Abstract
Objective
Extending the duration of isoniazid preventive therapy (IPT) among people living with HIV (PLHIV) may improve its effectiveness at both the individual and population level, but could also increase selective pressure in favor of isoniazid resistant tuberculosis (TB) strains. The objective of this study was to determine the relative importance of these two effects.
Methods
Transmission dynamic model
Design
We created a mathematical model of TB transmission incorporating HIV incidence and treatment, mixed strain latent TB infections, and four different phenotypes of TB drug resistance (pan-susceptible, isoniazid mono-resistant, rifampicin mono-resistant, and multi-drug resistant). We used this model to project the effects of IPT duration on the incidence of isoniazid-sensitive and -resistant TB as well as mortality among PLHIV. We evaluated the sensitivity of our baseline model, which was calibrated to data from Botswana, to different assumptions about the future trajectory of the TB epidemic.
Results
Our model suggests that, in the context of a declining TB epidemic such as that currently observed in Botswana, the incidence and mortality benefits of continuous IPT for PLHIV are likely to outweigh the potential resistance risks associated with long duration IPT. However, should TB epidemics fail to remain in control, as was observed during the initial emergence of HIV, the selective pressure imposed by widespread use of continuous IPT on isoniazid resistant TB incidence may erode its initial benefits.
Conclusions
Resistance concerns are likely insufficient to rule out use of continuous IPT when coupled with effective TB treatment, case finding, and HIV control.
Keywords: antibiotic resistance, mathematical model, preventive therapy, coinfection, selective pressure, competition
Introduction
The World Health Organization currently recommends at least 6–9 months of isoniazid preventive therapy (IPT) for all people living with HIV (PLHIV) deemed unlikely to have active tuberculosis (TB) on the basis of symptom screening [1]. Several clinical trials have demonstrated an individual-level efficacy of IPT for preventing TB among PLHIV [2]. Longer follow-up studies on the risks of TB after stopping IPT, however, suggest that the duration of protection post-IPT varies based on setting and may be lost almost immediately [3–6]. Community-wide IPT was demonstrated to have no effect on TB incidence within the Thibela study, an observation that has been at least partly attributed to rapid loss of protection from re-infection after IPT and could also suggest that 9 months of IPT are insufficient to clear latent TB strains among PLHIV [7–10].
A continuous, lifelong course of IPT has been suggested as a potential way to increase the community-wide impact of IPT [7]. At the individual level, clinical trials have shown an increased efficacy of 36 months of IPT, intended as a proxy for lifelong treatment, compared to the standard 6-month regimen [11, 12]. Despite these potential benefits, prolonging the course of IPT could exacerbate concerns about the risk of side effects and potential for increased isoniazid resistance. Martinson et al. found a greater risk of serious adverse effects on continuous IPT as compared to shorter duration regimens [13]. While the analysis of published literature included in WHO IPT guidelines concluded that IPT does not increase the risk of isoniazid-resistant TB among IPT recipients (graded “strong recommendation, moderate quality of evidence”), this analysis was based on clinical trials of IPT that used stricter criteria to exclude active TB than the WHO recommended symptom-screening algorithm. Furthermore, the included studies were not powered to assess risks of resistance [1, 14]. This analysis also did not consider the potential competitive advantage that community-wide IPT could confer to isoniazid resistant TB strains at the population level [15–17].
Several modeling studies have previously assessed the potential impact of widespread IPT use among PLHIV on the incidence of both isoniazid sensitive and isoniazid resistant TB [16, 18, 19]. However, these studies have not specifically investigated the impact of different IPT durations, and have not accounted for multiple pathways to multi-drug resistant (MDR) TB. They have also typically offered little guidance as to the conditions under which the potential benefits of IPT are most likely to outweigh increased risks of resistance. Because many of these models were constructed and parameterized before widespread availability of antiretroviral therapy (ART) and at time when TB incidence was rising, the applicability of their findings to conditions in which TB incidence is declining is unclear.
For this analysis, we created a mathematical model to assess the potential impact of variable durations of IPT on overall mortality among PLHIV over a range of epidemic scenarios. Unlike previous models, our analysis explicitly explores the potential multi-faceted effects of IPT on the incidence of pan-sensitive, isoniazid mono-resistant, rifampicin mono-resistant, and MDR TB. The motivation for this work was to understand whether the benefits of longer durations of IPT are likely to outweigh resistance risks in settings where HIV treatment has improved TB control. Our baseline scenario was chosen and the most uncertain parameters were estimated based on historical trends and future projections of the TB-HIV co-epidemic in Botswana.
Methods
To assess the potential impact of continuous vs. 6-month IPT on the incidence of isoniazid resistant TB in Botswana, we created a compartmental transmission model accounting for the natural history of TB, the incidence of HIV and uptake of ART, and the acquisition and transmission of TB drug resistance in this setting. Our modeling strategy is described briefly below and in more detail in the Appendix. The model was implemented in R version 3.2.0 as a series of delay differential equations numerically integrated using package deSolve.
Model Overview
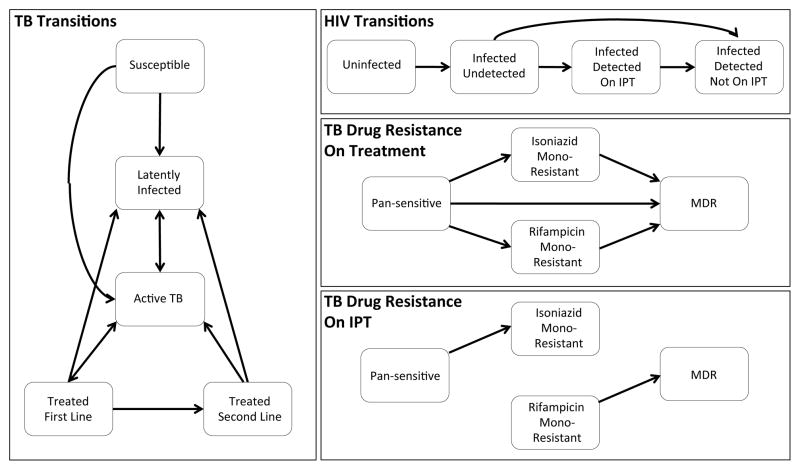
The basic structure of our model is shown in Fig 1. With respect to TB, individuals in the model may be fully susceptible, latently infected, actively infected, or receiving treatment. Initial infection moves individuals from the susceptible compartment to either the active TB (fast progression) or latently infected (slow progression) compartment. People who are latently infected may become actively infected via either reactivation or reinfection. We assume that initial infection affords partial but incomplete protection against future reinfection.
Figure 1.
Model structure
Episodes of active TB in the model may result in death, spontaneous cure, or initiation on treatment. Treatment episodes may result in successful cure, leading to return to latent infection, or in treatment failure, resulting in relapse to active disease either with or without acquired resistance. The treatment a patient receives depends on their drug susceptibility profile and whether drug resistance is detected by their healthcare provider. We assume that all newly diagnosed patients initially receive first-line TB treatment, but starting in 2008 allow a proportion of individuals failing their initial treatment course to receive drug susceptibility testing and appropriate retreatment [20].
With respect to HIV, individuals in the model may be uninfected, infected and undetected (i.e. not receiving ART), or infected and detected (i.e. receiving ART if eligible). Individuals with detected HIV are also eligible for IPT. Our model of HIV is not a transmission model in that the number of new HIV infections does not reflect the interaction between susceptible and infected individuals in the model, but is instead based on UNAIDS Botswana HIV incidence projections (UNAIDS 2015, unpublished data).
We account for four phenotypes of drug resistant TB in this model: pan-sensitive, isoniazid mono-resistant, rifampicin mono-resistant, and multi-drug resistant (MDR, resistant to both isoniazid and rifampicin). We assume that patients receiving treatment are at risk of developing resistance to both isoniazid and rifampicin. We also assume that patients receiving IPT are at risk of developing resistance to isoniazid, with rates depending on whether they are latently infected or have active TB disease (we assume imperfect sensitivity of symptom screening such that a small number of individuals with active disease may be initiated on IPT [21]). During the latent stage, individuals in the model may be infected by multiple strains with the same or varying resistance types; however, we assume that progression to active infection acts as a bottleneck, with only one strain dominating (as in [16]). Specifically, we assume that the dominant strain is determined at the time of each (re)infection event, but may switch if IPT is applied to a latently infected individual with a dominant strain that is isoniazid sensitive and non-dominant strain that is isoniazid resistant.
All individuals are assumed to enter the model HIV susceptible at age 15. We allow individuals to be latently infected with at most one TB strain at the time of model entry, with rates determined by the annual risk of infection over the previous 15 years. We do not include a detailed demographic model, and instead allow for a rate of entry that maintains a fairly consistent population size throughout our predictions.
Historical IPT use in Botswana is incorporated from 2004–2008 by allowing patients started on ARVs during that time to receive IPT for a mean duration of 3 months [22, 23]. Otherwise we assume no individuals receive IPT until 2017. We focus our analysis on the potential impact of different IPT strategies from 2017 onwards.
Parameterization
We allow the rates of TB infection, progression, and other natural history parameters to vary depending on whether a person is HIV uninfected, HIV infected and undetected, or HIV infected and detected. Treatment success is also allowed to vary based on both HIV status and resistance pattern. We assume that the majority of these parameters are known with certainty, with values chosen based on a review of the literature. The remaining 18 parameters were assigned prior distributions based on this literature review. Our estimates of these parameters were then refined using Bayesian melding [24, 25] by comparing model outputs to published estimates of TB incidence, TB prevalence, HIV prevalence, HIV prevalence in TB cases, and the coverage of antiretroviral therapy in Botswana from 1990–2013, as well as data from four TB drug resistance surveys conducted over the same time period [26–28].
Several of our parameters were allowed to vary over time to reflect observed trends in TB and HIV control in Botswana. These parameters include the rate of HIV infection, the rate at which PLHIV are started on ART, the TB case detection rate, and the rate of second-line treatment, and are discussed in more detail in the Appendix.
IPT Implementation & Impact
Individuals receiving IPT experience several different effects (Table 1). First, we assume that individuals cannot be infected or reinfected by isoniazid sensitive TB strains while receiving IPT. Second, we assume that the reactivation rate of pre-existing isoniazid sensitive infections is reduced for individuals receiving IPT. IPT may either clear these strains completely or suppress them only during the time that the individual is receiving IPT [8, 9]. If IPT is able to clear these strains, individuals may either retain or lose partial immunity to reinfection. Latently infected individuals may acquire resistance to isoniazid at a low rate; this rate is much higher for individuals with active TB inadvertently receiving IPT. Actively infected individuals receiving IPT may also be cured at low rates reflecting those of the initial trials of isoniazid alone [29, 30]. We allow individuals receiving IPT to experience a small excess mortality rate due to adverse effects, but do not assess other potentially burdensome aspects of long-term IPT use on individuals or the health system.
Table 1.
IPT-related parameters.
| Name | Description | Value | References |
|---|---|---|---|
| v | Risk ratio: DS reactivations allowed while on IPT | Prior: 0.28 (0.10 0.8) Posterior: 0.29 (0.1,0.8) |
[11] (TST+) |
| (not coded) | Proportion DS reinfections allowed on IPT | 0 | assumed |
| alipt | rate of INH resistance on IPT among people with latent TB (year−1) | Prior: 0.025 (0.001, 0.049) Posterior: 0.025 (0.001, 0.05) |
[14] (smaller than resistant rate on 1st line therapy) |
| a | Rate of INH resistance on IPT among people with active TB (year−1) | Same as rifampicin-resistant TB on 1st line therapy | [33] |
| γlipt | Rate of removal of latent TB strains on IPT (year−1) | Prior: 2.5 (0.125, 4.875) Posterior: 1.0 (0.03, 4.4) |
[8] |
| γipt | Cure rate of active TB on IPT (year−1) | Same as rifampicin- resistant TB on 1st line therapy | [33] |
| m_ipt_m | Susceptibility to reinfection after removal of latent TB strains 1: same as susceptible 0: same as latently infected |
Prior: 0.5 (0.025, 0.975) Posterior: 0.52 (0.02, 0.97) |
Assume no information |
| μi | mortality rate with detected HIV, on IPT (year−1) | 0.0006 + baseline mortality rate with detected HIV | [11] |
| μti | mortality rate with active TB, with detected HIV, on IPT (year−1) | Same as 1st line treatment rifampicin-resistant TB (10.6% of people die during therapy) | [34–38] |
| θl | proportion of people with latent TB who receive IPT upon HIV detection (>1 dose) | Before 2004: 0 2004–2008: 0.9 2008–2017: 0 2017 on (IPT scenarios): 0.9 |
assumed |
| θs | proportion of TB susceptibles who receive IPT upon HIV detection (>1 dose) | Same as θl | [1]; WHO recommendations don’t require TST |
| ϕ | proportion of people who start IPT after successfully finishing treatment | 0 | assumed |
| w | rate of IPT completion/dropout (year−1) | 2.147 (6 month), 0.147 (realistic continuous), or 0 (ideal continuous) | Dropout based on [13] |
| e | Rate allowed to start IPT from detected HIV compartment (year−1) | Pre-2017: 0 2017-2017.5: 2.77 Post 2017.5: 0 |
Assume 75% of detected people may start IPT |
Distributions are reported as median (2.5th percentile, 97.5th percentile). A complete list of all parameters can be found in the Appendix.
Beginning in 2017, we implement and compare four different IPT scenarios: no IPT, short-term IPT (mean duration 6 months plus additional dropout), realistic continuous IPT accounting for dropout (median duration 4.7 years, similar to [13]), and perfect continuous IPT assuming no dropout. A sensitivity analysis assuming lower adherence rates is included in the Appendix. We introduce a brief catch-up period in the beginning of 2017 to allow individuals already started on ART to receive IPT; from that point forward, people may only receive IPT upon HIV detection. Individuals may choose not to receive IPT, and those with active TB may be detected by symptom screening prior to IPT initiation and instead started on treatment for active TB. This paper does not address the effect of secondary IPT after completion of treatment for active disease.
Outcomes
The outcomes we investigated included TB incidence (both overall and by resistance type), mortality rate among PLHIV, and cumulative mortality among PLHIV. Because population-level changes in resistance may not be evident for several years, we present our results from the start of IPT in 2017 through 2050, recognizing that the recommended treatment regimens may very well change within that time. We present results both for a baseline scenario motivated by the epidemic in Botswana and for scenarios with higher future transmission, reflecting trends more similar to those seen in the pre-ART era.
Results
Here we describe the results of our analysis both for our baseline Botswana scenario, in which transmission is declining based on WHO estimates and our model predictions, and for scenarios with higher transmission post-2017.
Baseline Botswana Results
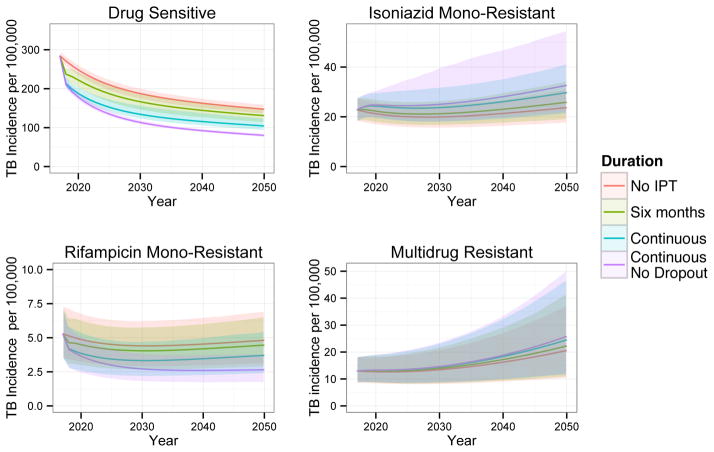
Figure 2 shows the projected incidence of pan-sensitive, rifampicin mono-resistant, isoniazid mono-resistant, and MDR TB in Botswana for the range of IPT durations. Our model projects that longer IPT durations will decrease the incidence of pan-sensitive and rifampicin mono-resistant TB through at least 2050 (assuming no changes in treatment, policy, etc). We also predict that longer durations of IPT will increase the incidence of isoniazid mono-resistant and MDR TB.
Figure 2.
The effect of IPT duration on TB incidence (new cases per year) by resistance phenotype under our baseline Botswana scenario. Solid lines display means and shaded regions display 95% quantiles of our posterior predictions.
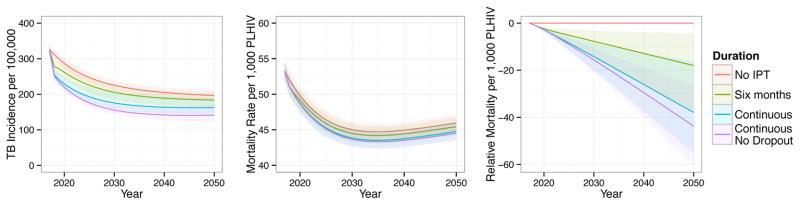
IPT has the greatest impact in absolute terms on the incidence of drug sensitive TB. Figure 3 shows projections of different IPT durations on overall TB incidence, mortality rate among PLHIV, and cumulative mortality among PLHIV relative to no IPT. Under our baseline scenario, we predict that longer durations of IPT will decrease the overall incidence of TB through 2050 despite increases in the incidence of isoniazid mono-resistant and MDR TB. We similarly predict that longer durations of IPT will provide overall mortality benefits to our population through at least 2050, suggesting that the projected increases in isoniazid resistance are not sufficient to outweigh the benefits of decreased overall TB incidence under this scenario.
Figure 3.
The composite effects of IPT duration on overall TB incidence (left, new cases per year), mortality rate among PLHIV (middle, deaths per year), and cumulative mortality among PLHIV (right, cumulative deaths per 1,000 PLHIV relative to no IPT). Solid lines display means and shaded regions display 95% quantiles of our posterior predictions.
The initial program providing IPT to PLHIV in Botswana beginning in 2004 was stopped in 2008 after an observed increase in isoniazid resistance between the 2002 and 2008 drug resistance surveys; it was unclear, however, how much of this increase could be attributed to the IPT program [22, 23, 26]. By comparing our baseline model results from these two time periods with a counterfactual scenario under which no IPT was provided, we estimate that 12.8% (95% quantiles 9.2%, 16.9%) of the increase in drug resistance from 2002 to 2008 was a result of the IPT program, with the remainder reflecting trends in treatment and transmission.
Sensitivity of Findings to Projected Epidemic Trajectory
Under our baseline scenario, we predict substantial decreases in overall TB incidence through 2050 even in the absence of IPT, reflecting UNAIDS HIV incidence projections and expanded access to antiretroviral therapy. However, these observed trends could be subject to unforeseen events or changes, whether technological, medical, or political; they also may limit the generalizability of our results to other settings. Therefore, we also explored the effect of IPT duration on the incidence of both isoniazid sensitive and isoniazid resistant TB under assumptions of constant or increasing TB transmission after 2017. Although the highest transmission scenario in particular is not reflected by current trends, we include these scenarios to facilitate comparison with the previous models that demonstrate the potential selective pressure exerted by IPT in favor of resistant strains [16, 18], and to provide projections for what could happen if conditions changed such that TB incidence was allowed to increase again.
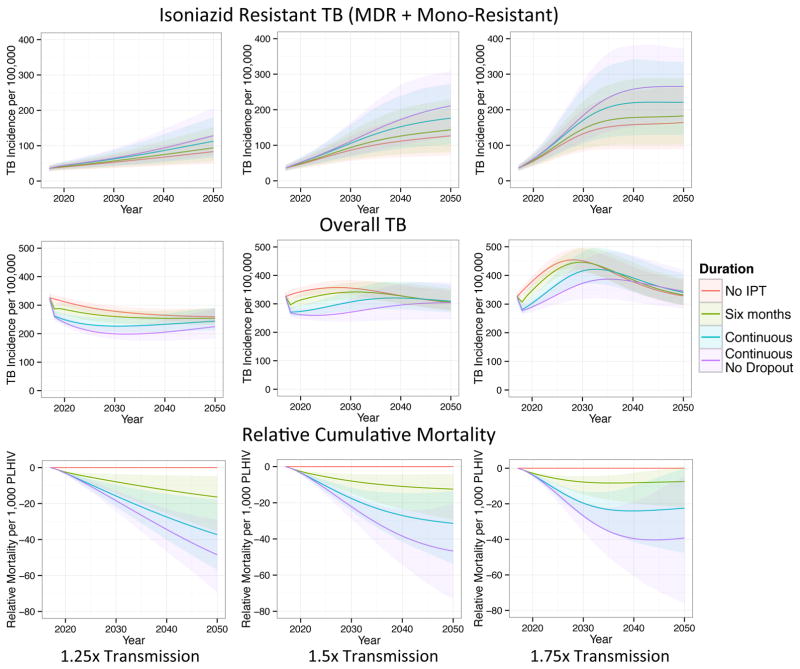
Figure 4 shows the incidence of isoniazid resistant (mono-resistant plus MDR) and overall TB, as well as cumulative mortality among PLHIV, under different durations of IPT when the transmission parameter is increased 1.25x, 1.5x, or 1.75x that of our baseline scenario beginning in 2017. Longer durations of IPT have a stronger effect on the incidence of isoniazid resistant TB under these higher transmission scenarios. When transmission is sufficiently high, the expected increase in isoniazid resistant TB outpaces the decrease in isoniazid sensitive TB within 25 years or less. However, the average cumulative mortality remains lowest for the longest IPT duration scenario through 2050 even for the highest transmission scenario..
Figure 4.
The effects of IPT duration on the incidence of isoniazid resistant and overall TB (new cases per year) and cumulative mortality relative to no IPT (cumulative deaths per 1,000 PLHIV) when the transmission parameter post-2017 is increased 1.25x, 1.5x, and 1.75x compared to our baseline scenario. When transmission is relatively high, longer durations of IPT can produce large increases in the incidence of isoniazid resistant TB, eroding their initial overall incidence benefits. Solid lines display means and shaded regions display 95% quantiles of our posterior predictions.
Discussion
We created a mathematical model to examine the potential impact of implementing IPT programs of varying durations among PLHIV in Botswana and explored the sensitivity of these results to assumptions about future TB transmission trends. Our model consistently predicts longer durations of IPT to decrease incidence of isoniazid sensitive TB and increase incidence of isoniazid resistant TB. However the relative importance of these two effects varies depending on the future trajectory of the epidemic. In a declining epidemic such as our baseline Botswana scenario, we predict the benefits of continuous IPT for PLHIV to outweigh the risks of increases in isoniazid resistance through at least 2050. If we allow for greater future transmission, however, the initial incidence and mortality benefits of longer IPT durations may subsequently be eroded by substantial increases in the incidence of isoniazid resistant TB. This finding likely reflects an increased importance of the selective pressure imposed by IPT relative to other resistance mechanisms when transmission is high [17], and is consistent with the results of previous models constructed at an earlier phase of the HIV epidemic when TB incidence was increasing [16, 18].
TB transmission trends may be affected by a large range of underlying parameters, including potential changes in HIV transmission, population structure, and standards of living, as well as the structural assumptions of our model. Our initial assumption was that the transmission parameter would remain fixed from 2017–2050, reflecting continued projected advances in HIV diagnosis and treatment as well as TB case detection and treatment policies that were assumed to be fairly well-functioning. If transmission were instead to increase as seen in the pre-ART era, the higher transmission scenarios may provide more realistic projections.
These results suggest that continuous IPT is likely to be most effective in preventing future TB transmission when coupled with strong TB and HIV control programs. Using continuous IPT in the absence of highly-effective TB and HIV case-finding and treatment, however, may result in substantial increases in the incidence of isoniazid resistant TB. Continuous IPT should be considered as one of a suite of tools that could be useful for more rapidly reducing the burden of HIV-associated TB, and does not decrease the importance of other interventions. We also suggest that IPT programs providing widespread and/or continuous IPT be accompanied by robust drug resistance surveillance, especially in settings with a high prevalence of HIV or where TB transmission is believed to be stable or increasing. Such surveillance programs should focus on the absolute incidence of isoniazid resistant TB, rather than the proportion of TB cases that are isoniazid resistant, as increases in the latter could also reflect expected declines in incidence of isoniazid sensitive TB.
Even under our most pessimistic high transmission scenario, however, the risks of increased isoniazid resistance seen in this analysis are not immediate. Longer durations of IPT are predicted to lower overall TB incidence and the mortality rate among PLHIV for at least 20 years on average, and the cumulative mortality advantage of continuous IPT could last much longer. The risks of resistance driven by widespread, long duration IPT should therefore be weighed against its potential immediate benefits. These future risks could be mitigated by future trends in TB research and treatment, particularly in the area of TB drug development. Though the use of different drugs for prevention and treatment may not currently be possible given the limited number of TB drugs available, continued drug development could make this a highly appealing option, either through the development of an effective alternative first-line regimen without isoniazid or perhaps even the targeted use of a new drug for prevention only [31, 32].
Apart from resistance, policymakers may have additional concerns about the feasibility of and costs associated with continuous IPT, which this model does not address. We allowed for a slightly increased mortality risk among people receiving IPT, but did not consider other side effects. We also did not fully explore the potential implications of poor adherence, though a sensitivity analysis (see Appendix) suggests mortality would be lowest assuming continuous IPT even if adherence were reduced.
IPT is a complicated intervention, with population-level impacts potentially affected by trends in TB, HIV, and drug resistance. We have attempted to strike a balance between simplicity and complexity in our analysis, with parameters informed by published estimates whenever possible and assigned wide prior ranges when not. The detailed structure of our model afforded us the opportunity to account for complexities avoided in previous models, such as the stepwise accumulation of mutations for resistance to isoniazid and rifampicin [16, 18, 19]. However, the increased complexity of this model also means that more parameters need to be specified, and many of the parameters assumed fixed may not actually be known with certainty. The uncertainty intervals presented here reflect these assumptions, and thus may underestimate the true uncertainty in our predictions. Similarly, the data used to estimate the most uncertain parameters were both limited in scope and based primarily on country-wide estimates rather than actual data points. Despite the complexity of the model, it also incorporates a number of strong simplifying assumptions and structural elements that may constrain the sorts of predictions we can make. In particular, this analysis does not incorporate a detailed model of demographic trends in Botswana. The HIV model is also simplified and does not fully account for the natural history of HIV infection. Furthermore, we relied on UNAIDS HIV incidence estimates rather than creating a full transmission model of HIV. These limitations suggest caution should be used in relying on the quantitative projections provided in this paper, but are less likely to affect the qualitative trends we report here.
In summary, our results suggest that if interventions using longer duration IPT among PLHIV could be brought to scale in Botswana or similar settings, we would observe a decrease the incidence of isoniazid sensitive (including rifampicin mono-resistant) TB through at least 2050. In higher transmission settings, however, IPT could result in large increases in the incidence of isoniazid resistant TB that could erode the initial reductions in TB incidence, though cumulative mortality remained lowest under the continuous IPT policy through 2050 for all explored scenarios. The benefits of continuous IPT are most likely to outweigh any increase in resistance when coupled with strong HIV and TB case-finding and treatment programs, continued TB drug development, and robust TB drug resistance surveillance.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institute of Health under award numbers T32AI007535 (AK) and R01AI112438 (TC and FWC) of the National Institute of Allergy and Infectious Diseases, award number KL2TR000140 of the National Center for Advancing Translational Sciences (FWC), and award number P30MH062294 of the National Institute of Mental Health (FWC), as well as the Bill and Melinda Gates Foundation/TB Modeling and Analysis Consortium under award number OPP1135288 (AK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We would like to acknowledge Marc Lipsitch and Megan Murray for their helpful comments throughout the development of this project.
Footnotes
Model and results presented previously at the 2015 Union World Conference on Lung Health, Cape Town, South Africa
Author contributions were as follows: AK TC conceived the study, AK developed the model with assistance from TC; FWC provided guidance for model calibration and interpretation; JS contributed data and to design of modeling scenarios; AK, FWC, TC interpreted model findings; AK wrote the first draft of the paper; AK, FWC, JS, TC contributed to writing final version of the paper.
References
- 1.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: 2011. [Google Scholar]
- 2.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010:Cd000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JL, Okwera A, Hom DL, Mayanja H, Mutuluuza Kityo C, Nsubuga P, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. Aids. 2001;15:2137–2147. doi: 10.1097/00002030-200111090-00009. [DOI] [PubMed] [Google Scholar]
- 4.Quigley MA, Mwinga A, Hosp M, Lisse I, Fuchs D, Porter JDH, et al. Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. Aids. 2001;15:215–222. doi: 10.1097/00002030-200101260-00011. [DOI] [PubMed] [Google Scholar]
- 5.Golub JE, Cohn S, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, et al. Long-term protection from isoniazid preventive therapy for tuberculosis in HIV-infected patients in a medium-burden tuberculosis setting: the TB/HIV in Rio (THRio) study. Clin Infect Dis. 2015;60:639–645. doi: 10.1093/cid/ciu849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Mosimaneotsile B, et al. Tuberculosis incidence after 36 months’ isoniazid prophylaxis in HIV-infected adults in Botswana: a posttrial observational analysis. Aids. 2015;29:351–359. doi: 10.1097/QAD.0000000000000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churchyard GJ, Fielding KL, Lewis JJ, Coetzee L, Corbett EL, Godfrey-Faussett P, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:301–310. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 8.Vynnycky E, Sumner T, Fielding KL, Lewis JJ, Cox AP, Hayes RJ, et al. Tuberculosis control in South African gold mines: mathematical modeling of a trial of community-wide isoniazid preventive therapy. Am J Epidemiol. 2015;181:619–632. doi: 10.1093/aje/kwu320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houben RM, Sumner T, Grant AD, White RG. Ability of preventive therapy to cure latent Mycobacterium tuberculosis infection in HIV-infected individuals in high-burden settings. Proc Natl Acad Sci U S A. 2014;111:5325–5330. doi: 10.1073/pnas.1317660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumner T, Houben RM, Rangaka MX, Maartens G, Boulle A, Wilkinson RJ, et al. Post-Treatment Effect of Isoniazid Preventive Therapy on Tuberculosis Incidence in Hiv-Infected Individuals on Antiretroviral Therapy. Aids. 2016 doi: 10.1097/QAD.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 11.Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 12.Boon SD, Matteelli A, Ford N, Getahun H. Continuous isoniazid for the treatment of latent tuberculosis infection in people living with HIV: a systematic review and meta-analysis. Aids. 2016 doi: 10.1097/QAD.0000000000000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365:11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis. 2006;12:744–751. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipsitch M, Samore MH. Antimicrobial use and antimicrobial resistance: a population perspective. Emerg Infect Dis. 2002;8:347–354. doi: 10.3201/eid0804.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills HL, Cohen T, Colijn C. Community-wide isoniazid preventive therapy drives drug-resistant tuberculosis: a model-based analysis. Sci Transl Med. 2013;5:180ra149. doi: 10.1126/scitranslmed.3005260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel A, Colijn C, Lipsitch M, Cohen T. How could preventive therapy affect the prevalence of drug resistance? Causes and consequences. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140306. doi: 10.1098/rstb.2014.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen T, Lipsitch M, Walensky RP, Murray M. Beneficial and perverse effects of isoniazid preventive therapy for latent tuberculosis infection in HIV-tuberculosis coinfected populations. Proc Natl Acad Sci U S A. 2006;103:7042–7047. doi: 10.1073/pnas.0600349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu S, Maru D, Poolman E, Galvani A. Primary and secondary tuberculosis preventive treatment in HIV clinics: simulating alternative strategies. Int J Tuberc Lung Dis. 2009;13:652–658. [PubMed] [Google Scholar]
- 20.Botswana National Tuberculosis Program. National Tuberculosis Program Annual Report 2012. 2012 [Google Scholar]
- 21.Getahun H, Kittikraisak W, Heilig CM, Corbett EL, Ayles H, Cain KP, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministry of Health Botswana. Isoniazid preventive therapy programme (IPT) evaluation report. 2008. [Google Scholar]
- 23.Ministry of Health Botswana. Isoniazid preventive therapy (IPT) progress report 2002-009. 2009. [Google Scholar]
- 24.Poole D, Raftery AE. Inference for Deterministic Simulation Models: The Bayesian Melding Approach. Journal of the American Statistical Association. 2000;95:1244–1255. [Google Scholar]
- 25.Alkema L, Raftery AE, Brown T. Bayesian melding for estimating uncertainty in national HIV prevalence estimates. Sex Transm Infect. 2008;84(Suppl 1):i11–i16. doi: 10.1136/sti.2008.029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzies HJ, Moalosi G, Anisimova V, Gammino V, Sentle C, Bachhuber MA, et al. Increase in anti-tuberculosis drug resistance in Botswana: results from the fourth National Drug Resistance Survey. Int J Tuberc Lung Dis. 2014;18:1026–1033. doi: 10.5588/ijtld.13.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNAIDS. AIDSinfo. http://aidsinfo.unaids.org.
- 28.World Health Organization. TB burden estimates. http://www.who.int/tb/country/data/download/en/
- 29.Sita Lumsden EG, Swoboda JA. Isoniazid in the treatment of pulmonary tuberculosis. Tubercle. 1952;33:322–329. doi: 10.1016/s0041-3879(52)80103-5. [DOI] [PubMed] [Google Scholar]
- 30.Treatment of pulmonary tuberculosis with isoniazid; an interim report to the Medical Research Council by their Tuberculosis Chemotherapy Trials Committee. Br Med J. 1952;2:735–746. [PMC free article] [PubMed] [Google Scholar]
- 31.Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, et al. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet. 2012;380:986–993. doi: 10.1016/S0140-6736(12)61080-0. [DOI] [PubMed] [Google Scholar]
- 32.Churchyard GJ, Friedland G, Fielding K, Nardell E. Opportunities afforded by new drugs for tuberculosis. Lancet Infect Dis. 2010;10:368–369. doi: 10.1016/S1473-3099(10)70095-5. [DOI] [PubMed] [Google Scholar]
- 33.A concurrent comparison of isoniazid plus PAS with three regimens of isoniazid alone in the domiciliary treatment of pulmonary tuberculosis in South India. Bull World Health Organ. 1960;23:535–585. [PMC free article] [PubMed] [Google Scholar]
- 34.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 35.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straetemans M, Glaziou P, Bierrenbach AL, Sismanidis C, van der Werf MJ. Assessing tuberculosis case fatality ratio: a meta-analysis. PLoS One. 2011;6:e20755. doi: 10.1371/journal.pone.0020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Churchyard GJ, Kleinschmidt I, Corbett EL, Murray J, Smit J, De Cock KM. Factors associated with an increased case-fatality rate in HIV-infected and non-infected South African gold miners with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:705–712. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.