Abstract
OBJECTIVE
Antibiotic treatment for asymptomatic bacteriuria (ASB) is prevalent but often in contrast to published guidelines. We evaluated risk factors for treatment of ASB.
DESIGN
Retrospective observational study
SETTING
A tertiary academic hospital, county hospital, and community hospital
PATIENTS
Hospitalized adults with bacteriuria
METHODS
Patients without documented symptoms of urinary tract infection per Infectious Disease Society of America (IDSA) criteria were classified as ASB. We examined ASB treatment risk factors, broad-spectrum antibiotic usage, and quantified diagnostic concordance between IDSA and National Healthcare Safety Network (NHSN) criteria.
RESULTS
Among 300 patients with bacteriuria, ASB was present in 71% by IDSA criteria. By NHSN criteria, 71% of patients had ASB; within-patient diagnostic concordance with IDSA was moderate (kappa = 0.52). After excluding those given antibiotics for non-urinary indications, antibiotics were given to 38% (62/164) with ASB. Factors significantly associated with ASB treatment were elevated urine white cell count (65 versus 24 white blood cells per high-powered field, p<0.01), hospital identity (Hospital C vs. A, OR 0.34, 95% CI 0.14–0.80, p=0.01), presence of leukocyte esterase (OR 5.48, 95% CI 2.35–12.79, p<0.01), presence of nitrites (OR 2.45, 95% CI 1.11–5.41, p=0.03), and E. coli on culture (OR 2.4, 95% CI 1.2–4.7, p=0.01). Of patients treated for ASB, broad-spectrum antibiotics were used in 84%.
CONCLUSIONS
ASB treatment was prevalent across diverse inpatient settings and contributed to broad-spectrum antibiotic use. Associating abnormal urinalysis results with the need for antibiotic treatment, regardless of symptoms, may drive unnecessary antibiotic use and provides an opportunity for antibiotic stewardship interventions.
The emergence of antibiotic resistance represents a major public health threat that is driven by antibiotic overuse.1 Antibiotics are among the most commonly prescribed medications in hospitalized patients, yet are frequently prescribed inappropriately.2 Positive urine cultures are a common trigger for antibiotic use in hospitalized patients,2,3 though data on overuse of antibiotics for asymptomatic bacteriuria (ASB) are limited. Guidelines recommend avoiding antibiotic therapy for bacteriuria in the absence of symptoms, with few exceptions such as during pregnancy.4–5 Despite these recommendations, antibiotic treatment of ASB is thought to be prevalent and to contribute to increasing antibiotic resistance, costs, and antibiotic-related adverse events, including Clostridium difficile infection.6–8
Data on antibiotic treatment for ASB are limited to observational data from academic centers or specific inpatient settings. An investigation from an academic Canadian hospital observed inappropriate antibiotics were given to 64% of patients with ASB.9 Treatment for ASB ranged between 32% – 52% in two observational studies in academic centers evaluating catheterized patients,10,11 and was observed in 33% of patients with enterococcal ASB.12 A recent study in two tertiary teaching hospitals identified antibiotic use in 59% of patients with ASB.13 Importantly, criteria for asymptomatic bacteriuria and exclusion criteria were not consistent across studies.
For these reasons, reducing overtreatment for ASB has been proposed as an area of focus for hospital antibiotic stewardship programs,14 which promote antibiotic use consistent with evidence-based guidelines. Additionally, reducing ASB overtreatment is part of the American Board of Internal Medicine “Choosing Wisely” campaign15, and has been proposed as a National Performance Measure.16 Previous investigations on treatment of ASB have been descriptive in nature with limited analyses of which inpatient populations with ASB are at highest risk for antibiotic treatment. Identification of populations at risk may help identify targets likely to benefit from future intervention to reduce antibiotic treatment of ASB.
Multiple criteria for symptomatic urinary tract infections (UTI) exist. The two major definitions are the clinically-oriented criteria from the Infectious Diseases Society of America (IDSA) and criteria from the National Healthcare Safety Network (NHSN), typically used for infection surveillance purposes. Both criteria rely on the presence of symptoms, not bacteriuria or pyuria alone, to diagnose symptomatic UTI and initiate antibiotic treatment per IDSA guidelines. Notable differences exist between these criteria; specifically, IDSA criteria require 1 or more bacterial species, whereas NHSN requires no more than 2 microorganisms (including yeast). Additionally, NHSN criteria consider a fever from any cause as consistent with symptomatic UTI, whereas IDSA criteria require fever without another recognized cause. While we are unaware of published data examining how these different criteria affect assessment of ASB prevalence and treatment, exploration of this topic may provide insight into strategies for reducing antibiotic overuse. Currently, the IDSA criteria for patient management guides clinician behavior, while hospitals often rely on NHSN criteria for surveillance and reporting.4,5,17
To improve understanding of ASB overtreatment as a potential focus for stewardship programs, we conducted a multi-center study to characterize the burden of antibiotic use for bacteriuria across three diverse inpatient hospital settings. We assessed the prevalence of and risk factors for antibiotic treatment for ASB, as well as how these factors vary by different ASB criteria.
METHODS
We performed a multi-center retrospective chart review across three diverse hospital settings: a 900-bed tertiary care academic teaching hospital, a 550-bed county hospital, and a 389-bed community hospital. Medical record review was performed on consecutive patients with positive urine cultures between September 2013 – April 2014. Positive urine cultures were identified at each centers’ Clinical Microbiology Laboratory. Criteria for inclusion for chart review were: age ≥ 18 years, an inpatient stay >1 calendar day, and a urine culture with >103 colony forming units. For patients with multiple positive urine cultures, the first positive culture was evaluated. Patients were excluded if they had an immunocompromising condition (neutropenia, solid organ or stem cell transplant recipient), pregnancy, recent or pending urologic procedure expected to cause mucosal bleeding, were discharged within one calendar day of urine culture collection, or had a suprapubic catheter, nephrostomy tube, or neobladder. One hundred cultures that fit the above criteria during a predetermined time period convenient to the center’s investigative team were obtained from each center. This study was approved by the Institutional Review Board of each participating hospital.
Symptomatic UTI were defined using criteria from the IDSA guidelines (Table 1).4,5 Patients with bacteriuria without a symptomatic UTI as defined by IDSA criteria were considered to have ASB. We also performed secondary analyses using NHSN definitions of ASB.17 Within-patient diagnostic concordance between IDSA and NHSN criteria for symptomatic UTI was calculated as the overall percent agreement and Cohen’s kappa value.
Table 1.
Diagnostic criteria used to define symptomatic urinary tract infection.
| IDSA criteria for symptomatic UTIa |
|
| NHSN criteria for symptomatic UTIb | Both conditions (1) and (2) below must be met
|
Urinalysis results do not factor into assessment of a symptomatic urinary tract infection
NHSN criteria are from 2013; criteria have been subsequently modified to remove urinalysis criteria, among other changes.
Only applies to patients age ≤65 years without an indwelling urinary catheter
With no other recognized cause
Only applies to patients without an indwelling urinary catheter
IDSA, Infectious Disease Society of America; UTI, urinary tract infection; NHSN, National Healthcare Safety Network; WBC, white blood cells.
Treatment of ASB was defined by medical record documentation of a clinician order for a specific antibiotic treatment for bacteriuria. Patients with a documented reason for antibiotics other than for ASB (e.g., pneumonia) were not considered to be treated for ASB. Our primary outcome was antibiotic treatment for ASB (using IDSA criteria) at either day 1 or day 4 (when urine cultures would be finalized) after cultures were taken. Patients on concurrent antibiotic treatment for ASB and non-UTI infection were excluded from bivariate analysis of risk factors for ASB treatment. We also measured the proportion of antibiotic use that was broad-spectrum. Based on consensus of investigators, we defined broad-spectrum antibiotics as beta-lactam/beta-lactamase inhibitor combinations, fluoroquinolones, 3rd and 4th generation cephalosporins, fosfomycin, and carbapenems. Multi-drug resistant pathogens were defined as vancomycin-resistant Enterococcus, methicillin-resistant Staphylococcus aureus, and extended-spectrum beta-lactamase-producing and carbapenemase-producing gram-negative bacteria.
Medical record review was conducted by clinicians (physicians at 2 hospitals, pharmacists at one) using a standardized data abstraction tool and entered into an electronic database (REDCap version 5.6.0). Data elements included patient age, gender, urine culture and urinalysis results, presence of urinary catheters, hospital location (intensive care unit (ICU) versus non-ICU), primary service (surgical versus non-surgical), and involvement of an Infectious Disease specialist on the day of urine culture collection or the subsequent day. Bacteriuria present between day of admission (hospital day 0) or by hospital day 2 were considered admission-onset; all others were considered hospital-onset. Physician documentation was reviewed to assess for UTI symptoms (Table 1) and concurrent conditions that may contribute to symptoms. Antibiotic treatment both at day 1 and at day 4 after urine culture collection was assessed.
An assessment of inter-rater reliability was performed at each hospital; 5% of data collected was reviewed by co-investigators experienced in medical record review, and overall agreement was 96%. Data were analyzed using the chi-squared test or Fisher exact test for categorical variables, and the t-test for continuous variables. Bivariate analysis was conducted to assess factors associated with ASB treatment by calculating odds ratios, 95% confidence intervals, and associated p-values. All calculations were performed using SAS statistical software version 9.3 (SAS Institute, Cary, NC).
RESULTS
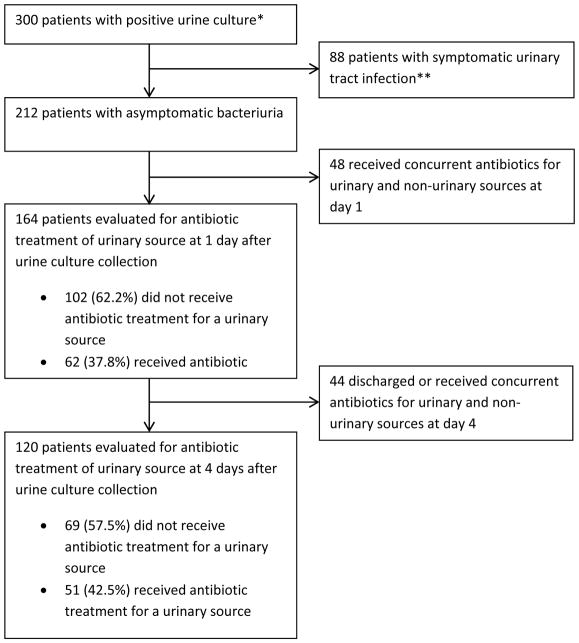
Among the 300 unique inpatients with positive urine cultures from three hospitals, the prevalence of ASB using IDSA criteria was 71% (212/300) (Figure 1). Table 2 summarizes the characteristics of all study patients with bacteriuria, regardless of symptoms. The mean age was 67 years; 66% of patients were female. Sixteen percent (49/300) of patients with bacteriuria were in the ICU, and 22% (67/300) were catheterized. Urine cultures were collected within the first 2 hospital days in 75% (225/300). Forty-four percent (133/300) of patients with bacteriuria were on antibiotics for a non-urinary source on day 1 following urine culture collection. Significant differences in patient characteristics between hospitals were seen regarding patient age, ICU status, surgical service, urethral catheterization, admission-onset bacteriuria, presence of infectious disease consultant, and frequency of use of broad-spectrum antibiotics for treatment of bacteriuria.
Figure 1. Patients included in the study analysis.
Summary of patients selected for analysis.
*Patients were adults with an inpatient stay > 1 calendar day who were non-immunocompromised and met all inclusion criteria, and no exclusion criteria. These criteria are outlined in the Methods section.
**Symptomatic urinary tract infection per Infectious Disease Society of America criteria, as outlined in Table 1.
Table 2.
Descriptive analysis of all study patients with bacteriuria.
| Total | Hospital A | Hospital B | Hospital C | p-value | |
|---|---|---|---|---|---|
| # Patients with bacteriuria | 300 | 100 | 100 | 100 | |
|
| |||||
| Age (years), median (range) | 66 (19– 104) | 79 (34– 99) | 57 (24– 91) | 70 (19– 104) | <0.0001 |
|
| |||||
| Age >65 years | 156 (52%) | 72 (72%) | 24 (24%) | 60 (60%) | <0.0001 |
|
| |||||
| Female gender | 199 (66%) | 71 (71%) | 57 (57%) | 71 (71%) | 0.06 |
|
| |||||
| ICU patients | 49 (16%) | 15 (15%) | 9 (9%) | 25 (25%) | 0.008 |
|
| |||||
| Surgical patients | 43 (14%) | 10 (10%) | 33 (33%) | 0 (0%) | 0.001 |
|
| |||||
| Catheterized patients | 67 (22%) | 15 (15%) | 33 (33%) | 19 (19%) | 0.006 |
|
| |||||
| Admission-onset bacteriuria | 225 (75%) | 87 (87%) | 46 (46%) | 92 (92%) | <0.0001 |
|
| |||||
| Multi-drug resistant pathogens on urine culture | 39 (13%) | 7 (7%) | 17 (17%) | 15 (15%) | 0.08 |
|
| |||||
| Patients receiving treatment for non-UTI infection at day 1 | 133 (44%) | 38 (38%) | 42 (42%) | 53 (53%) | 0.09 |
|
| |||||
| Participation of infectious diseases specialist | 36 (12%) | 29 (29%) | 3 (3%) | 4 (4%) | <0.0001 |
|
| |||||
| Patients with symptomatic UTI by IDSA criteria | 88 (29%) | 31 (31%) | 23 (23%) | 34 (34%) | 0.21 |
|
| |||||
| Patients with symptomatic UTI by NHSN criteria | 87 (29%) | 34 (34%) | 35 (35%) | 18 (18%) | 0.01 |
|
| |||||
| Treatment for ASB (IDSA criteria) at day 1, n = 164 | 62 (38%)* | 25 (51%)* | 25 (36%)* | 12 (26%)* | 0.04 |
|
| |||||
| Treatment for ASB (IDSA criteria) at day 4, n = 120 | 51 (42%)* | 19 (49%)* | 19 (38%)* | 13 (42%)* | 0.02 |
|
| |||||
| Broad-spectrum antibiotic use** | 158 (53%) | 62 (62%) | 42 (42%) | 54 (54%) | 0.02 |
| --Beta-lactam/beta-lactamase inhibitor combinations | 27 | 15 | 0 | 12 | 0.0005 |
| --Fluoroquinolones | 33 | 16 | 1 | 16 | 0.0005 |
| --3rd and 4th generation cephalosporins | 89 | 27 | 38 | 24 | 0.07 |
| --Fosfomycin | 1 | 0 | 1 | 0 | 0.33 |
| --Carbapenems | 11 | 4 | 2 | 5 | 0.64 |
Data are number (%) unless otherwise indicated.
Percentage represents proportion of ASB patients who received antibiotic therapy, excluding those on concurrent treatment for both UTI and non-UTI indications.
Broad-spectrum antibiotic use refers to receipt of beta-lactam/beta-lactamase inhibitor combinations, fluoroquinolones, 3rd and 4th generation cephalosporins, fosfomycin, or carbapenems, for any indication during the study period. SD, standard deviation; ICU, intensive care unit; UTI, urinary tract infection; UTI, urinary tract infection; IDSA, Infectious Disease Society of America; NHSN, National Healthcare Safety Network; ASB, asymptomatic bacteriuria.
The most frequent organism isolated from urine culture was Escherichia coli (n=119), followed by Klebsiella pneumoniae (n=36), Enterococcus spp. (n=28), and Proteus mirabilis (n=20). Polymicrobial urine culture results were present in 38% (115/300), and urine cultures with less than 105 colony-forming units represented 28% (83/300). A urinalysis associated with the urine culture was present in 94% (281/300); leukocyte esterase was detected in 76% (213/281), and nitrites were detected in 27% (77/281). Microscopy identified a leukocyte count of 5 or more cells per high-powered field in 70% (198/281).
Of the 212 patients with ASB, and after excluding 48 patients on antibiotics for both UTI and non-UTI indications, the proportion of patients with ASB that received antibiotics for positive urine culture was 38% (62/164) at one day after urine culture collection. This proportion increased slightly by day 4 to 43% (51/120, p=0.59).
Of patients who received antibiotics for urinary sources at day 1 after urine culture, the prevalence of treatment with broad-spectrum antibiotics was 84% for both those without symptoms (52/62) and for those with symptoms (49/58). For ASB, broad-spectrum antibiotics used were predominantly third and fourth generation cephalosporins (n=32), followed by fluoroquinolones (n=13), similar to what was observed in those treated for a symptomatic UTI. Multi-drug resistant pathogens comprised 13% (22/164) of all ASB cultures, also similar to what was observed in patients with symptomatic UTI (13%, 11/88).
In bivariate analysis, variables associated with ASB treatment were hospital identity (Hospital C vs. Hospital A, OR 0.34, 95% CI 0.14–0.80, p=0.01), presence of leukocyte esterase (OR 5.48, 95% CI 2.35–12.79, p<0.01), positive nitrite (OR 2.45, 95% CI 1.11–5.41, p=0.03), elevated mean urine leukocyte count (65 versus 24 white blood cells per high-powered field, p<0.01), and the presence of E. coli as the primary urine pathogen (OR 2.4, 95% CI 1.2–4.7, p=0.01). (Table 3)
Table 3.
Bivariate analysis of patients with asymptomatic bacteriuria to evaluate variables associated with treatment at Day 1 using IDSA criteria.
| Characteristic | ASB treated (n=62) | ASB not treated (n=102) | Odds Ratio (95% CI) | p- value |
|---|---|---|---|---|
| Study site | ||||
| Hospital A | 25 | 24 | Reference | |
| Hospital B | 25 | 44 | 0.55 (0.26–1.15) | 0.11 |
| Hospital C | 12 | 34 | 0.34 (0.14–0.80) | 0.01 |
|
| ||||
| Urinalysis results: | ||||
| Leukocyte count (mean)* | 65 | 24 | -- | <0.01 |
| Leukocyte esterase positive | 52 | 51 | 5.48 (2.35– 12.79) | <0.01 |
| Nitrite positive | 18 | 14 | 2.45 (1.11–5.41) | <0.01 |
|
| ||||
| Age (years, mean) | 67 | 64 | 1.01 (0.99–1.1) | 0.20 |
|
| ||||
| Female gender | 45 | 65 | 1.5 (0.8–3.0) | 0.30 |
|
| ||||
| Non-surgical service | 51 | 87 | 0.8 (0.3–1.9) | 0.61 |
|
| ||||
| Non-ICU patient at time of urine culture | 58 | 85 | 2.9 (0.93–9.1) | 0.07 |
|
| ||||
| Hospital-onset bacteriuria** | 17 | 30 | 0.91 (0.5–1.8) | 0.78 |
|
| ||||
| Presence of indwelling urinary catheter | 18 | 21 | 1.6 (0.76–3.3) | 0.22 |
|
| ||||
| Involvement of infectious diseases specialist during hospital admission | 4 | 10 | 0.64 (0.19–2.1) | 0.46 |
|
| ||||
| Pathogen type | ||||
| Escherichia coli | 27 | 25 | 2.4 (1.2–4.7) | 0.01 |
| Enterococcus species | 5 | 16 | 0.47 (0.16–1.4) | 0.16 |
| Other gram-negative rods | 14 | 16 | 1.57 (0.71–3.5) | 0.27 |
|
| ||||
| Urine culture with multi- drug resistant pathogen | 10 | 12 | 1.4 (0.58–3.6) | 0.43 |
Measured as white blood cells per high-powered field.
Hospital-onset was defined as presence of bacteriuria after hospital day 2. ASB, asymptomatic bacteriuria; ICU, intensive care unit.
Overall rates of ASB prevalence were no different when IDSA criteria were compared to NHSN surveillance criteria (71% versus 71%, respectively). Additionally, overall rates of ASB treatment were also similar when either criterion was applied (38% versus 41%, respectively). Within-patient concordance between IDSA and NHSN criteria was 66% (Cohen’s kappa value 0.52). When bivariate analysis was performed with NHSN, instead of IDSA, criteria, the same factors (hospital identity, elevated urine leukocyte count, presence of leukocyte esterase and nitrite) were associated with ASB treatment.
DISCUSSION
In our study of hospitalized patients with positive urine cultures across three different types of hospitals, we observed frequent antibiotic use in patients with positive urine cultures without documented symptoms of UTI. Broad-spectrum antibiotics comprised a high-proportion (84%) of treatment, regardless of the presence or absence of UTI symptoms. The proportion of patients treated with antibiotics for ASB did not change from day 1 to day 4 following urine culture, a time by which most urine cultures would have been finalized. Treatment for ASB was not significantly associated with patient characteristics such as age, gender, presence of a urethral catheter, surgical versus non-surgical service, ICU versus non-ICU setting, or admission-onset bacteriuria but instead primarily based on results of urine studies. Our findings are comparable with observations from more limited categories of ASB, such as catheterized patients10,11 or patients with enterococcal ASB.12
We found that abnormal urinalysis findings were associated with ASB treatment, a finding consistent with prior observations.10,12,13 We hypothesize this finding reflects widespread physician misperception that pyuria with bacteriuria defines infections requiring antibiotic treatment, despite clear guideline recommendations that in the absence of symptoms, pyuria is not an indication for treatment (level A-II evidence).4,5 Our hypothesis is supported by physician surveys that demonstrate considerable variability in management of bacteriuria,18 as well as overall suboptimal knowledge regarding management of catheter-associated bacteriuria19 and the use of urine testing and subsequent treatment.20
E. coli was the predominant organism isolated from urine culture in our study (40%) and was associated with an increased risk of treatment for ASB. E. coli is the most prevalent agent of uncomplicated cystitis, pyelonephritis, catheter-associated UTIs, as well as ASB.4,5,21 Its known role as the predominant causative pathogen in symptomatic UTI may contribute to a bias towards treatment of asymptomatic patients with E. coli identified in urine culture.
We believe this to be the largest study to specifically address how treatment of ASB contributes to broad-spectrum antibiotic usage. Dalen et al. observed ciprofloxacin to be the most common antibiotic used in 15 cases of overtreatment for ASB (33%), and Werner et al. identified that among all inpatient fluoroquinolone use, 30% was for ASB.11,22 Likewise, we observed broad-spectrum antibiotics comprise a substantial proportion of ASB treatment, thereby potentially contributing to the emergence of multi-drug resistant pathogens, and increased rates of C. difficile infection.8 The latter is of particular concern, given older age is an established risk factor for C. difficile infection, and our median patient age was 67 years.23
Multi-drug resistant organisms were identified in 13% (22/164) of patients with ASB, a similar proportion to that observed in patients with symptomatic UTI (13%, 11/88). Overall, the most frequent multi-drug resistant pathogen was extended-spectrum beta-lactamase producing gram-negative rods (26), followed by vancomycin-resistant Enterococcus (9). We did not observe a significant association between presence of multi-drug resistant urinary pathogens and risk for treatment of ASB, though we were underpowered to test this association. It remains unclear if ASB with multi-drug resistant bacteriuria represents a risk factor for treatment. A recent investigation of enterococcal ASB found vancomycin-resistance did not impact rates of ASB treatment.24
To our knowledge, our investigation is the first to directly compare ASB prevalence using both the IDSA and NHSN criteria. Although ASB prevalence and ASB treatment rates were similar when comparing the IDSA to NHSN criteria, the diagnostic concordance between criteria was only moderate (kappa 0.52).25 Diagnostic discordance was largely attributed to differences in the number of organisms present on urine cultures. This finding may have implications for antibiotic stewardship programs that utilize NHSN surveillance criteria to identify cases for potential intervention; we postulate clinicians may be more comfortable referencing the clinically-oriented IDSA guidelines, rather than NHSN surveillance criteria, when faced with antibiotic stewardship program treatment recommendations.
We did not find an association between an infectious disease physician participation in the patient’s care and reduced frequency of ASB treatment, though our study was underpowered to detect such an association. Educational efforts using infectious diseases specialists have been shown to reduce the incidence of inappropriate ASB treatment.26 Our observations suggest that infectious disease specialists are only involved in a small proportion of patients with ASB (8%, 14/164), thus limiting the opportunity to impact overall hospital antibiotic usage. This supports the role for a stewardship program that promotes guideline-concordant antibiotic use targeting all clinicians.
We observed significant differences in baseline patient characteristics across each hospital in our three-hospital survey. Differences were observed in age, as well as in proportion of patients in the ICU, on a surgical service, with a urethral catheter, and with admission-onset bacteriuria (Table 2). Despite these underlying differences, treatment for ASB remained frequent at each hospital, ranging from 26–51%. Additionally, bivariate analysis found only results of urine studies, and not patient-specific variables such as age or presence of a catheter, were associated with antibiotic treatment for ASB. Together, these findings suggest ample stewardship opportunities exist across a wide variety of inpatient settings.
Strengths of this study include its multi-center design encompassing a diverse inpatient setting and incorporating both catheterized and non-catheterized inpatients. This study represents one of the largest evaluations of appropriate ASB antibiotic treatment in hospitalized patients, and the only study to specifically address issues pertinent to antibiotic stewardship programs, including the impact on broad-spectrum antibiotic usage and how different criteria might impact interventions. We used standardized criteria to assess for UTI symptoms, and utilized a standardized data collection tool that yielded high inter-rater reliability. Limitations include the retrospective study design and possibility that physicians under-documented UTI symptoms, contributing to an overestimate of ASB. The diagnosis of non-urinary infections was not standardized and relied on clinical documentation, which may have impacted our assessment of ASB treatment prevalence. This study excluded immunocompromised patients, pregnant women, and those with advanced urologic conditions, limiting the generalizability of our findings to these patient populations. However, the IDSA treatment recommendations also exclude these patient groups, thus enhancing the validity of our analysis. Finally, we excluded patients who were discharged within one day of urine culture collection, so our findings do not reflect the burden of antibiotic usage for ASB in the outpatient setting.
This study has important implications for the emerging field of antibiotic stewardship. First, opportunities for improving guideline-concordant antibiotic therapy for bacteriuria exist across a wide-spectrum of hospitalized patients. These opportunities appear most abundant in reducing third/fourth-generation cephalosporin and fluoroquinolone use predominantly in patients >65 years, which are both established risk factors for C. difficile infection.23,27 Thus, reducing antibiotic use for ASB aligns well with the primary goal of antibiotic stewardship, which is to optimize antibiotic use and minimize unintended consequences.28 Second, educational efforts targeting appropriate interpretation of urinalysis results may be effective, since this appears to be the predominant risk factor associated with ASB treatment. Education for clinicians should reinforce that the diagnosis of a UTI requiring antibiotics is not a laboratory diagnosis based on urinalysis results, but a clinical diagnosis based on symptoms. Third, criteria developed for epidemiologic infection surveillance (such as NHSN criteria) are not always consistent with treatment guidelines developed for clinical management (such as IDSA guidelines) of individual patients. Stewardship interventions must be mindful of these discrepancies and not rely only on surveillance criteria for identifying opportunities to reduce antibiotic use.
In conclusion, we observed treatment for ASB to be common across diverse adult inpatient populations, and to contribute to the use of broad-spectrum antibiotics. Our analyses reveal that for a substantial group of hospitalized patients, the results of urine studies appear to drive antibiotic treatment regardless of the absence of symptoms. Treatment of bacteriuria with pyuria in the absence of symptoms conflicts with evidence-based clinical guideline recommendations. While infectious disease specialists can model appropriate care for hospitalized patients with UTIs, our data show that infectious disease specialists are involved in only a small minority of hospitalized patients with ASB. Since antibiotic management of bacteriuria involves a diverse set of clinicians, antibiotic stewardship interventions applied to a broad spectrum of clinical specialties has the potential to improve guideline-concordant antibiotic therapy and reduce ASB treatment. Given the high prevalence of treatment among patients with ASB, additional evaluation of programs to reduce ASB treatment may be critical to ongoing efforts to halt unnecessary antibiotic use.
Acknowledgments
Financial Support: This research was supported by the UCLA Clinical and Translational Science Institute, NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124.
Footnotes
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
Additional Contributions: None.
This work was presented in part at IDWeek; October 9, 2014; Philadelphia, Pennsylvania.
References
- 1.Centers for Disease Control and Prevention. [Accessed on 23 June 2014];Antibiotic resistant threats in the United States. 2013 http://www.cdc.gov/drugresistance/threat-report-2013/index.html.
- 2.Fridkin S, Baggs J, Fagan R, et al. Vital Signs: Improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014 Mar 7;63(9):194–200. [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi T, Flanders SA, Markovitz E, Saint S, Kaul DR. Importance of urinary tract infection to antibiotic use among hospitalized patients. Infect Control Hosp Epidemiol. 2009;30:193–195. doi: 10.1086/593951. [DOI] [PubMed] [Google Scholar]
- 4.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooten TM. Infectious Diseases Society of America Guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 5.Hooten TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines for the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 6.Dull RB, Friedman SK, Risoldi ZM, Rice EC, Starlin RC, Destache CJ. Antimicrobial treatment of asymptomatic bacteriuria in noncatheterized adults: a systematic review. Pharmacotherapy. 2014;34:941–960. doi: 10.1002/phar.1437. [DOI] [PubMed] [Google Scholar]
- 7.Trautner BW, Grigoryan L. Approach to a positive urine culture in a patient without urinary symptoms. Infect Dis Clin North Am. 2014;28:15–31. doi: 10.1016/j.idc.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaughnessy MK, Amundson WH, Kuskowski MA, DeCarolis DD, Johnson JR, Drekonja DM. Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34:109–116. doi: 10.1086/669089. [DOI] [PubMed] [Google Scholar]
- 9.Silver SA, Baillie L, Simor AE. Positive urine cultures: A major cause of inappropriate antimicrobial use in hospitals? Can J Infect Dis Med Microbiol. 2009;20:107–111. doi: 10.1155/2009/702545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cope M, Cevallos ME, Cadle RM, et al. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clin Infect Dis. 2009;48:1182–1188. doi: 10.1086/597403. [DOI] [PubMed] [Google Scholar]
- 11.Dalen DM, Zvonar RK, Jessamine PG. An evaluation of the management of asymptomatic catheter-associated bacteriuria and candiduria at the Ottawa Hospital. Can J Infect Dis Med Microbiol. 2005;16:166–170. doi: 10.1155/2005/868179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin E, Bhusal Y, Horwitz D, Shelburne SA, Trautner BW. Overtreatment of Enterococcal Bacteriuria. Arch Intern Med. 2012;172:33–38. doi: 10.1001/archinternmed.2011.565. [DOI] [PubMed] [Google Scholar]
- 13.Ifran N, Brooks A, Mithoowani S, Celetti SJ, Main C, Mertz D. A controlled quasi-experimental study of an educational intervention to reduce the unnecessary use of antimicrobials for asymptomatic bacteriuria. PLOS ONE. 2015;10:e0132071. doi: 10.1371/journal.pone.0132071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.File TM, Solomkin JS, Cosgrove SE. Strategies for improving antimicrobial use and the role of antimicrobial stewardship programs. Clin Infect Dis. 2011;53:S15–S22. doi: 10.1093/cid/cir364. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed October 2, 2015];Choosing Wisely website. www.choosingwisely.org.
- 16.Gross PA, Patel B. Reducing antibiotic overuse: a call for a national performance measure for not treating asymptomatic bacteriuria. Clin Infect Dis. 2007;45:1335–1137. doi: 10.1086/522183. [DOI] [PubMed] [Google Scholar]
- 17.Catheter-associated urinary tract infection (CAUTI) event. [Accessed 30 August 2013];National Healthcare Safety Network website. http://www.cdc.gov/nhsn/acute-care-hospital/CAUTI/index.html.
- 18.Chant C, Dos Santos CC, Saccucci P, Smith OM, Marshall JC, Friedrich JO. Discordance between perception and treatment practices associated with intensive care unit-acquired bacteriuria and funguria: A Canadian physician survey. Crit Care Med. 2008;36:1158–1167. doi: 10.1097/CCM.0b013e3181692af9. [DOI] [PubMed] [Google Scholar]
- 19.Trautner BW, Petersen NJ, Hysong SJ, Horwitz D, Kelly PA, Naik AD. Overtreatment of asymptomatic bacteriuria: Identifying provider barriers to evidence-based care. Am J Infect Control. 2014;42:653–658. doi: 10.1016/j.ajic.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Drekonja DM, Abbo LM, Kuskowski MA, Gnadt C, Shukla B, Johnson JR. A survey of resident physician’s knowledge regarding urine testing and subsequent antimicrobial treatment. Am J Infect Control. 2013;41:892–896. doi: 10.1016/j.ajic.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 22.Werner NL, Hecker MT, Sethi AK, Donskey CJ. Unnecessary use of fluoroquinolone antibiotics in hospitalized patients. BMC Infect Dis. 2011;11:187. doi: 10.1186/1471-2334-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 24.Khair HN, VanTassell P, Henderson JP, Warren DK, Marschall J for the CDC Prevention Epicenters Program. Vancomycin resistance has no influence on outcomes of enterococcal bacteriuria. J Hosp Infect. 2013;85:183–188. doi: 10.1016/j.jhin.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 26.Pavese P, Saurel N, Labarere J, et al. Does an educational session with an Infectious Diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with postitive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596–599. doi: 10.1086/597514. [DOI] [PubMed] [Google Scholar]
- 27.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:881–891. doi: 10.1093/jac/dkt477. [DOI] [PubMed] [Google Scholar]
- 28.Dellit TH, Owens RC, McGowan JE, et al. Infectious Disease Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]