Abstract
Because carbonyl groups can participate in both hydrogen bonds and n→π* interactions, these two interactions likely affect one another. Herein, enhancement of an amidic n→π* interaction is shown to reduce the ability of β-keto amides to tautomerize to the enol, indicating decreased hydrogen-bonding capacity of the amide carbonyl group. Thus, an n→π* interaction can have a significant effect on the strength of a hydrogen bond to the same carbonyl group.
Graphical Abstract
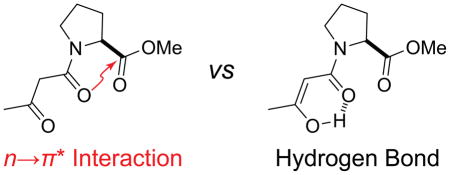
Intermolecular interactions are responsible for a wide variety of chemical and biological phenomena, motivating characterization of these interactions and their interplay.1 Hydrogen bonding has commanded special attention due to its ability to organize complex molecular systems like proteins.2 The strength of individual hydrogen bonds is crucial for establishing the structure and ensuring the stability of proteins, and has been the focus of extensive research and debate.3 The varied literature on the subject suggests that even small differences in the energies of individual hydrogen bonds could cause dramatic changes in protein stability when propagated throughout an entire protein.
In proteins, hydrogen bonds often form simultaneously with carbonyl–carbonyl n→π* interactions.4 In an n→π* interaction, lone pair (n) electron density from a carbonyl oxygen is donated into the π* antibonding orbital of an adjacent carbonyl group,5 thereby releasing upwards of 0.27 kcal/mol of stabilizing energy.6 These interactions are particularly prevalent in α-helices,7 where over 70% of adjacent residues are poised to engage in n→π* interactions4a along with the canonical hydrogen bond within the main chain.8 In the α-helix, a carbonyl oxygen donates electron density to two different acceptors. Accordingly, changes in the strength of one of these interactions should affect the strength of the other.
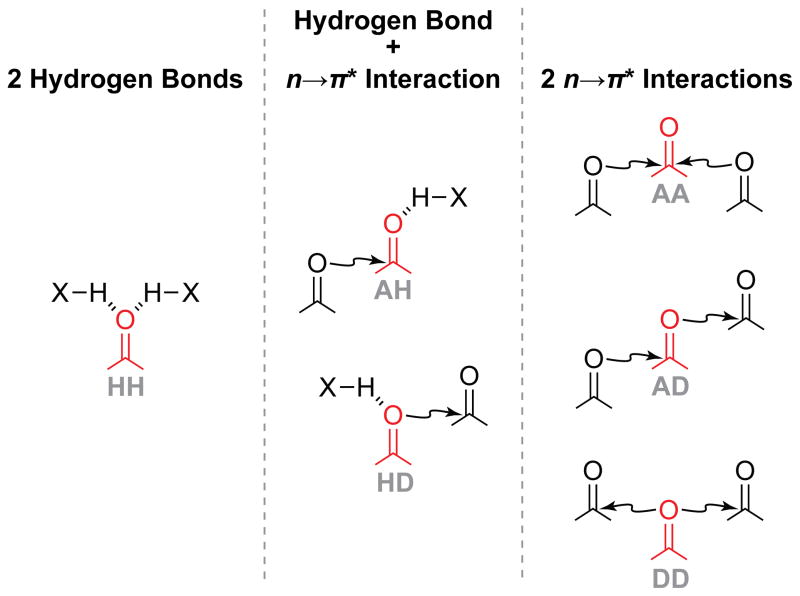
Context is known to affect hydrogen-bonding energies.9 Less characterized is the influence of other noncovalent interactions on hydrogen bonds. Given the prevalence of hydrogen bonds and n→π* interactions in proteins, their interplay is especially important and can occur in several distinct modes (Figure 1). Donation of a hydrogen bond to the oxygen of a carbonyl group is known to enhance the ability of that carbonyl group to accept an n→π* interaction (AH in Figure 1).10 The influence of an n→π* interaction on the ability of the carbonyl electron-pair donor to accept a hydrogen bond is, however, unknown (HD in Figure 1).
Figure 1.
Interplay of hydrogen bonds and n→π* interactions with a single carbonyl group. The carbonyl group can accept a hydrogen bond (H), accept an n→π* interaction (A), or donate an n→π* interaction (D). HH,11 AH,10 and AA12 have been explored previously. HD is explored herein.
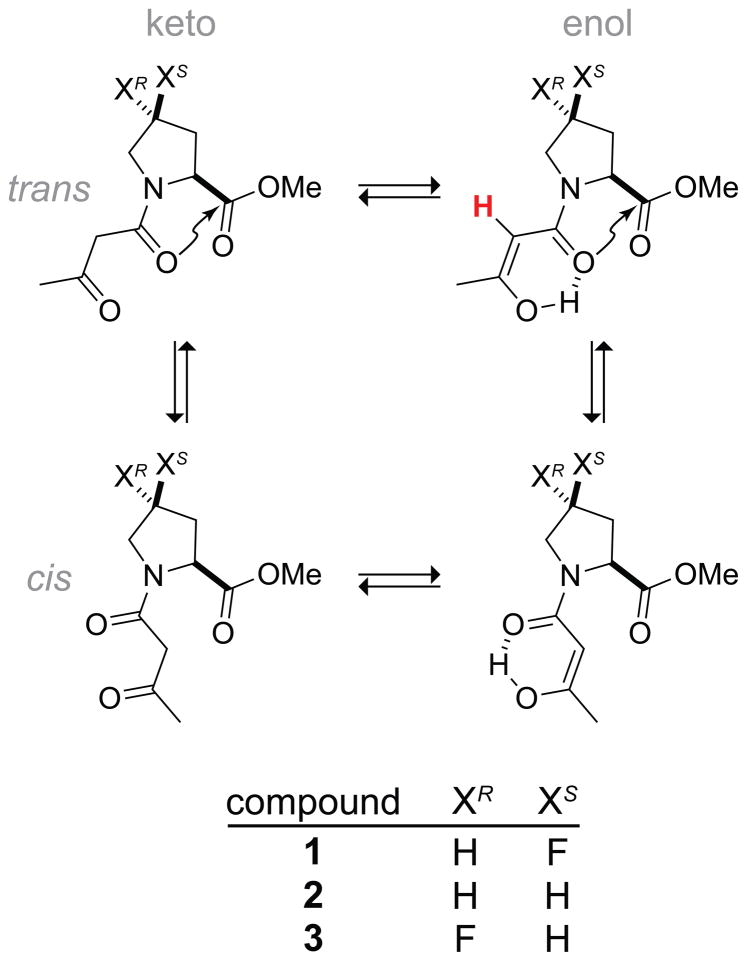
Because the electron density of the carbonyl oxygen is limited, we hypothesized that increases in the strength of the n→π* interaction would attenuate hydrogen bonds involving that same oxygen atom. We set out to test this hypothesis by constructing a model system in which both interactions could form. Inspired by recent work on N-acyl homoserine lactones,13 we designed and synthesized β-keto amides 1–3 for our analyses (Figure 2).
Figure 2.
Keto/enol tautomerization and trans/cis isomerization of β-keto amides of substituted prolines. An n→π* interaction can occur in the trans isomer. Integration of the 1H NMR signal of the alkenyl proton (red) in the trans enol species reports on the effect of the n→π* interaction on the hydrogen bond.
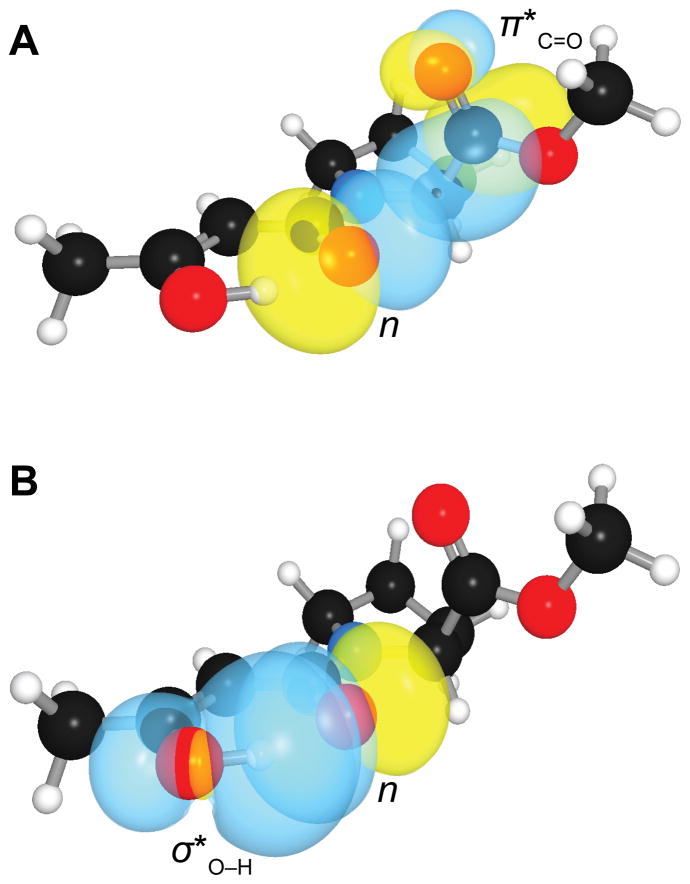
We next performed a natural bond orbital (NBO) analysis14 on β-keto amides 1–3. We optimized relevant structures in vacuo with density functional theory calculations at the B3LYP/6-311+G(2d,p) level of theory.15 We observed that the amide group can donate an n→π* interaction to the terminal ester (Figure 3A). In addition, upon enolization of the β-keto amide, the amide carbonyl group can accept a hydrogen bond from the enol (Figure 3B).16 Because the enol tautomer is stabilized by the internal hydrogen bond, the ability of the amide carbonyl group to act as a hydrogen-bond acceptor should affect the extent to which the β-keto amide undergoes tautomerization. As the keto and enol tautomers interconvert slowly,16 it is possible to distinguish them by using NMR spectroscopy, affording a convenient readout of the hydrogen-bonding capacity of the central carbonyl group.
Figure 3.
Three-dimensional orbital rendering of the trans endo conformation of the enol tautomer of β-keto amide 2 in its optimized geometry. (A) Overlap of the carbonyl n and carbonyl π* orbitals to form an n→π* interaction. (B) Overlap of the carbonyl n and hydroxyl σ* orbitals to form a hydrogen bond. Images were created with the program NBOView 1.1.
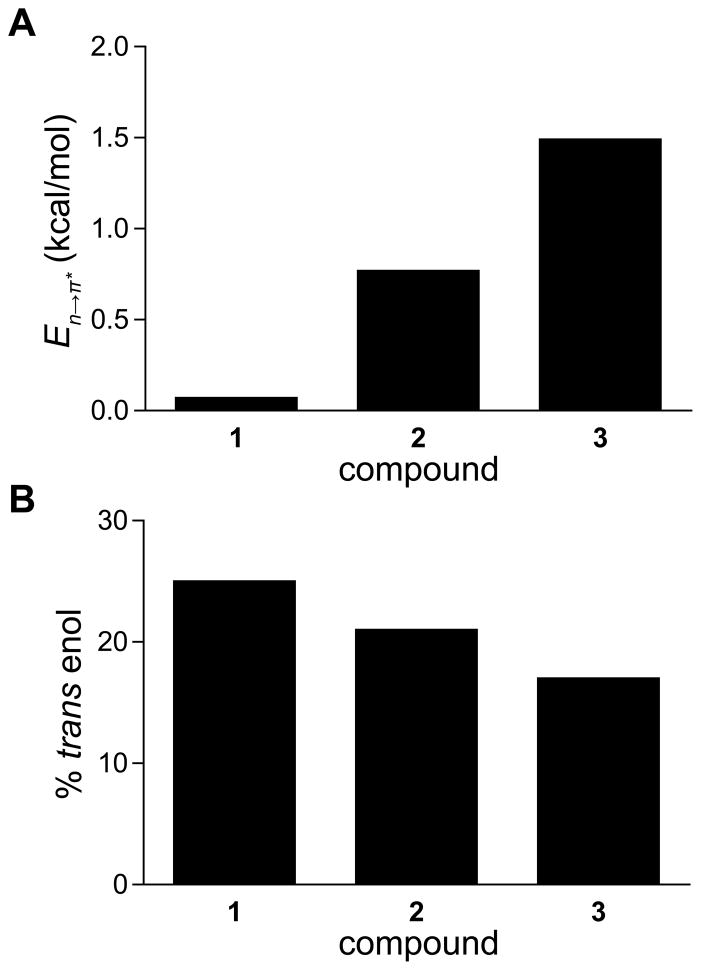
Having established enolization as a potential readout of hydrogen-bond capacity, we perturbed the n→π* interaction by employing a strategy evoked previously.17 Specifically, we incorporated fluoro groups on the pyrrolidine ring, thereby inducing a gauche effect to modulate the ring pucker of the heterocycle.18 An electronegative substituent installed at the 4-position with S stereochemistry (1) enforces an endo pucker of the pyrrolidine ring, which alters the backbone ϕ dihedral angle (C′i–1–Ni–Cαi–C′i) and thereby increases the distance between the carbonyl groups on either side of the ring.17b The ψ dihedral angle (Ni–Cαi–C′i–Ni+1) changes as well, due to transannular repulsion between the 4S substituent and the carbonyl group of the ester moiety.17b,19 The result is an attenuation of the n→π* interaction. Conversely, electronegative substituents at the 4-position with R stereochemistry (3) enforce an exo ring pucker, which decreases the distance between adjacent carbonyl groups without steric repulsion, enhancing the n→π* interaction.17b NBO analysis15 confirmed that there is an increase in the strength of the n→π* interaction from 1 to 2 and from 2 to 3 (Figure 4A).
Figure 4.
(A) Energies of n→π* interactions in β-keto amides 1–3, as calculated in vacuo from second-order perturbation theory implemented by NBO 5.9.20 (B) Percentage of β-keto amides 1–3 populating the trans enol form in CDCl3 at 25 °C, as determined by 1H NMR spectroscopy. Repeated integration yields values within 1% of those depicted.
The 1H NMR spectra of these compounds are congested, not only due to the complex multiplicity patterns of pyrrolidine rings, but also due to the presence of four distinct species (Figure 2). In addition to the keto and enol forms of the β-keto amide, the tertiary amide populates both the cis and trans conformations, increasing spectral complexity. Importantly, however, the alkenyl protons of the of β-keto amide are well-resolved and easily assigned by their chemical shift (δ ~5.0 ppm), multiplicity (singlet), and carbon correlations in 1H–13C HMBC spectra, providing a quantitation of the population of the enol species. Here, we focused specifically on integration of the 1H NMR signal from the trans enol, which reports on the form of the molecule in which both the n→π* interaction and the hydrogen bond are present (Figure 2). Moreover, 1H NMR signals for the α-proton, as well as the γ-proton in compounds 1 and 3, are well resolved from other proton signals. For these two particular protons, the 1H NMR signals for all four species coincide, allowing for quantitation of the total amount of β-keto amide in a sample.
We observed a decrease in the population of the trans enol species upon enhancement of the n→π* interaction (Figure 4). In principle, the decrease in trans enol population could result from either decreased population of the trans conformation relative to the cis or decreased population of the enol relative to the keto tautomer. Enhancement of the n→π* interaction is known to cause an increase in the trans conformation,17 so a decrease in the population of the trans enol form must arise from a decrease in the enol tautomer upon enhancement of the n→π* interaction. The confounding increase in the trans conformation upon enhancement of the n→π* interaction indicates that the decrease in trans enol population underestimates the effect of the n→π* interaction on keto–enol tautomerization, and therefore also underestimates its effect on the strength of the competing hydrogen bond. As it is, an increase in n→π* energy of 0.7 kcal/mol results in a ~20% decrease in the population of the hydrogen-bonded tautomer, suggesting a substantial interplay between n→π* interactions and hydrogen bonding. These results demonstrate that n→π* interactions do compete with hydrogen bonds for carbonyl electron density.
The observed competition between hydrogen bonds and n→π* interactions demonstrates that the energy of an individual n→π* interaction is significant and can affect other intermolecular interactions. Indeed, these data suggest that changes in the strength of competing hydrogen bonds can provide an indirect readout of the strength of an n→π* interaction, thereby enabling an additional strategy for characterizing these interactions, supplementing extant analyses by computation or consideration of high-resolution crystal structures.17c,21 These data are also prognostic of a broad impact of n→π* interactions, which, through their competition with hydrogen bonds, likely affect the folding and stability of many protein segments. For example, our findings are consistent with recent work demonstrating that stronger hydrogen-bonding energies are correlated with weaker and less frequent formation of n→π* interactions in proteins,22 and indicate that the canonical hydrogen bonds in an α-helix8 are undermined by concurrent n→π* interactions. We also conclude that computational hydrogen-bonding potentials could be improved by considering the possible presence of a competing n→π* interaction. Moreover, molecular mechanics methods could benefit from explicit accounting for n→π* interactions.
Supplementary Material
Acknowledgments
This work was supported by grant R01 AR044276 (NIH). R.W.N. was supported by Biotechnology Training Grant T32 GM008349 (NIH) and by an ACS Division of Organic Chemistry Graduate Fellowship. The National Magnetic Resonance Facility at Madison is supported by grant P41 GM103399 (NIH). High-performance computing is supported by grant CHE-0840494 (NSF).
Footnotes
ASSOCIATED CONTENT
Synthetic and computational procedures, characterization data. The Supporting Information is available free of charge on the ACS Publications website.
References
- 1.(a) Riley KE, Pitoňák M, Jurečka P, Hobza P. Chem Rev. 2010;110:5023–5063. doi: 10.1021/cr1000173. [DOI] [PubMed] [Google Scholar]; (b) Scheiner S, editor. Noncovalent Forces. Springer; Switzerland: 2015. [Google Scholar]
- 2.(a) Mirsky A, Pauling L. Proc Natl Acad Sci USA. 1936;22:439–447. doi: 10.1073/pnas.22.7.439. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kabsch W, Sander C. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]; (c) Dill KA. Biochemistry. 1990;29:7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]; (b) Karshikoff A. Non-covalent Interactions in Proteins. Imperial College Press; London: 2006. [Google Scholar]
- 3.(a) McDonald IK, Thornton JM. J Mol Biol. 1994;238:777–793. doi: 10.1006/jmbi.1994.1334. [DOI] [PubMed] [Google Scholar]; (b) Baldwin RL. J Biol Chem. 2003;278:17581–17588. doi: 10.1074/jbc.X200009200. [DOI] [PubMed] [Google Scholar]; (c) Bolen DW, Rose GD. Annu Rev Biochem. 2008:339–362. doi: 10.1146/annurev.biochem.77.061306.131357. [DOI] [PubMed] [Google Scholar]
- 4.(a) Bartlett GJ, Choudhary A, Raines RT, Woolfson DN. Nat Chem Biol. 2010;6:615–620. doi: 10.1038/nchembio.406. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bartlett GJ, Woolfson DN. Protein Sci. 2016;25:887–897. doi: 10.1002/pro.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Hinderaker MP, Raines RT. Protein Sci. 2003;12:1188–1194. doi: 10.1110/ps.0241903. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Singh SK, Das A. Phys Chem Chem Phys. 2015;17:9596–9612. doi: 10.1039/c4cp05536e. [DOI] [PubMed] [Google Scholar]
- 6.Newberry RW, VanVeller B, Guzei IA, Raines RT. J Am Chem Soc. 2013;135:7843–7846. doi: 10.1021/ja4033583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhary A, Raines RT. Protein Sci. 2011;20:1077–1081. doi: 10.1002/pro.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauling L, Corey RB, Branson HR. Proc Natl Acad Sci USA. 1951;37:205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Fersht AR, Shi UP, Knill-Jones J, Lowe DM, Wilkinson AJ, Blow DM, Brick P, Carter P, Waye MMY, Winter G. Nature. 1985;314:235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]; (b) Gilli P, Pretto L, Bertolasi V, Gilli G. Acc Chem Res. 2009;42:33–44. doi: 10.1021/ar800001k. [DOI] [PubMed] [Google Scholar]
- 10.(a) Kuemin M, Nagel YA, Schweizer S, Monnard FW, Ochsenfeld C, Wennemers H. Angew Chem, Int Ed. 2010;49:6324–6327. doi: 10.1002/anie.201001851. [DOI] [PubMed] [Google Scholar]; (b) Shoulders MD, Kotch FW, Choudhary A, Guzei IA, Raines RT. J Am Chem Soc. 2010;132:10857–10865. doi: 10.1021/ja103082y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Erdmann RS, Wennemers H. J Am Chem Soc. 2012;134:17117–17124. doi: 10.1021/ja3066418. [DOI] [PubMed] [Google Scholar]; (d) Siebler C, Erdmann RS, Wennemers H. Angew Chem, Int Ed. 2014;53:10340–10344. doi: 10.1002/anie.201404935. [DOI] [PubMed] [Google Scholar]; (e) Siebler C, Trapp N, Wennemers H. J Pept Sci. 2015;21:208–211. doi: 10.1002/psc.2734. [DOI] [PubMed] [Google Scholar]
- 11.(a) Takemoto Y. Org Biomol Chem. 2005;3:4299–4306. doi: 10.1039/b511216h. [DOI] [PubMed] [Google Scholar]; (b) Feldblum ES, Arkin IT. Proc Natl Acad Sci USA. 2014;111:4085–4090. doi: 10.1073/pnas.1319827111. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kuster DJ, Liu C, Fang Z, Ponder JW, Marshall GR. PLoS ONE. 2015;10:e0123146. doi: 10.1371/journal.pone.0123146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Pollock SB, Kent SBH. Chem Commun. 2011;47:2342–2344. doi: 10.1039/c0cc04120c. [DOI] [PubMed] [Google Scholar]; (b) Choudhary A, Fry CG, Kamer KJ, Raines RT. Chem Commun. 2013;49:8166–8168. doi: 10.1039/c3cc44573a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Newberry RW, Raines RT. ACS Chem Biol. 2014;9:880–883. doi: 10.1021/cb500022u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Newberry RW, Raines RT. Acta Crystallogr. 2016;E72:136–139. doi: 10.1107/S2056989015024913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Weinhold F. In: Encyclopedia of Computational Chemistry. Schleyer PvR, Allinger NL, Clark T, Gasteiger J, Kollman PA, Shaefer HF, III, Schreiner PR., editors. Vol. 3. John Wiley & Sons; Chichester, UK: 1998. pp. 1792–1811. [Google Scholar]; (b) Weinhold F, Landis CR. Valency and Bonding: A Natural Bond Orbital Donor–Acceptor Perspective. Cambridge University Press; Cambridge, UK: 2005. [Google Scholar]
- 15.Reed AE, Curtiss LA, Weinhold F. Chem Rev. 1988;88:899–926. [Google Scholar]
- 16.(a) Wengenroth H, Meier H. Chem Ber. 1990:123. [Google Scholar]; (b) Hynes MJ, Clarke EMJ. Chem Soc Perkin Trans. 1994;2:901–904. [Google Scholar]; (c) Laurella SL, Latorre C, Dietrich R, Furlong JJP, Allegretti PE. J Phys Org Chem. 2012;25:1365–1373. [Google Scholar]
- 17.(a) Bretscher LE, Jenkins CL, Taylor KM, DeRider ML, Raines RT. J Am Chem Soc. 2001;123:777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]; (b) DeRider ML, Wilkens SJ, Waddell MJ, Bretscher LE, Weinhold F, Raines RT, Markley JL. J Am Chem Soc. 2002;124:2497–2505. doi: 10.1021/ja0166904. [DOI] [PubMed] [Google Scholar]; (c) Choudhary A, Gandla D, Krow GR, Raines RT. J Am Chem Soc. 2009;131:7244–7246. doi: 10.1021/ja901188y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newberry RW, Raines RT. Top Heterocy Chem. 2016. [DOI] [Google Scholar]
- 19.(a) Erdmann RS, Wennemers H. Angew Chem, Int Ed. 2011;50:6835–6838. doi: 10.1002/anie.201008118. [DOI] [PubMed] [Google Scholar]; (b) Siebler C, Maryasin B, Kuemin M, Erdmann RS, Rigling C, Grünenfelder C, Ochsenfeld C, Wennemers H. Chem Sci. 2015;6:6725–6730. doi: 10.1039/c5sc02211h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, JrPeralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian, Inc; Wallingford, CT: 2009. [Google Scholar]; (b) Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F. Theoretical Chemistry Institute, University of Wisconsin–Madison; Madison, WI: 2012. [Google Scholar]
- 21.(a) Newberry RW, Bartlett GJ, Vanveller B, Woolfson DN, Raines RT. Protein Sci. 2014;23:284–288. doi: 10.1002/pro.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Choudhary A, Newberry RW, Raines RT. Org Lett. 2014;16:3421–3423. doi: 10.1021/ol5012967. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wilhelm P, Lewandowski B, Trapp N, Wennemers H. J Am Chem Soc. 2014;136:15829–15832. doi: 10.1021/ja507405j. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett GJ, Newberry RW, VanVeller B, Raines RT, Woolfson DN. J Am Chem Soc. 2013;135:18682–18688. doi: 10.1021/ja4106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.