfonamides from aryl boronic acids, see: Beaulieu C, Guay D, Wang Z, Evans DA. Tetrahedron Lett. 2004;45:3233. Bandgar BP, Bettigeri SV, Phopase J. Org. Lett. 2004;6:2105. doi: 10.1021/ol049692c. Huang F, Batey RA. Tetrahedron Lett. 2007;48:7667. ref. [7c,d]
- 9.For a review on NHCs in gold catalysis, see: Marion N, Nolan SP. Chem. Soc. Rev. 2008;37:1776–1782. doi: 10.1039/b711132k.
- 10.A gold sulfinate previously reported in the literature (Ref. [5]) was also structurally characterized. See the Supporting Information for further details.
- 11.Roth KE, Blum SA. Organometallics. 2010;29:1712–1716. [Google Scholar]
- 12.For a review of SO2 insertion into metal – carbon bonds, see: Wojcicki A. Adv. Organomet. Chem. 1974;12:31–81.
- 13.See the Supporting Information for a complete survey of gold catalysts and solvents.
- 14.Phosphine-supported gold alkoxides are rare and have only been isolated using electron-deficient alkoxide ligands: Komiya S, Iwata M, Sone T, Fukuoka A. J. Chem. Soc. Chem. Commun. 1992:1109–1110.
- 15.Byrd WE. Inorg. Chem. 1962;1:762–768. [Google Scholar]
- 16.Reaction of IPrAu(4-CNCH6H4) with SO2 also results in no reaction. See the Supporting Information for further details.
- 17.For examples of pharmaceuticals possessing both indazolyl and sulfonyl moieties, see: Rueeger H, Lueoend R, Rogel O, Rondeau J-M, Möbitz H, Machauer R, Jacobsen L, Staufenbiel M, Desrayaud S, Neumann U. J. Med. Chem. 2012;55:3364–3386. doi: 10.1021/jm300069y. Spinks D, Torrie LS, Thompson S, Harrison JR, Frearson JA, Read KD, Fairlamb AH, Wyatt PG, Gilbert IH. Chem Med Chem. 2012;7:95–106. doi: 10.1002/cmdc.201100420.
- 18.The two libraries provided a 93% (sulfone synthesis) and 100% (sulfonamide synthesis) success rate as measured by product successfully isolated and characterized (1H and 13C NMR spectroscopy, and LCMS).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.