Abstract
Purpose
To develop a complete and consistent prescription drug monitoring program (PDMP) data set for use by drug safety researchers in evaluating patterns of high-risk use and potential abuse of scheduled drugs.
Methods
Using publically available data references from the US Food and Drug Administration and the Centers for Disease Control and Prevention, we developed a strategic methodology to assign drug categories based on pharmaceutical class for the majority of prescriptions in the PDMP data set. We augmented data elements required to calculate morphine milligram equivalents and assigned duration of action (short-acting or long acting) properties for a majority of opioids in the data set.
Results
About 10% of prescriptions in the PDMP data set did not have a vendor-assigned drug category, and 20% of opioid prescriptions were missing data needed to calculate risk metrics. Using inclusive methods, 19,133,167 (>99.9%) of prescriptions in the PDMP data set were assigned a drug category. For the opioid category, augmenting data elements resulted in 10,760,669 (99.8%) having required values to calculate MME and evaluate duration of action properties.
Conclusions
Drug safety researchers who require a complete and consistent PDMP data set can use the methods described here to ensure that prescriptions of interest are assigned consistent drug categories and complete opioid risk variable values.
Keywords: prescription drug monitoring program, data sources, metrics, prescription opioid, prescription drug abuse, diversion measurement
Forty-nine states in the United States (U.S.) and some Canadian provinces operate prescription drug monitoring programs (PDMPs). These programs collect and manage data for controlled medications dispensed by outpatient pharmacies. Such data are being used by public health agencies and health systems to evaluate prescription drug consumption.1 Epidemiologists and clinical researchers are using PDMP data, alone and in combination with other data sets, to identify high-risk patterns of prescription drug use, trends in prescribing patterns, and health outcomes related to the opioid use epidemic occurring in North America.2,3
These PDMP systems are prepared and maintained by commercial vendors, who classify prescriptions, typically based on therapeutic category. An example would be a narcotic analgesic. Thus, a query of opioid prescriptions from the PDMP using a therapeutic classification would include narcotic analgesics, but exclude opioid-containing antitussive prescriptions (e.g., hydrocodone-homatropine tablets), though clinical experience suggests these drugs are also subject to misuse. For research purposes, pharmaceutical class may better capture risk potential, because it is based on drug mechanism of action and overall pharmaceutical effects, rather than on intended treatment application.
In exploring Oregon’s PDMP vendor-assigned drug categories, we found several opioid and non-opioid prescriptions with potential for increased risk which were not assigned a category using the initial therapeutic assignment of the vendor. A more complete pharmacologic classification became important to detect potentially risky prescribing patterns for our research on PDMP utilization, prescribing patterns, and patient outcomes.
To calculate opioid risk metrics we needed to convert opioid doses into morphine milligram equivalents (MME), and distinguish duration of action (long-acting or short-acting opioids). These features are known to be associated with overdose risks.4,5 Assigning these features required complete pharmacologic classification of opioid prescriptions and assignment of MME Conversion Factors, Strength per Unit values, and Duration of Action categories. The Centers for Disease Control and Prevention (CDC) Conversion Reference Table provided some of this information, but for a limited number of opioids.6
We therefore sought to develop a more complete and consistent PDMP analysis data set for use by drug safety researcher. Our goals were:
To categorize a larger fraction of PDMP prescriptions using the improved pharmacologic classification methodology and a reference from the U.S. Food and Drug Administration.
To assign risk variable values to a larger fraction of the opioid prescriptions using a reference from the U.S. Centers for Disease Control and Prevention.
METHODS
The PDMP data set included prescription information for schedule II, III, and IV controlled substances and pseudoephedrine dispensed to Oregon residents from October 2011 through February 2014. Each prescription included information regarding the patient, prescriber, pharmacy, quantity dispensed, and other drug properties, including National Drug Code (NDC). In addition, the data set included a vendor-assigned drug categorization for a subset of prescriptions. Because PDMPs are regulated and run by individual states, there is variation in available data. For example, during our study period, neither the prescription days supply nor prescriber specialty beyond licensing credential, such as physician or dentist, were available in the Oregon PDMP. The Oregon Health Authority Public Health Division prepared the de-identified data set for use by researchers.
Drug category assignment
We used the U.S. Food and Drug Administration’s NDC drug reference table for pharmacologic classification.7 This table includes detailed information about drugs available in the US. We first found it necessary to remove extra zeros from NDC numbers in the PDMP (11 characters) to align them with NDC numbers in the drug reference table (10 characters). This process, and an explanation of the two formats, has been described elsewhere.8
Next we used pharmaceutical class (PharmClasses field in the NDC drug reference table) to identify and assign pharmacologic drug categories to PDMP prescriptions. We identified categories of interest based on known or suspected association with overdose risk, including benzodiazepines, non-benzodiazepine sedatives, opioids, other (e.g. carisoprodol), or “not assigned.” If a prescription could not be linked to the NDC drug reference table, the study pharmacist used vendor classification and other data set information (e.g., information in the drug name) to assist in classification.
Opioid risk variable augmentation
To calculate measures needed for research on potential opioid risk, we used the CDC Conversion Reference Table. Prescriptions in the PDMP data set were linked to the Conversion Reference Table using NDC codes. Required variables included MME Conversion Factor and Strength per Unit to calculate MME dispensed in a given time frame, as well as Duration of Action to evaluate potential risk associated with drug duration properties. We found it necessary to augment data in the CDC Conversion Reference Table because 19.9% of opioid prescriptions were missing one or more required variable. To assign missing variable values, the study pharmacist used known pharmaceutical properties and the values available within the CDC Conversion Reference Table.
Missing MME Conversion Factors were assigned based on values associated with drugs having the same generic name and route of administration. Missing Strength per Unit values were assigned using details contained within the drug name and strength field. Missing Duration of Action variables were assigned based on drugs with the same name, strength, and route of administration. For example, prescriptions missing risk variable values that had drug name and strength field “HYDROCODONE-ACETAMINOPHEN 10mg-650mg” were assigned a MME Conversion Factor=1, a Strength per Unit=10, and a Duration of Action=short-acting, similar to other hydrocodone-acetaminophen 10mg-650mg tablets in the CDC Conversion Reference Table.
After drugs were categorized and missing opioid prescription risk variable values were assigned, we performed data quality checks to ensure that newly assigned values were consistent with existing values for like-drugs, that all variables were assigned, and that assigned drug categories aligned with vendor categories when available. A clinician not otherwise involved in the study then reviewed the augmented table.
Final preparatory steps
Prior to de-identification, a Public Health Division analyst removed erroneously included prescriptions (e.g. transfers between pharmacies, invalid or institutional Drug Enforcement Agency numbers, duplicates, pets receiving human drugs). The PDMP data were then linked with vital records and a hospital discharge registry, using probabilistic methods (based on patient name, age, and zip code). After linking, data that could identify individual patients, prescribers, or pharmacies were removed to create a de-identified dataset for researchers.
Data analysis
Data management was performed using SAS 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Drug category assignment
In the initial data set of 19,134,105 prescriptions provided by the vendor, 1,987,098 (10.4%) of prescriptions were not classified. This included 137,026 (0.7%) of prescriptions subsequently determined to contain an opioid, 87,147 (0.5%) subsequently determined to contain non-benzodiazepine sedative, and 1,795 (<0.1%) subsequently determined to contain a benzodiazepine. Although the increase in prescriptions for each category was relatively small, the improvement in risk detection was important because each prescription contributes to patient risk analysis, whether for calculating total opioid MMEs or detecting risky co-prescribing. Using our methods for assigning drug category by pharmaceutical class, we were able to categorize 19,133,167 (>99.9%) of prescriptions into a drug category. [Figure 1: PDMP Drug Category Methodology]
Figure 1.
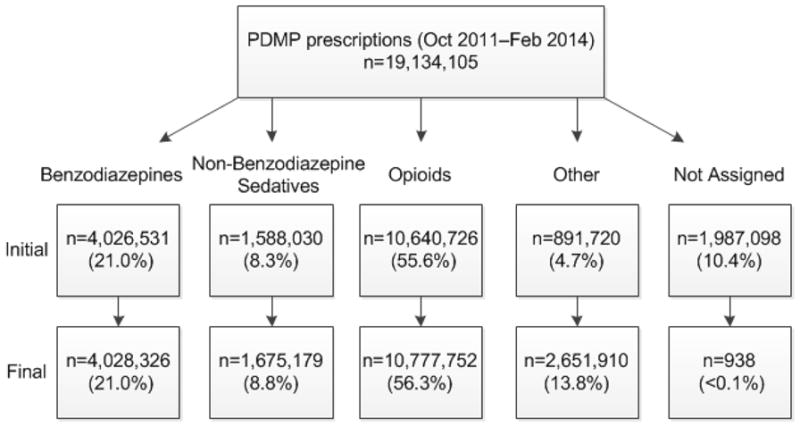
PDMP drug category methodology showing initial and final distribution of PDMP prescriptions within each drug category.
Drug category methodology showing initial and final distribution of prescription drug monitoring program prescriptions within each drug category. Drug categories were assigned using National Drug Code drug reference table definitions. The drug product, alone or in combination with other chemicals, included substances in the following pharmaceutical classes: Benzodiazepines, Non-benzodiazepine sedatives (e.g., zaleplon, zolpidem, or barbiturates), Opioids (e.g. opioid agonists, partial opioid agonists), Other: schedule II stimulants (8.2%), pseudoephedrine (1.8%) schedule III-IV appetite suppressants (1%), the muscle relaxant carisoprodol (<1%), and other scheduled medications not otherwise categorized (1.9%).
Opioid risk variable augmentation
Using the original CDC Conversion Reference Table to assign opioid risk variables, data required to calculate risk metrics were not assigned for 19.9% of opioid prescriptions. The prescriptions with missing risk variables included a wide variety of opioids with more than 100 unique drug products. The study pharmacist was able to augment variables for most opioid prescriptions using information in the drug name field (e.g. name, strength, and route). This included greater than 99.8% availability for the MME Conversion Factor, the Strength per Unit, and the Duration of Action variables. [Table 1: Opioid Risk Variable Methodology]
Table 1.
Opioid risk variable methodology shows the unaugmented and augmented distribution of opioid prescriptions with a risk variable value assigned or not assigned.
| Oregon PDMP Opioid Prescriptions (October 2011–February 2014), n=10,777,752 | ||||
|---|---|---|---|---|
| Unaugmented | Augmented | |||
| Opioid Risk Variable | Assigned | Not Assigned | Assigned | Not Assigned |
| MME Conversion Factor | 8,631,283 (80.1%) | 2,146,469 (19.9%) | 10,775,644 (>99.9%) | 2,108 (<0.1%) |
| Strength per Unit | 8,631,283 (80.1%) | 2,146,469 (19.9%) | 10,760,669 (99.8%) | 17,083 (0.2%) |
| Duration of Action | 8,631,283 (80.1%) | 2,146,469 (19.9%) | 10,777,193 (>99.9%) | 559 (<0.1%) |
DISCUSSION
Starting with a PDMP database prepared by a commercial vendor, we identified several key additional elements needed for our pharmacoepidemiologic research. Our approach successfully categorized nearly 2 million prescriptions (10.4% of PDMP prescriptions) that had previously been uncategorized. Our methods also augmented more than 2 million opioid prescriptions (19.9% of opioid prescriptions) with required risk variables not previously available. Our research risk metrics relied on summing morphine equivalents for all opioid prescriptions and detecting concomitant use of benzodiazepines, non-benzodiazepine sedatives, and carisoprodol. It was important to categorize nearly all PDMP prescriptions to prevent underestimating patient risk.
Our approach has some limitations. It may not be applicable to all researchers, depending on research aims. The data provided in the Oregon PDMP may differ from data in other states, due to variations in vendor contracts. Prescribers in Oregon may have different prescribing patterns than other states, so others may find a different initial magnitude of uncategorized prescriptions or missing opioid risk values. The clinical pharmacist’s manual review process is subject to variability and human error, despite consultation with another clinician and data quality checks for consistency.
Despite these limitations, our experience and approach may help others prepare complete and consistent PDMP analytic data sets for pharmacoepidemiologic research.
Ethics Statement
These efforts are part of a larger study approved by the institutional review boards at Oregon Health & Science University and Oregon Health Authority, Public Health Division.
Key Points.
Developing a complete and consistent prescription drug monitoring program (PDMP) analysis data set is essential for studying prescription drug abuse. Using a strategic methodology of drug category assignment and augmentation of opioid risk variable information, a complete and consistent data set can be created.
This methodology allows for a more inclusive and consistent method for categorization according to pharmaceutical class rather than therapeutic class. A publically available U.S. Food and Drug Administration drug database provides sufficient drug property information to categorize most prescriptions in a PDMP data set.
A publically available U.S. Centers for Disease Control and Prevention conversion reference can be augmented to create a complete opioid prescription data set for calculating opioid risk metrics.
Acknowledgments
Sponsor of Research:
Supported by the National Institutes of Health, National Institute for Drug Abuse through Grant # 1 R01 DA031208-01A1, and by the National Center for Research Resources and the National Center for Advancing Translational Sciences through Grant UL1RR024140.
Footnotes
Disclosure and Conflicts of Interest:
None of the authors have conflicts of interest to declare.
Prior Postings and Presentations:
Use of Prescription Drug Monitoring Programs to improve patient care and outcomes: A strategic methodology for drug risk metric research poster presented on May 28, 2015, at PDMP Researcher’s Forum in Washington D.C.
References
- 1.US Department of Health and Human Services, Behavioral Health Coordinating Committee. Addressing Prescription Drug Abuse in the United States: Current Activities and Future Opportunities. [Accessed 11 August 2015];2013 Sep; http://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf.
- 2.Haegerich TM, Paulozzi LJ, Manns BJ, et al. What we know and don’t know about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145C:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Number of poisoning deaths involving opioid analgesics and other drugs or substances—United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2013;62(12):234. [Google Scholar]
- 4.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175(4):608–615. doi: 10.1001/jamainternmed.2014.8071. [DOI] [PubMed] [Google Scholar]
- 6.National Center for Injury Prevention and Control. A compilation of opioid analgesic formulations with morphine milligram equivalent conversion factors, 2014 version. Atlanta, GA: Centers for Disease Control and Prevention; [Accessed Conversion Reference Table.xlsx 15 September 2014]. CDC Conversion Reference Table. http://www.pdmpassist.org/pdf/BJA_performance_measure_aid_MME_conversion.pdf. [Google Scholar]
- 7.US Food and Drug Administration. [Accessed 14 June 2014];National Drug Code Database File. http://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm.
- 8.US Food and Drug Administration. [Accessed 29 April 2015];National Drug Code Database Background Information. http://www.fda.gov/drugs/developmentapprovalprocess/ucm070829.


