Abstract
Threat induces a state of sustained anxiety that can disrupt cognitive processing, and reciprocally, cognitive processing can modulate an anxiety response to threat. These effects depend on the level of cognitive engagement, which itself varies as a function of task difficulty. In adults, we recently showed that induced anxiety impaired working memory accuracy at low- and medium- but not high-load. Conversely, increasing the task load reduced the physiological correlates of anxiety (anxiety-potentiated startle). The present work examines such threat-cognition interactions as a function of age. We expected threat to more strongly impact working memory in younger individuals by virtue of putatively restricted cognitive resources and weaker emotion regulation. This was tested by examining the influence of age on the interaction of anxiety and working memory in 25 adolescents (10 to 17 years) and 25 adults (22 to 46 years). Working memory load was manipulated using a verbal n-back task. Anxiety was induced using the threat of an aversive loud scream and measured via eye-blink startle. Findings revealed that, in both age groups, accuracy was lower during threat than safe conditions at low- and medium- but not high-load, and reaction times were faster during threat than safe conditions at high-load but did not differ at other loads. Additionally, anxiety-potentiated startle was greater during low- and medium- than high-load. Thus, the interactions of anxiety with working memory appear similar in adolescents and adults. Whether these similarities reflect common neural mechanisms would need to be assessed using functional neuroimaging.
Keywords: cognitive load, adolescence, threat, anxiety, working memory
Anxiety facilitates adaptive defensive behaviors under threat, but excessive anxiety can negatively impact cognition. For example, individuals with clinical anxiety and high-trait anxiety, relative to non-anxious individuals, manifest perturbed attention and memory (Airaksinen, Larsson, & Forsell, 2005; Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Eysenck, Derakshan, Santos, & Calvo, 2007; Mogg & Bradley, 2005). Even in healthy adults, induced anxiety can impair working memory (Clarke & Johnstone, 2013; Vytal, Cornwell, Arkin, & Grillon, 2012; Vytal, Cornwell, Letkiewicz, Arkin, & Grillon, 2013). Here, we examine the interaction of sustained anxiety, induced by the anticipation of an aversive stimulus, and working memory in adolescents vs. adults.
Research has begun to map interactions of cognition with sustained anxiety using behavioral and psychophysiological measures in healthy adults (see Vytal et al., 2013). In prior work with healthy adults, Vytal and colleagues (2012) examined the interaction of verbal working memory load (low, medium, and high) with induced sustained anxiety evoked by the threat of electric shock. Reaction time (RT) did not differ between threat and safe (no threat) conditions for any load. However, threat impaired accuracy in low- and medium- but not high-load tasks (Vytal et al., 2012). Thus, threat altered performance in tasks that required fewer cognitive resources (low- and medium-load) compared to the task that required the most resources (high-load). This pattern is consistent with the notion of limited cognitive resources (Bishop, 2009; Eysenck & Calvo, 1992; Lavie, Hirst, de Fockert, & Viding, 2004; Pessoa, 2009; Vytal et al., 2012). Accordingly, available cognitive resources under lower loads allow for the processing of threat, which can, in turn, interfere with performance. In contrast, the full consumption of resources at a higher load precludes threat processing and thus performance interference. Consistent with this theory, Vytal and colleagues (2012) also found that anxiety decreased as working memory load increased; this was evidenced by reduced anxiety-potentiated startle, a robust measure of anxiety (Grillon & Baas, 2003).
The ability to efficiently perform a cognitive task under threat may differ between adults and adolescents, whose executive function is still maturing. This question is important because adolescence is a period when many anxiety disorders arise (Beesdo, Knappe, & Pine, 2009; Kessler, et al., 2005), and it is also a critical period for learning, with the acquisition of academic, social, and self-related knowledge. One could then expect adolescents to have an enhanced sensitivity to elevated anxiety state when performing cognitive tasks. Of note, there is an important distinction between anxiety and fear, which engage different neural mechanisms subserving distinct functions. During anxiety, a sustained response occurs to a distal threat, while during fear, a phasic response occurs to an imminent threat (Davis, Walker, Miles, & Grillon, 2010). The developmental work on the interaction of fear with cognitive performance has been conducted on performance interference by an imminent threat stimulus, such as a fearful face (Hare, et al., 2008; Mueller, et al., 2012; Mueller, et al., 2015). Little is known about the effect of induced, sustained anxiety on cognitive function during development. The current study examines this question by comparing adolescents and adults on working memory in a prolonged threat and safe context.
Adolescents exhibit unique cognitive and emotional characteristics (e.g., Steinberg, 2005). These characteristics have guided the formulation of theoretical models of brain maturation during this period (Casey, Getz, & Galvan, 2008; Ernst & Fudge, 2009). Working memory improves during adolescence (Huizinga, Dolan, & van der Molen, 2006; Luna, Garver, Urban, Lazar, & Sweeney, 2004; Luna, Padmanabhan, & O’Hearn, 2010), suggesting an increasing availability of cognitive resources and processing efficiency (RT) with age (Ferrer et al., 2013; Luna et al., 2004). In addition, emotion regulation also improves with age (Steinberg, 2005; Yurgelun-Todd, 2007). Most relevant here, fear learning and extinction may vary across adolescent development (see reviews, King, Pattwell, Sun, Glatt, & Lee, 2013; Shechner, Hong, Britton, Pine, & Fox, 2014). These changes have been reported in response to discrete threat stimuli, such as aversive pictures and sounds. Taken together, prior research suggests that adolescents and adults might differ in terms of how emotion, particularly anxiety, interferes with cognition.
The present work compares healthy adolescents with healthy adults using the experimental design employed by Vytal and colleagues (2012), with adaptations for use with pediatric populations. The hypotheses are three-fold. First, we expect to replicate the findings by Vytal and colleagues (2012) in adults. Namely, we expect working memory accuracy to be reduced during threat vs. safe conditions at low and medium cognitive loads, but to be similar during threat and safe conditions at high cognitive load. Additionally, we do not expect to find differences in RT between threat and safe conditions for any load. Second, based on ongoing cognitive maturation in adolescents, we expect lower working memory accuracy and longer RTs in adolescents compared to adults. Third, regarding the main question of how induced anxiety modulates cognitive function in adolescents compared to adults, three alternative predictions are considered: (1) On the basis of putatively restricted cognitive resources in adolescence, threat may not be processed at any cognitive load, with resources being fully devoted to cognitive processes. As a result, adolescent performance (accuracy and RT) would not differ between the threat and safe condition at any cognitive load. (2) On the basis of weaker emotion regulation in adolescents (Steinberg, 2005), resources might be prioritized for the processing of threat over cognitive performance, resulting in poorer performance (accuracy and RT) during threat vs. safe conditions of all cognitive loads, and higher induced anxiety (anxiety-potentiated startle) in adolescents compared to adults. (3) Finally, adolescents may not differ from adults, indicating that the processes of interference of sustained anxiety with cognitive performance are already mature in adolescence.
Method
Participants
Twenty-five healthy adolescents (age 10 to 17 years; 13 male) and 25 healthy adults (age 22 to 46 years; 12 male) successfully completed the study (see Table 1 for demographics). Three additional adolescents and three additional adults were excluded because of incomplete responses or unreliable physiological responses (see below). Adolescents were recruited through a county public school system and general advertisements, and adults were recruited through general advertisements. The two groups did not differ on sex1 distribution or IQ (ps > 0.1).
Table 1.
Adolescent and Adult Demographics
| Demographic | Adolescents (n = 25) | Adults (n = 25) |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age in years | 13.96 (1.95) | 28.85 (6.14) |
| IQ | 117.04 (10.57) | 116.28 (12.44) |
| STAI-T | 28.0 (9.20) i | 26.44 (4.98) ii |
Notes: STAI-T = Spielberger State-Trait Anxiety Inventory trait subscale.
child version of STAI-T,
adult version of STAI-T.
Subjects were not taking any psychoactive medications and did not have a current psychiatric disorder as determined by the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 1997) for adolescents and the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Williams, & Gibbon, 1995) for adults. All subjects had an IQ score greater than 80 (Vocabulary and Matrix reasoning subtests; Wechsler, 1999). Subjects were also assessed for trait anxiety using the trait subscale of the State-Trait Anxiety Inventory (STAI-T) (for adolescents, Spielberger, Edwards, Montuori, & Lushene, 1973; for adults, Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). The range of possible scores for the child and adult STAI-T versions differ, limiting direct comparisons of trait anxiety between groups. However, the mean trait score for each group (see Table 1) was within the lowest quarter range of possible scores.
The protocol was approved by the National Institute of Mental Health Institutional Review Board. Written consent was obtained from adult participants and from parents of adolescents, and written assent was obtained from adolescent participants. Subjects were compensated financially for participation in the study.
Procedure
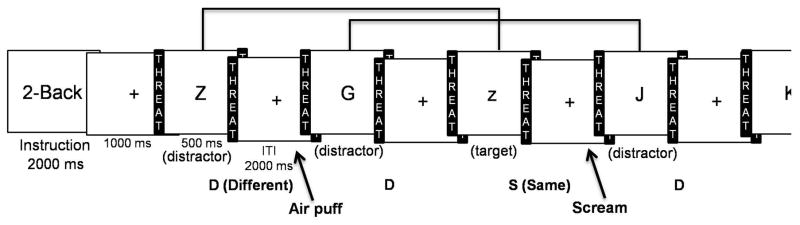
The Memory-threat task (Figure 1a and 1b) was identical to the task used by Vytal and colleagues (2012) with the exception of the threat stimulus and startle probe (see below). In short, verbal working memory was assessed using a visual verbal n-back task with varying levels of cognitive load (no-load (0-load), 1-back (low-load), 2-back (medium-load), and 3-back (high-load)). A single letter was presented serially. During the no-load task, subjects were told to just look at the letters without making a response. For the three working memory tasks, subjects were asked to make a button response of “S” (for same) or “D” (for different) based on the letter one (1-back), two (2-back), or three trials back (3-back) (see Figure 1a for an example of the 2-back task).
Figure 1.
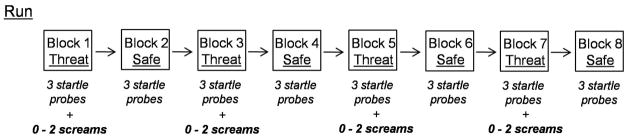
Memory-threat task. (a) The figure provides an illustration of the Memory-threat task during the threat condition of a partial 2-back block. Participants responded to every letter indicating whether the currently presented letter was the same as (S; the target) or different from (D; the distractor) the letter presented two letters back (e.g., same = z and Z; different = J and G). The letters presented at the beginning of the block, for which a response could not be made (i.e., going n letters back would fall onto the instruction screen, were considered to be distractors. The startle probe and threat stimulus were presented during the ITI. (b) The figure provides an illustration of one run (8 blocks) of the Memory-threat task. Blocks interchanged between the threat and safe conditions. Three startle probes were presented in every block, and 0 to 2 loud screams were presented during the threat blocks.
The task employed a within-subjects design. It consisted of four runs with eight blocks per run. The eight blocks were comprised of four threat and four safe blocks. Threat and safe blocks were alternated (see Figure 1b). Each cognitive load was presented twice per run, once in a threat block and the other in a safe block. An instruction screen indicated the upcoming type of n-back task (i.e., no-load, 1-back, 2-back, or 3-back) for each block. Runs and blocks were counterbalanced.
The threat condition consisted of the unpredictable presentation of a loud shrieking scream (95dB, 1000ms) through headphones. This is in contrast to the threat of shock used in our previous study (Vytal et al., 2012). Given our adolescent group, the threat of a loud scream (vs. shock) was a more appropriate stimulus. The loud scream has been successfully applied in conditioning studies with adolescents (Britton, Lissek, Grillon, Norcross, & Pine, 2011; Lau et al., 2008) and in studies with adults (Lissek et al., 2005; Massar, Mol, Kenemans, & Baas, 2011). Prior to beginning the task, the loud scream was presented to acquaint the subject with the stimulus and to prompt a state of aversion toward it that otherwise would not have been known until the presentation of the loud scream during the task. Zero to two loud screams were presented during each threat block, with a total of two loud screams in each run. In order to prevent desensitization to the threat stimulus, only two loud screams were presented during each run. During the safe condition, subjects were told that the loud scream would not be presented. The threat condition was indicated to the subject by the word THREAT written within a colored border on the right and left sides of the screen, and the safe condition was similarly demarcated with the word SAFE.
Startle, specifically the eye-blink response, was probed with an air-puff to the forehead (Lissek et al., 2005). Unlike in prior studies which used an auditory probe (Vytal et al., 2012; Vytal et al., 2013), a tactile probe was employed in this study in order to present the startle probe and the threat stimulus in different sensory modalities, and avoid potential issues of threat stimulus generalization. The air-puff to the forehead provides a good substitute to the auditory white noise probe in eliciting a startle response (Lissek et al., 2005). The air-puff was administered through a tube attached to a helmet worn by the subject, and directed at the forehead. Responses to the air-puff were measured via electromyographic recordings of the reflexive startle eye-blink response (e.g., Vytal et al., 2012). These recordings were measured by placing a ground electrode on the left arm and two electrodes beneath the left eye to record orbicularis oculi muscle activity. In order to obtain a moderate to large eye-blink response, the air-puff was presented initially to the subject at 4 pounds per square inch (psi). If a startle response was not obtained at 4 psi, the psi was increased by increments of 2 psi until an appropriate response was evoked. Prior to the task, participants were exposed to a 5-minute habituation period during which they received five startle probes. This served to prevent artificially high startle responses to initial presentations of the startle probe. Similarly, during each run, three habituation startle probes were presented prior to the beginning of the task. During the task, three unpredictable startle probes were presented during inter-trial intervals of each block, for a total of 24 per run.
After the 5-minute habituation period, subjects were given instructions for the verbal working memory task. After all task-related questions were answered, subjects completed a paper version of the task to determine proficiency. This was followed by a short practice session on the computer, and finally, the task. At the end of each task run, subjects were asked to indicate “how anxious” they felt during the threat and safe conditions (on a scale of 1 = not at all to 9 = extremely).
PsychLab (Contact Precision Instruments, London, United Kingdom) was used to present the startle probe and threat stimulus, and Presentation® software (Version 0.70, www.neurobs.com) was used to present the n-back task and manage the presentation of the probe and stimulus.
Data Reduction
Working memory performance was indexed by two variables, RT (for correct responses only) and accuracy. A correct response indicated that subjects correctly identified whether or not the current letter matched the letter presented 1 trial (1-back), 2 trials (2-back) or 3 trials (3-back) back, depending on the task. We only analyzed RT for correct responses. Incorrect responses may originate from a number of processes, such as impulsivity, attention lapses, or faulty memory, making it difficult to interpret this measure. Accuracy was the percent of correct responses, i.e., the number of correct responses divided by the total number of trials. Two adolescents were excluded because of incomplete performance data.
Anxious arousal was indexed by eye-blink responses. These responses were recorded during the 1-back, 2-back, and 3-back tasks, and also during the baseline condition in the absence of the task (no-load). Eye-blink responses were processed similarly to our previous work (Vytal et al., 2012). Eye-blink data were taken at 1,000 Hz, filtered at 30–500 Hz, and smoothed and rectified with a 20 ms time constant. Peak eye-blink magnitude was determined in the 20–100 ms time frame following startle probe onset relative to baseline (average baseline magnitude for the 50 ms immediately preceding startle probe onset). Trials in which the electromyographic activity during baseline was excessive (three times greater than the standard deviation of the mean) were excluded because this type of response represents a spontaneous blink that is not in response to the startle probe (i.e., the subject was in the process of blinking when the startle probe was presented). Subjects with noisy eye-blink data (one adult) or low mean startle amplitudes (< 2.5 μV) (one adolescent and two adults) were excluded. Responses were averaged within each condition of each load. Eye-blink magnitude T-scores were calculated to normalize data and to account for individual differences in startle variability.
Additionally, T-scores of eye-blink magnitude from the safe condition were subtracted from that during the threat condition [threat – safe]. This difference score, the anxiety-potentiated startle, provided a physiological measure of anxiety (Grillon, Ameli, Woods, Merikangas, & Davis, 1991; Grillon, Baas, Lissek, Smith, & Milstein, 2004).
Data Analysis
The effectiveness of the threat manipulation was assessed using the “how anxious” subjective ratings. These responses were analyzed using a repeated-measures analysis of variance (rANOVA) with Run (run 1, run 2, run 3, run 4) and Condition (threat, safe) as the within-subjects factors, and Group (adolescents, adults) as the between-subjects factor. To determine self-reported anxiety between conditions, “how anxious” responses were averaged across runs, and a rANOVA with a within-subjects factor of Condition and a between-subjects factor of Group was conducted.
RT and accuracy were analyzed using a rANOVA with Condition and Load (1-back, 2-back, 3-back) as the within-subjects factors, and Group as the between-subjects factor. Additionally, difference scores of performance (RT, accuracy) between threat and safe conditions [threat – safe] were calculated and were used for conducting correlations between performance (RT, accuracy) and anxiety-potentiated startle.
Eye-blink startle responses were analyzed using a rANOVA with Condition and Load (no-load, 1-back, 2-back, 3-back) as the within-subjects factors, and Group as the between-subjects factor. Lastly, in order to determine changes in physiological anxiety based on cognitive load, anxiety-potentiated startle was analyzed using a rANOVA with a within-subjects factor of Load (no-load, 1-back, 2-back, 3-back) and a between-subjects factor of Group.
Statistical tests used an alpha = 0.05. Greenhouse-Geisser corrections were made for rANOVAs when violations of sphericity were detected (uncorrected degrees of freedom and a corrected p value are reported). Bonferroni corrections were used to adjust for multiple comparisons during post-hoc tests. Hochberg’s procedure (Hochberg, 1988) was used to make corrections for multiple comparisons during correlational analyses.
Results
Anxiety Manipulation (Threat of Scream)
Two measures were used to ascertain the aversiveness of the threat stimulus and its subsequent ability to induce anxiety during the threat condition. First, “how anxious” ratings did not significantly decrease from run 1 to run 4 (N = 44; p > .1), indicating that subjects experienced constant levels of anxiety throughout the task. Additionally, as expected, “how anxious” ratings averaged across runs (N = 49) showed a main effect of Condition (F(1,47) = 68.48, p < .001, ηp2 = .59), which reflected greater anxiety ratings during the threat (mean = 3.98) than safe (mean = 1.97) condition. No Group differences emerged for these measures (ps > .1), indicating that the threat stimulus was subjectively equally aversive for adolescents and adults. Second, eye-blink startle responses were greater during the threat than safe condition (F(1,48) = 59.17, p < .001, ηp2 = .55). These results confirmed that the loud scream successfully induced anxiety.
Working Memory Reaction Time
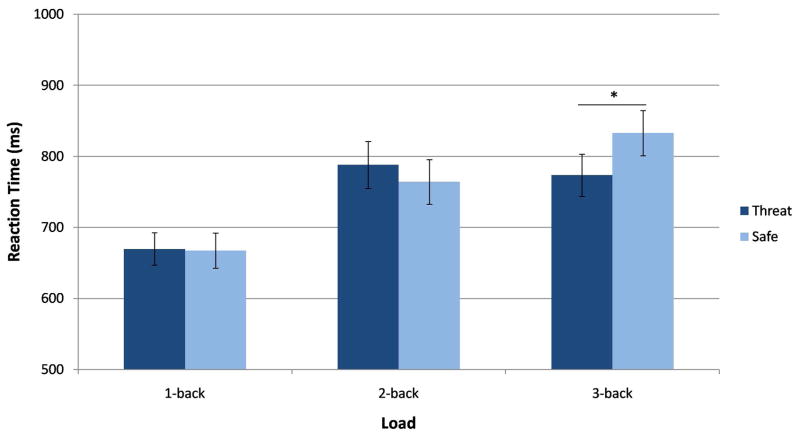
A Condition by Load by Group rANOVA conducted on RT of correct responses (Figure S1 in supplementary material) did not reveal a significant 3-way interaction (F(2,96) = .46, p > .1, ηp2 = .01). However, a significant 2-way Condition by Load interaction (F(2,96) = 13.88, p < .001, ηp2 = .22) did emerge. To better understand this interaction, each load was examined separately. Findings showed that RT was faster in the threat vs. safe condition for the 3-back task (p < .001), but not different between these conditions in the 1-back (p > .1) or 2-back task (p > .05) (Figure 2).
Figure 2.
Mean reaction time by Condition and Load. The Condition by Load interaction showed a faster reaction time during the threat than safe condition on the 3-back task, but not the 1-back or 2-back tasks across age. N = 50, *p < .001, error bars represent ± SEM.
In addition, Load had an expected significant main effect (F(2,96) = 42.82, p < .001, ε= .86, ηp2 = .47), consisting of longer RTs as load increased. Post-hoc tests revealed significant RT differences between the 1-back and 2-back tasks and between the 1-back and 3-back tasks (ps < .001), but not between the 2-back and 3-back tasks (p > .05). Group had no significant effects on RT, either as a main effect (F(1,48) = .76, p > .1, ηp2 = .02) or in interaction with the other factors (ps > .1).
Working Memory Accuracy
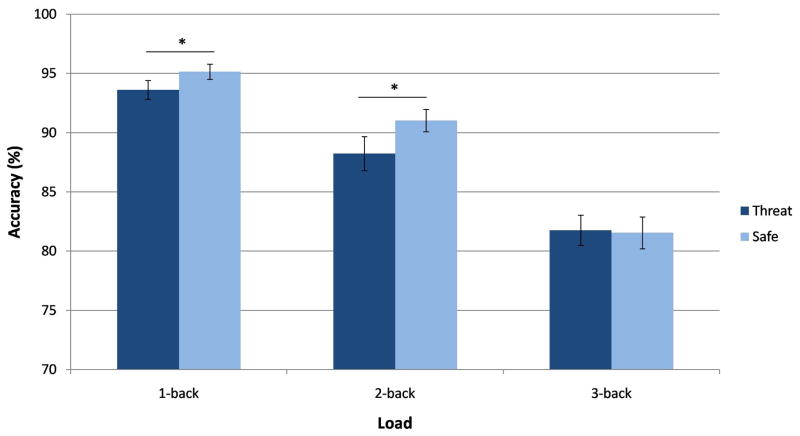
Similarly to RT, a 3-way Condition by Load by Group interaction was not found for accuracy (F(2,96) = .44, p > .1, ηp2 = .009) (Figure S2 in supplementary material). However, a trend emerged for the Condition by Load interaction (F(2,96) = 2.84, p = .064, ηp2 = .056). Based on the significant Condition by Load interaction on RT, and because of our a priori hypothesis of the differential influence of threat on cognitive load in adults, we further examined the Condition by Load interaction on accuracy. When each load was examined separately, we found that accuracy was lower for the threat than safe conditions in the 1-back and 2-back tasks (ps < .05), but not for the 3-back task (p > .1), in which accuracy was similar for the threat and safe conditions (Figure 3). In other words, high cognitive load prevented threat from disrupting working memory accuracy.
Figure 3.
Mean accuracy by Condition and Load. The Condition by Load trend showed lower accuracy during the threat than safe condition on the 1-back and 2-back tasks, but not the 3-back task across age. N = 50, *p < .05, error bars represent ± SEM.
In addition, main effects emerged for Group (F(1,48) = 6.84, p < .05, ηp2 = .13), Condition (F(1,48) = 7.98, p < .01, ηp2 = .14), and Load (F(2,96) = 96.93, p < .001, ε = .89, ηp2 = .67). With regard to Group, accuracy was higher in adults than adolescents. The main effect of Condition indicated greater accuracy during safe than threat conditions. Finally, the main effect of Load indicated that accuracy decreased as load increased. Post-hoc tests confirmed that all three levels of load differed significantly from one another (ps < .001).
Startle Response
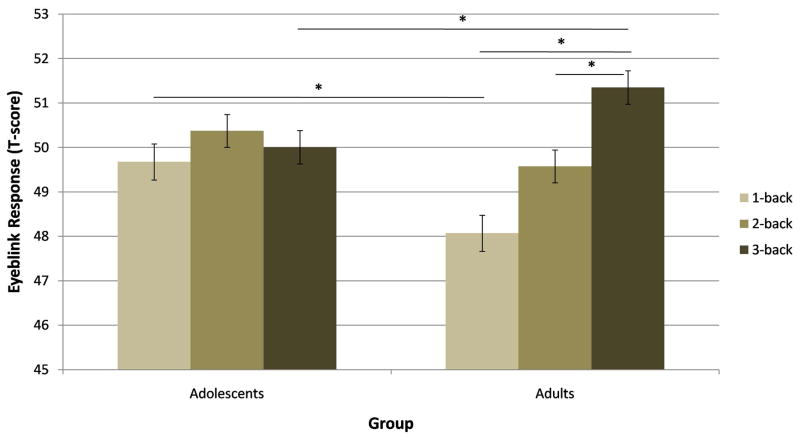
We analyzed eye-blink startle responses using a rANOVA with Condition and Load as the within-subjects factors, and Group as the between-subjects factor. The Condition by Load by Group rANOVA analysis (F(3,144) = 1.41, p > .1, ηp2 = .03) (Figure S3 in supplementary material) revealed two significant interactions of Condition by Load and Load by Group. As expected, the Condition by Load interaction (F(3,144) = 25.01, p < .001, ηp2 = .34) indicated significantly greater startle magnitude during the threat than safe condition for the 1-back and 2-back tasks (ps < .001), but not for the 3-back task (p > .1). Thus, threat did not modulate startle during the 3-back task (see Table 2). The Load by Group interaction (F(3,144) = 3.75, p < .05, ηp2 = .072) indicated that adults showed an increased startle response as load increased from 1-back to 3-back, while adolescents showed no modulation by load (Figure 4a).
Table 2.
Eye-blink Startle Response (T-score) Means in the Threat and Safe Condition by Load and Group
| Condition | Load | Adolescents (n = 25) | Adults (n = 25) | |
|---|---|---|---|---|
| Mean (SEM) | Mean (SEM) | |||
| Threat | 1-back | 52.37 | (0.69) | 50.87 (0.69) |
| 2-back | 52.55 | (0.65) | 52.30 (0.65) | |
| 3-back | 50.02 | (0.54) | 51.41 (0.54) | |
| Safe | 1-back | 46.98 | (0.47) | 45.27 (0.47) |
| 2-back | 48.20 | (0.44) | 46.85 (0.44) | |
| 3-back | 49.99 | (0.61) | 51.29 (0.61) | |
Figure 4.
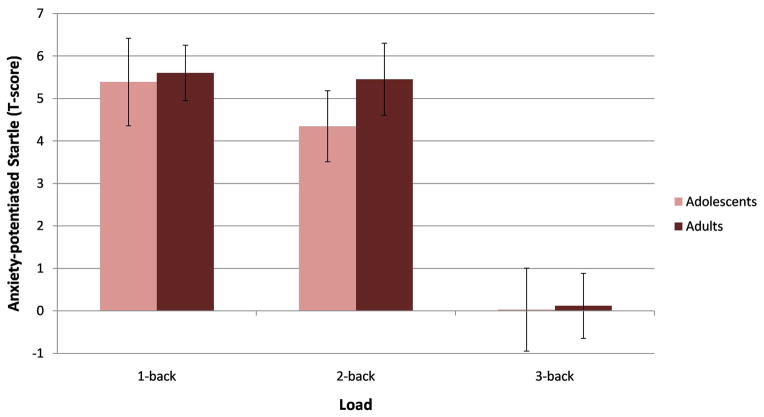
Mean startle response by Load and Group. (a) Adults had greater eye-blink responses during the 3-back task than the 1-back or 2-back tasks. Adolescents showed no differences in eye-blink responses as a function of load. In addition, the startle response was lower in adults than in adolescents in the 1-back task, while the opposite was found for the 3-back task. No difference was found between adolescents and adults for the 2-back task. N = 50, *p < .05, error bars represent ± SEM. (b) Both adults and adolescents exhibited a blockade of the effects of anxiety on startle (anxiety-potentiated startle = [threat – safe] startle) during the 3-back task. N = 50, error bars represent ± SEM.
We then examined anxiety-potentiated startle, the difference in startle during the threat and safe conditions ([threat – safe] startle). This difference score was analyzed using a rANOVA, with Load as the within-subjects factor and Group as the between-subjects factor. Results revealed a main effect of Load (F(3,144) = 24.96, p < .001, ηp2 = .34) that showed greater anxiety-potentiated startle during the 1-back and 2-back tasks compared to the 3-back task (ps < .01) (Figure 4b).
Working Memory Performance and Startle
Correlations were conducted between the difference scores [threat – safe] in performance (RT, accuracy) and anxiety-potentiated startle. No significant correlations emerged.
Discussion
The present study used a modified methodology adapted for pediatric research to test three hypotheses regarding the effects of threat on working memory performance in adolescents and adults. First, in adults, we expected cognitive load to interact with threat, consistent with our prior findings (Vytal et al., 2012). Indeed, interactions replicated those previously found (Vytal et al., 2012). Second, we predicted that adolescents would exhibit lower accuracy and longer RTs than adults on the working memory task. Accordingly, findings showed lower working memory accuracy in adolescents than adults, but no differences in RT. Finally, regarding the primary research question, we expected the effects of induced anxiety on working memory to differ between adolescents and adults. However, no such interactions of age with condition and cognitive load emerged.
Cognitive Load on Anxiety and Performance Across Groups
As expected from our prior work (Vytal et al., 2012), only high cognitive load blocked the detrimental influence of anxiety on working memory accuracy. Accuracy was lower during threat than safe conditions in the 1-back and 2-back tasks, but did not differ between conditions in the 3-back task. The blockade was accompanied by an abolition of the anxiety-potentiated startle at high cognitive load. These findings support the conclusion that high cognitive load blocked the deleterious effect of anxiety on accuracy. In addition, RT was improved (shortened) during threat vs. safe in the 3-back task, and did not differ between conditions in the 1-back or 2-back task.
The effects of induced anxiety on accuracy and RT have theoretical implications, particularly with regards to the attentional control theory (Eysenck et al., 2007). This theory proposes that anxiety disrupts mainly RT as a way to maintain accuracy on a cognitive task, akin to a trade-off effect. RT (efficiency) indexes the level of effort deployed to achieve a given level of accuracy (efficacy). Our findings are inconsistent with this schema. We found that the high-load task normalized accuracy in the threat condition to the same level as in the safe condition, but this was not at the expense of a slower RT. Rather, RT was speeded up, suggesting better endogenous attention. This pattern raises two possibilities that are not necessarily mutually exclusive. First, state-anxiety may facilitate top-down control in highly demanding tasks. Second, during a difficult task, cognitive resources may be primarily allocated to task performance, minimizing threat processing. This mechanism may manifest as a diversion away from threat, with a subsequent reduction in state-anxiety.
Finally, the main theoretical interpretation of our prior and current findings rests on the idea of limited resources (Bishop, 2009; Eysenck & Calvo, 1992; Lavie et al., 2004; Pessoa, 2009; Vytal et al., 2012). Accordingly, low-load cognitive tasks leave resources available for threat to be processed and to interfere with cognitive performance, whereas high-load cognitive tasks deplete cognitive resources such that threat cannot be processed and thus does not interfere with performance. This interpretation fits both our working memory accuracy findings and the abolition of the anxiety-potentiated startle response during the 3-back task.
Age Effects
Adolescents and adults did not differ in terms of how cognitive load impacted the influence of anxiety on working memory performance. Adolescents demonstrated a similar regulatory capacity as adults, suggesting that the mechanisms that modulate the interaction of cognitive load with anxiety may already be mature in adolescence. Conceivably, the neural systems models, which describe in adolescents a unique balance between a relatively immature and less efficient executive control system and a relatively hyper-responsive emotion-related subcortical system (Casey et al., 2008; Ernst & Fudge, 2009), may not substantially influence the processes examined here. However, developmental differences may occur in situations of higher cognitive load and/or greater anxiety. Although overall working memory accuracy was significantly higher in adults than adolescents, the interactions with Group were not significant, indicating a lack of age effects. This result may suggest that the threat and/or cognitive load may not have been high enough to reveal interactions by age. Regarding threat processing, it is conceivable that developmental differences in response to imminent threat (King et al., 2013) might be lagging behind the maturation of the neural substrates of prolonged periods of threat (Grillon et al., 2004). Alternatively, adolescents may use different strategies, engaging distinct regulatory mechanisms to produce an adult-like behavioral output (i.e., the modulation of performance as a function of anxiety and cognitive load). This question could be addressed in future neuroimaging work by identifying which neural mechanisms are affected by threat in adolescents vs. adults.
Finally, an unexpected effect of age emerged on eye-blink startle reactivity. Specifically, adolescents did not modulate their startle reactivity by working memory load across conditions. In contrast, adults exhibited a linear increase of startle reactivity with increasing working memory load, consistent with increasing arousal with task difficulty. Eye-blink startle reactivity was higher in the low- and medium-load tasks and lower in the high-load task in adolescents compared to adults. This suggests that perhaps adolescents have an overall higher arousal response to low- and medium-load tasks, energizing their performance on such tasks and pushing them to perform similarly to adults. This explanation is highly speculative, and before further interpretation, the finding needs to be replicated and better delineated. Additionally, neuroimaging studies might bring some insight to the mechanism underlying this age difference on startle reactivity.
Strengths and Limitations
This study presents both strengths and limitations. Regarding strengths, we used a well-established method of anxiety induction and measurement (Grillon & Baas, 2003). This method includes the manipulation of anxiety using a within-subject design, where subjects serve as their own control. Hence, results are less likely to be influenced by idiosyncratic variability or errors associated with individual differences. Furthermore, the method was successfully modified to be used with pediatric populations. The threat stimulus was an aversive loud scream instead of an electric shock, and the startle probe was an air-puff to the forehead instead of white noise. Finally, we had clear hypotheses based on a prior similar study and replicated findings from that study (Vytal et al., 2012). A few limitations should be mentioned. First, the age range of the adolescent group was relatively large (10 to 17 years), and unfortunately, the sample size was too small to reliably examine age effects during this period. Although collapsing across pediatric ages, as done here, is often used in the literature (e.g., Hardin, et al., 2009; Jazbec, McClure, Hardin, Pine, & Ernst, 2005; Jazbec, et al., 2006; Mueller, et al., 2015), it prevents us from refining the trajectories of developmental changes. Future research should examine large pediatric samples to capture these changes, which are reported in some executive functions during adolescence (Huizinga et al., 2006; Luna et al., 2004). Second, participants self-reported moderate anxiety ratings during threat. Future studies may manipulate the intensity of the threat stimulus to investigate changes in cognitive processing and startle response as a function of anxiety level. Third, this study can only inform our understanding of processes in healthy individuals and does not directly address pathological anxiety. However, the study’s findings beg the question of how these effects of state anxiety on cognitive performance manifest in adolescents and adults with anxiety disorders. Conceivably, the adaptive mechanisms found in our healthy sample might break down in pathological anxiety, with threat impairing performance at all cognitive loads, including high-load. Future studies should include clinically anxious patients to probe how processing resources shift during cognitively demanding tasks, and whether this shift accompanies reduced physiological anxiety. This would aid in the development of treatments for clinically anxious individuals that may reduce the cognitive impairments associated with daily functioning, such as working memory. Additionally and more specific to adolescents, related research and treatments may help in reducing the association of anxiety with poor school achievement (Owens, Stevenson, Hadwin, & Norgate, 2012).
In sum, the present results suggest that adolescents may prioritize resources for cognitive over emotional processes, similarly to adults. Like adults, adolescents did not show a prioritization of resources toward stimulus-driven (threat) effects during high cognitive load, as was found during low and medium cognitive loads. They maintained goal-oriented behaviors during high cognitive load, moving their focus away from the impending threat, which in turn minimized anxiety (anxiety-potentiated startle). This was in contrast to low and medium cognitive loads, which were accompanied by greater anxiety. Future work could examine the neural mechanisms underlying the robust behavioral effects identified here and in our previous work, and may provide mechanisms useful for designing cognitive strategies to decrease anxiety.
Supplementary Material
Acknowledgments
This study was funded by the Intramural Research Program of the National Institute of Mental Health (Grant MH002798, Proposal 1ziamh002781).
Footnotes
Sex was also examined based on animal and human research that suggests that stress may impact working memory differently in females and males (Schoofs, Pabst, Brand, & Wolf, 2013; Shansky, Rubinow, Brennan, & Arnsten, 2006). Therefore, sex was included as a between-subjects factor in the data analyses. However, it did not influence the results and was thus removed as a factor from the presented analyses.
The authors declare no conflict of interest.
References
- Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: Evidence of episodic memory dysfunction. Journal of Psychiatric Research. 2005;39:207–214. doi: 10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatric Clinics of North America. 2009;32:483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: The role of threat appraisal and fear learning. Depression and Anxiety. 2011;28:5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Johnstone T. Prefrontal inhibition of threat processing reduces working memory interference. Frontiers in Human Neuroscience. 2013;7:228. doi: 10.3389/fnhum.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience & Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cognition & Emotion. 1992;6:409–434. doi: 10.1080/02699939208409696. [DOI] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Whitaker KJ, Steele JS, Green CT, Wendelken C, Bunge SA. White matter maturation supports the development of reasoning ability through its influence on processing speed. Developmental Science. 2013;16:941–951. doi: 10.1111/desc.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/S1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Hardin MG, Mandell D, Mueller SC, Dahl RE, Pine DS, Ernst M. Inhibitory control in anxious and healthy adolescents is modulated by incentive and incidental affective stimuli. Journal of Child Psychology Psychiatry. 2009;50:1550–1558. doi: 10.1111/j.1469-7610.2009.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. doi: 10.1093/biomet/75.4.800. [DOI] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: An antisaccade task. Biological Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, … Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- King EC, Pattwell SS, Sun A, Glatt CE, Lee FS. Nonlinear developmental trajectory of fear learning and memory. Annals of the New York Academy of Sciences. 2013;1304:62–69. doi: 10.1111/nyas.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, … Pine DS. Fear conditioning in adolescents with anxiety disorders: Results from a novel experimental paradigm. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Baas JMP, Pine DS, Orme K, Dvir S, Nugent M, … Grillon C. Airpuff startle probes: An efficacious and less aversive alternative to white-noise. Biological Psychology. 2005;68:283–297. doi: 10.1016/j.biopsycho.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massar SAA, Mol NM, Kenemans JL, Baas JMP. Attentional bias in high- and low-anxious individuals: Evidence for threat-induced effects on engagement and disengagement. Cognition & Emotion. 2011;25:805–817. doi: 10.1080/02699931.2010.515065. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognitive Therapy and Research. 2005;29:29–45. doi: 10.1007/s10608-005-1646-y. [DOI] [Google Scholar]
- Mueller SC, Hardin MG, Mogg K, Benson V, Bradley BP, Reinholdt-Dunne ML, … Ernst M. The influence of emotional stimuli on attention orienting and inhibitory control in pediatric anxiety. Journal of Child Psychology and Psychiatry. 2012;53:856–863. doi: 10.1111/j.1469-7610.2012.02541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Shechner T, Rosen D, Nelson EE, Pine DS, Ernst M. Incidental threat during visuospatial working memory in adolescent anxiety: An emotional memory-guided saccade task. Depression and Anxiety. 2015;32:289–295. doi: 10.1002/da.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M, Stevenson J, Hadwin JA, Norgate R. Anxiety and depression in academic performance: An exploration of the mediating factors of worry and working memory. School Psychology International. 2012;33:433–449. doi: 10.1177/0143034311427433. [DOI] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs D, Pabst S, Brand M, Wolf OT. Working memory is differentially affected by stress in men and women. Behavioural Brain Research. 2013;241:144–153. doi: 10.1016/j.bbr.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AFT. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behavioral and Brain Functions. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Hong M, Britton JC, Pine DS, Fox NA. Fear conditioning and extinction across development: Evidence from human studies and animal models. Biological Psychology. 2014;100:1–12. doi: 10.1016/j.biopsycho.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Edwards CD, Montuori J, Lushene R. State-Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologists Press; 1973. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Vytal K, Cornwell B, Arkin N, Grillon C. Describing the interplay between anxiety and cognition: From impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology. 2012;49:842–852. doi: 10.1111/j.1469-8986.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal KE, Cornwell BR, Letkiewicz AM, Arkin NE, Grillon C. The complex interaction between anxiety and cognition: Insight from spatial and verbal working memory. Frontiers in Human Neuroscience. 2013;7:93. doi: 10.3389/fnhum.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current Opinion in Neurobiology. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.