Abstract
Objectives
Infections cause significant morbidity and mortality in neonatal intensive care units (NICUs). The association between nursery design and nosocomial infections has not been delineated. We hypothesized that rates of colonization by methicillin-resistant Staphylococcus aureus (MRSA), late-onset sepsis, and mortality are reduced in single-patient rooms.
Design
Retrospective cohort study.
Setting
NICU in a tertiary referral center.
Methods
Our NICU is organized into single-patient and open-unit rooms. Clinical datasets including bed location and microbiology results were examined over a 29-month period. Differences in outcomes between bed configurations were determined by Chi-square and Cox regression.
Patients
All NICU patients.
Results
Among 1823 patients representing 55,166 patient-days, single-patient and open-unit models had similar incidences of MRSA colonization and MRSA colonization-free survival times. Average daily census was associated with MRSA colonization rates only in single-patient rooms (hazard ratio 1.31, p=0.039), while hand hygiene compliance on room entry and exit was associated with lower colonization rates independent of bed configuration (hazard ratios 0.834 and 0.719 per 1% higher compliance, respectively). Late-onset sepsis rates were similar in single-patient and open-unit models as were sepsis-free survival and the combined outcome of sepsis or death. After controlling for demographic, clinical and unit-based variables, multivariate Cox regression demonstrated that bed configuration had no effect on MRSA colonization, late-onset sepsis, or mortality.
Conclusions
MRSA colonization rate was impacted by hand hygiene compliance, regardless of room configuration, while average daily census only affected infants in single-patient rooms. Single-patient rooms did not reduce the rates of MRSA colonization, late-onset sepsis or death.
INTRODUCTION
Late-onset infections continue to cause substantial morbidity and mortality in neonatal intensive care units (NICUs),1–3 increasing length of stay and costs.4 While many studies have examined the impact of environmental factors on nosocomial infections, the cornerstone of which is proper hand hygiene by healthcare workers,5 the role of room configuration is less well defined. Many pathogens are transmitted via surfaces and fomites,6 and multi-patient rooms are more difficult to decontaminate because of their greater number of surfaces and higher traffic. These concerns contributed to single-patient rooms becoming the standard design in healthcare facilities.7, 8 While improvements in air quality and nosocomial infections have been attributed to the change from open-unit to single-patient room facilities,8, 9 these NICU bed configurations have not been directly compared contemporaneously. Our NICU, which has both single-patient and open-unit beds, provided an opportunity to test the hypothesis that infants in single-patient rooms have a lower risk of methicillin-resistant Staphylococcus aureus (MRSA) colonization, late-onset sepsis, and death.
PATIENTS AND METHODS
Study Location
The NICU at St. Louis Children’s Hospital has 73 beds that can flex to 81 beds during times of high census. Thirty-six beds are in single-patient rooms while 3 open-unit areas have 9 or 14 beds, with flexible beds organized in an 8-bed open-unit model.
Open-unit and single-patient rooms were staffed by the same groups of nurses, residents, nurse practitioners, fellows and attending physicians. Patients were assigned to one of four multidisciplinary teams. Nurse-to-patient staffing ratios are 1:1–3, depending on illness severity, and all patients in a nursing assignment are in the same bed configuration. Staffing was similar across bed configurations. Bed assignment was based on staffing and bed availability without regard to diagnosis, acuity or bed configuration.
Patients
All patients who resided in the NICU from July 1, 2009 to November 30, 2011 were included, regardless of admission or discharge date. The study was approved by the Washington University Human Research Protection Office.
Data Acquisition
Billing and coding data from the hospital management information system (HMIS) were retrospectively queried for the study interval to determine dates of birth, admission, discharge and death, room location, gender, race, ethnicity, insurance type (Medicaid, private, uninsured), as well as ICD-9 diagnosis codes that contain gestational age and birth weight information. Additionally, data were gathered from the hospital infection control service to identify all patients colonized with MRSA during the study period and the rates of hand hygiene compliance during patient encounters. Apgar score, temperature on admission, and initial blood gas results were gathered from our NICU’s National Institute of Child Health and Human Development dataset10 and the Clinical Investigation Data Exploration Repository.11 Finally, patient-specific information regarding all positive cerebrospinal fluid (CSF) and blood cultures from NICU patients for the study interval was provided by the microbiology laboratory information system (Cerner Millennium, Kansas City, MO).
HMIS data included daily room assignments, allowing for each patient’s NICU room assignment and bed configuration type to be tracked on a day-by-day basis. Patients who transferred between open-unit and single-patient rooms had all data removed from the analyses.
MRSA Genotyping
Anterior nares swab cultures were used to screen for MRSA colonization as part of routine infection control measures on admission and weekly thereafter, per institution protocol. The first MRSA recovered from each subject was frozen for future analysis. DNA was extracted from bacterial isolates using the BiOstic™ Bacteremia DNA Isolation Kit (MoBio Laboratories) according to the manufacturers’ directions. Repetitive sequence PCR (repPCR) was then performed as previously described, using approximately 100 ng of DNA, a Ready-to-go RAPD analysis bead (GE), and primer RW3A in a final reaction volume of 25 μL.12, 13 The repPCR products were resolved using the Agilent 2100 Bioanalyzer, and banding patterns were analyzed using Diversilab software version 3.4 (bioMérieux) to measure strain similarity. Isolates with similarity indices > 95% were considered identical.
The Diversilab software compared the DNA banding pattern of each isolate to all other isolates and assembled this into a two-dimensional scatterplot. Those with high similarity indices clustered closer than those with low similarity indices. This allowed visualization of genotype clustering within a set of isolates.14
Barrier Precautions
All patients, regardless of MRSA colonization status, were cared for using standard precautions. In addition, infants colonized with MRSA were placed in contact isolation. These policies applied to all members of the staff, families and visitors. No visitor restriction occurred for either group of patients. Use of alcohol foam or hand washing stations on room entry and exit is standard of care. Compliance with hand hygiene was assessed by direct observation of repeated patient encounters by members of the hospital infection control committee and included all providers. Observations of compliance occurred weekdays during the day shift and covered all areas of the NICU. A provider who exited one bed space, performed hand hygiene, and entered another bed space remained compliant as long as no other surfaces were contacted during this transition.
Definitions
Confirmed Late-onset Sepsis
Confirmed late-onset sepsis (CLOS) was defined as a having a culture-positive bacterial infection of the blood or CSF on or after 72 hours of life for which the patient was treated with antibiotics for 5 or more days.3, 15 Episodes of positive bacterial cultures not meeting this definition and non-pathogenic bacteria typically considered contaminants were removed from further analysis.
Illness Severity Indices
Maximum Acuity Score
Acuity scores were based on level of care required by each patient, and consisted of type and level of ventilator assistance, presence or absence of central lines, need for and frequency of laboratory draws, and patient monitoring. Scores ranged from 2 to 4 with higher scores indicating greater level of resources. For each patient, the maximum acuity score throughout their stay was used in the analysis.
CRIB-II Score
The CRIB-II score (clinical risk index for babies) is an aggregation of clinical and laboratory data that is used to provide risk adjustment of mortality and neurologic dysfunction across institutions.16–18 It is a sum of scores for the combination of birth weight, gestational age, and gender; admission temperature; and base excess. Scores range from 0 to 27 with higher scores associated with higher mortality risk. For patients born after > 32 weeks gestation or whose birth weights were in excess of 3000 grams, only the temperature and base excess portions of the CRIB-II were used.19 CRIB-II scores were available for 1128 patients. Because this was a subset of patients, multivariate regressions were performed with and without this variable, which minimally affected the significance of the models.
Mean Colonization Pressure and Average Census
Colonization pressure is the ratio of MRSA positive patient-days to total patient-days, expressed as a percentage. The mean colonization pressure (MCP) is the arithmetic mean of this ratio over a patient’s hospitalization. The MCP was calculated for the entire unit and for the patient-specific bed configuration. Average census for the entire unit and the patient-specific bed configuration used the average census of the respective areas during the patient’s admission.
Statistical Analysis
The outcomes of time to MRSA colonization, CLOS, and combined CLOS or death were compared between patients in single-patient rooms and those in open-unit rooms. Kaplan-Meier curves yielding log-rank tests as well as univariate, bivariate, and multivariate Cox regressions were used to determine these time-dependent outcomes. Additionally, time-independent incidences of these endpoints were compared using Chi-square tests and Fisher’s exact tests, where appropriate. ANOVA was used to determine differences between bed configurations in CRIB-II scores, 5-minute Apgar scores, average daily census, MCP, and hand hygiene compliance upon room entry and room exit. Pearson Chi-square and log-rank tests with alpha values of 0.05 and two-sided tests were used for power calculations. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Demographics
The 1823 subjects representing 55,166 patient days were included in this analysis. Twenty-seven patients (1.5%) transferred between single-patient and open-unit layouts were excluded from further analysis. Patients in single-patient and open-unit rooms were similar in terms of birth weight, gestational age at birth, gender, race, insurance type, and illness severity based on CRIB-II score, 5-minute Apgar score, and maximum acuity score (Table 1).
TABLE 1.
Summary of Characteristics by Bed Configuration
| Single-patient configuration | Open-unit configuration | P-value | |
|---|---|---|---|
| Demographics | |||
| Birth Weight (grams), n (%) | |||
| < 500 | 2 (0) | 5 (1) | 0.48 |
| 500–749 | 55 (6) | 54 (6) | |
| 750–999 | 69 (8) | 64 (7) | |
| 1000–1249 | 60 (7) | 53 (6) | |
| 1250–1499 | 38 (4) | 44 (5) | |
| 1500–1749 | 36 (4) | 48 (5) | |
| 1750–1999 | 40 (4) | 43 (5) | |
| 2000–2499 | 94 (10) | 107 (12) | |
| 2500–4499 | 514 (56) | 459 (52) | |
| 4500 and higher | 4 (0) | 7 (1) | |
| Gestational Age (weeks), n (%) | |||
| < 24 | 20 (2) | 16 (2) | 0.54 |
| 24 | 27 (3) | 19 (2) | |
| 25–26 | 60 (7) | 62 (7) | |
| 27–28 | 62 (7) | 69 (8) | |
| 29–30 | 44 (5) | 57 (6) | |
| 31–32 | 63 (7) | 70 (8) | |
| 33–34 | 77 (8) | 84 (10) | |
| 35–36 | 105 (12) | 107 (12) | |
| 37–40 | 431 (47) | 383 (43) | |
| >40 | 23 (3) | 17 (2) | |
| Gender, n (%) | |||
| Female | 377 (41) | 379 (43) | 0.51 |
| Male | 535 (59) | 505 (57) | |
| Ethnicity, n (%) | |||
| African American | 234 (26) | 246 (28) | 0.58 |
| Caucasian | 629 (69) | 600 (68) | |
| Other | 49 (5) | 38 (4) | |
| Insurance, n (%) | |||
| Medicaid | 535 (59) | 509 (58) | 0.53 |
| Other insurance | 377 (41) | 375 (42) | |
| Clinical Characteristics | |||
| CRIB-II Score, median (IQR) | 3 (2,7) | 2 (1,7) | 0.09 |
| n=563 | n=565 | ||
| 5-minute Apgar Score, median (IQR) | 8 (6,9) | 8 (6,9) | 0.998 |
| Maximum Acuity Score, n (%) | |||
| 2 | 521 (57) | 542 (61) | 0.17 |
| 3 | 190 (21) | 159 (18) | |
| 4 | 201 (22) | 183 (21) | |
| Unit Characteristics | |||
| Patients, n (%) | 912 (51) | 884 (49) | 0.51 |
| Patient-Days, n (%) | 27,950 (52) | 26,200 (48) | <0.001 |
| Daily Census, median (IQR) | 32 (30,35) | 31 (26,35) | <0.001 |
| Mean Colonization Pressure, median (IQR) | 2.7% (0%, 3.7%) | 3.6% (1.2%, 6.9%) | <0.001 |
| Hand Hygiene Adherence | |||
| Room Entry, median (IQR) | 100.0% (96.8%, 100%) | 98.5% (96.9%, 100%) | 0.75 |
| Room Exit, median (IQR) | 100.0% (100%, 100%) | 100.0% (98.6%, 100%) | 0.052 |
IQR = interquartile range. p-values from Chi Square and ANOVA.
The median daily census over the study period was significantly greater in the single-patient rooms than open-unit rooms (32 vs. 31, ANOVA p<0.001). MCP was significantly smaller in the single-patient rooms (2.7% vs 3.6%, ANOVA p<0.001).
A median of 48 hand hygiene assessments upon room entry (interquartile range 37–60) and 53 hand hygiene assessments upon room exit (interquartile range 42–71) per bed configuration per month were available throughout the study period. Hand hygiene compliance upon room entry did not differ significantly between staff assigned to the different bed configurations. At room exit, hand hygiene compliance was slightly higher in single-patient rooms at Q1 (100% vs 98.6%) while median and Q3 were 100% for both groups (ANOVA p=0.052).
MRSA
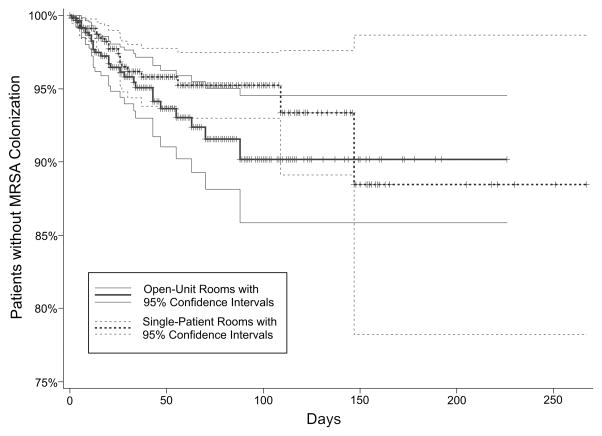
The incidence of MRSA colonization in single-patient and open-unit rooms was similar (2.1% vs. 3.3%, respectively, Chi-square p=0.11). Figure 1 demonstrates the similarity in MRSA-free survival over time between the two bed configurations. Univariate Cox regression showed no difference in MRSA colonization rates between bed configurations (Chi-square p=0.10); this similarity persisted when controlling for demographic (birth weight, gestational age, gender, race, insurance type), patient-driven (CRIB-II score, 5-minute Apgar score, and maximum acuity) and unit-driven (average census, MCP, and hand hygiene adherence) variables (Table 2). Average daily census was the only variable to interact significantly with bed configuration in the bivariate analyses. Within the subset of patients located in single-patient rooms, each additional one patient in the average census during their hospitalization correlated with 31% greater MRSA colonization rate (hazard ratio [HR] 1.31, 95% confidence interval 1.02–1.68, p=0.039). This correlation was not seen within the open-unit configuration. A Cox regression model for MRSA colonization using bed configuration and average census was not significant.
FIGURE 1.
Patients without methicillin-resistant Staphylococcus aureus (MRSA) colonization by bed configuration. Data are presented as a Kaplan-Meier plot, with patients censored at death or discharge (log-rank p=0.19).
TABLE 2.
Cox Proportional Hazards Model for MRSA Colonization
| Covariate(s) in model | Bed Configuration p-value1 |
Covariate p-value2 |
Interaction p-value3 |
|---|---|---|---|
| Bed Configuration | 0.10 | ------- | ------- |
| Bed Configuration + Gender | 0.11 | 0.08 | 0.63 |
| Bed Configuration + Ethnicity | 0.10 | 0.90 | 0.65 |
| Bed Configuration + Birth Weight | 0.12 | 0.79 | 0.95 |
| Bed Configuration + Gestational Age | 0.09 | 0.75 | 0.90 |
| Bed Configuration + Insurance | 0.10 | 0.83 | 0.83 |
| Bed Configuration + CRIB-II score | 0.31 | 0.55 | 0.68 |
| Bed Configuration + 5-minute Apgar | 0.10 | 0.21 | 0.51 |
| Bed Configuration + Acuity | 0.10 | 0.87 | 0.28 |
| Bed Configuration + Average census (side) | 0.11 | 0.90 | 0.024 |
| Bed Configuration + Average census (entire unit) | 0.11 | 0.84 | 0.14 |
| Bed Configuration + MCP (side) | 0.28 | 0.13 | 0.61 |
| Bed Configuration + MCP (entire unit) | 0.10 | 0.15 | 0.11 |
| Bed Configuration + Hand Hygiene (room entry) | 0.13 | 0.01 | 0.44 |
| Bed Configuration + Hand Hygiene (room exit) | 0.59 | 0.001 | 0.98 |
| Bed Configuration + all covariates | 0.84 | ------- | ------- |
CRIB = clinical risk index for babies. MCP = mean colonization pressure. Cox regression models for methicillin-resistant Staphylococcus aureus (MRSA) colonization using univariate, bivariate, and multivariate analyses of bed configuration.
Chi-square p-values are shown for bed configuration in Cox proportional hazard models.
Chi-square p-values are shown for listed covariates in bivariate Cox proportional hazard models.
Chi-square p-values are provided for bivariate model interactions, which model the effect on MRSA colonization when subgroups of one of the two covariates are selected.
The only variables to affect MRSA colonization involved hand hygiene. Lower rates of MRSA colonization of 16% (HR 0.834, 95% CI 0.731–0.951, p=0.0068) and 28% (HR 0.719, 95% CI 0.611–0.846, p<0.0001) were associated with 1% greater hand hygiene compliance on room entry or exit, respectively. This was independent of and similar across bed configurations.
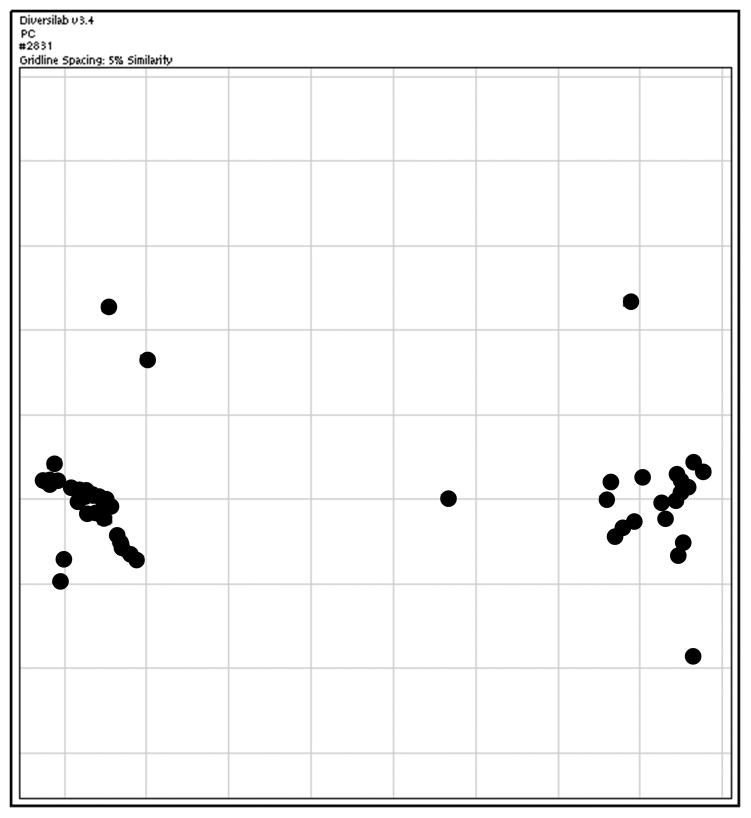
A two-dimensional scatter plot of the MRSA genotypes (Figure 2) demonstrated that 44 of the 51 isolates (86%) clustered into two distinct genotypes. There was no difference in MRSA genotypes between single-patient and open-unit rooms (two-tailed Fisher’s exact test p=0.63) (TABLE 3).
FIGURE 2.
Scatterplot of methicillin-resistant Staphylococcus aureus (MRSA) genotype similarity plotted by Diversilab version 3.4. Grid markings represent 5% genotypic difference. DNA banding patterns produced by repetitive sequence PCR (repPCR) are compared to construct this scatterplot, allowing visualization of genotype clustering within a set of isolates. Isolates with similar banding patterns cluster closer together than those with more divergent banding patterns.
TABLE 3.
MRSA Genotypes by Bed Configuration
| Genotype Group | Single-Patient (n = 19) | Open-Unit (n = 29) | Total |
|---|---|---|---|
| 1* | 10 | 14 | 24 |
| 2 | 6 | 11 | 17 |
| 3 | 1 | 0 | 1 |
| 4 | 0 | 1 | 1 |
| 5 | 1 | 0 | 1 |
| 6 | 0 | 2 | 2 |
| 7 | 1 | 1 | 2 |
MRSA = methicillin-resistant Staphylococcus aureus.
An additional 3 patients from genotype group 1 spent time in both bed configurations and were excluded from the analysis. Fisher’s exact test p=0.63.
Late-onset Sepsis
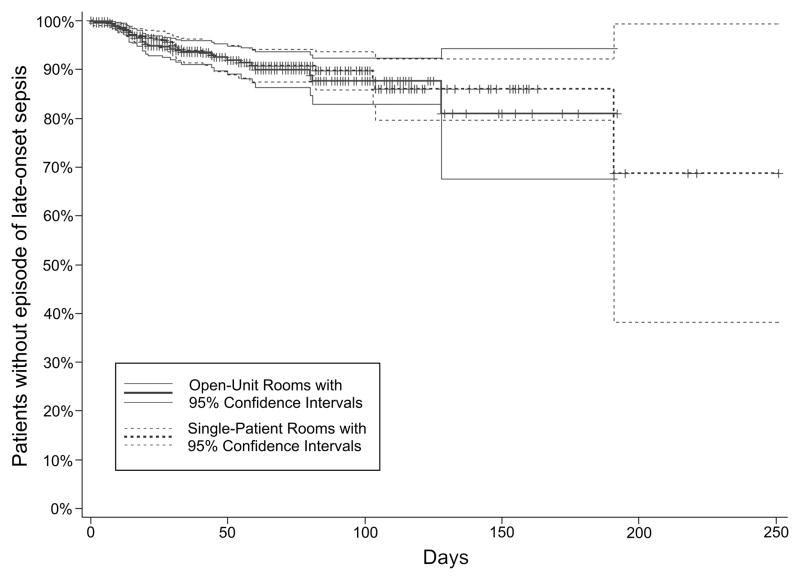
The rates of CLOS in single-patient and open-unit rooms were similar (3.9% vs. 4.1%, respectively, Chi-Square p=0.89). Coagulase-negative Staphylococcus species was the most frequent pathogen recovered from cultures in both configurations at 43% of all positive cultures. Methicillin-susceptible Staphylococcus aureus and Streptococcus agalactiae (group B streptococci (GBS)) were the next most frequent gram-positive (11% and 8%, respectively) and Escherichia coli was the most frequent gram-negative (10%) pathogens. Overall, 56 gram-positive and 16 gram-negative bacterial pathogens were isolated from blood and/or CSF (Supplemental Table 1). Of the 72 patients with CLOS, 6 had more than one episode. With few patients having multiple episodes and concerns that an earlier course of sepsis and antibiotics might influence a second episode of CLOS, only the first episode was entered into the analysis. A Kaplan-Meier plot (FIGURE 3) demonstrates similarity of survival when controlling only for bed configuration (log-rank p=0.78). This similarity holds in the univariate, bivariate, and multivariate Cox regression models when controlling for demographic, patient-driven, and unit-driven variables (TABLE 4). Lower birth weight and greater CRIB-II scores and acuity scores correlated with higher rates of CLOS, but only independently of bed configuration.
FIGURE 3.
Patients without late-onset sepsis by bed configuration. Data are presented as a Kaplan-Meier plot, with patients censored at death or discharge (log-rank p=0.78).
TABLE 4.
Cox Proportional Hazards Model for Late-onset Sepsis and Combined Outcome of Late-onset sepsis or Death
| Covariate(s) in model | Late-onset sepsis
|
Combined late-onset sepsis or death
|
||||
|---|---|---|---|---|---|---|
| Bed Configuration p-value1 |
Covariate p-value2 |
Interaction p-value3 |
Bed Configuration p-value1 |
Covariate p-value2 |
Interaction p-value3 |
|
| Bed Configuration | 0.78 | ------- | ------- | 0.76 | ------- | ------- |
| Bed Configuration + Gender | 0.77 | 0.78 | 0.53 | 0.79 | 0.02 | 0.88 |
| Bed Configuration + Ethnicity | 0.87 | 0.87 | 1.00 | 0.76 | 0.92 | 0.37 |
| Bed Configuration + Birth Weight | 0.62 | 0.04 | 0.52 | 0.98 | <0.0001 | 0.05 |
| Bed Configuration + Gestational Age | 0.61 | 0.06 | 0.24 | 0.93 | <0.0001 | 0.32 |
| Bed Configuration + Insurance | 0.78 | 1.00 | 0.99 | 0.77 | 0.60 | 0.99 |
| Bed Configuration + CRIB-II score | 0.17 | 0.0007 | 0.22 | 0.50 | <0.0001 | 0.11 |
| Bed Configuration + 5-minute Apgar | 0.65 | 0.13 | 0.39 | 0.96 | <0.0001 | 0.56 |
| Bed Configuration + Acuity | 0.57 | 0.005 | 0.12 | 0.64 | <0.0001 | 0.07 |
| Bed Configuration + Average census (side) | 0.69 | 0.32 | 0.80 | 0.76 | 0.98 | 0.94 |
| Bed Configuration + Average census (unit) | 0.85 | 0.15 | 0.93 | 0.78 | 0.68 | 0.44 |
| Bed Configuration + Hand Hygiene (room entry) | 0.82 | 0.41 | 0.52 | 0.68 | 0.13 | 0.98 |
| Bed Configuration + Hand Hygiene (room exit) | 1.00 | 0.41 | 0.96 | 0.80 | 0.91 | 0.90 |
| Bed Configuration + all covariates | 0.58 | ------- | ------- | 0.13 | ------- | ------- |
CRIB = clinical risk index for babies. MCP = mean colonization pressure. Cox regression models for late-onset sepsis and the combined outcome of late-onset sepsis or death using univariate, bivariate, and multivariate analyses of bed configuration.
Chi-square p-values are shown for bed configuration in Cox proportional hazard models.
Chi-square p-values are shown for listed covariates in bivariate Cox proportional hazard models.
Chi-square p-values are provided for bivariate model interactions, which model the effect on outcomes when subgroups of one of the two covariates are selected.
Late-onset Sepsis or Death
To ensure that death did not mask differences in rates of sepsis between bed configurations, a combined outcome of CLOS or death was used. The combined outcome was similar in single-patient and open-unit rooms (11.6% vs. 10.8%, respectively, Chi-square p=0.56). This similarity also existed in the univariate, bivariate, and multivariate Cox regression models (TABLE 4). Lower birth weight, early gestational age, male gender, higher acuity and CRIB-II score, and lower 5-minute Apgar score correlated with greater rates of CLOS or death.
DISCUSSION
This study was the first attempt to examine the association between bed configuration within an NICU and rates of MRSA colonization and CLOS using the same study period for both groups, while accounting for illness severity. Vietri, et al, reported similarity in MRSA colonization rates between bed configurations before and after conversion of an adult ICU from an open-unit configuration to single- or double-patient rooms.20 Domanico, et al, also compared intervals and found lower MRSA colonization rates in a NICU when converting from open-unit configuration to single-patient rooms.9 The contemporaneous comparison of the two bed configurations in our study minimized potential bias from changing clinical practices or practitioners.
In this analysis, single-patient rooms were not associated with reduced MRSA colonization, CLOS or the combined outcome of CLOS or death when compared with open-unit beds. The overall incidence of MRSA colonization in this study (2.7%) was similar to the 1.1–5.6% colonization rates reported by other NICUs in the U.S. 21–23 but less than 20–41% rates in Taiwan and Japan.24, 25 Colonization pressure was significantly higher in the open-unit configuration. Although this and insurance provider have been shown to be risk factors for MRSA colonization in and out of the NICU,26, 27 only the average daily census was positively associated with increasing MRSA colonization, and only in the single-patient configuration (Table 2). The only variable to significantly impact MRSA colonization rates was hand hygiene compliance upon room entry and exit; bed configuration, however, did not contribute to these models.
Rates of CLOS and the combined outcome of CLOS or death also did not differ between bed configurations. During the study, the overall rate of CLOS and the combined rate of CLOS or death were 4.0% and 11.2%, respectively. Of note, this study was performed in an era of focused efforts to decease central-line associated blood stream infections.
The large sample size of this study and the contemporaneous housing provide the power to detect clinically significant hazard ratios for each of our outcomes. The retrospective design of this study offers perspective into the clinical impact of our results while controlling for secular changes. For all three of the outcomes, our sample size had >80% power to detect a hazard ratio of 1.25, i.e. a difference of 25% in the outcomes between bed configurations would have been detected. While an argument can be made that any reduction in the outcomes we selected (death, sepsis) might be considered to be worth an investment in single-patient rooms, proving that room configuration plays a role in averting undesirable outcomes would require an enormous study population. For instance, a 10% reduction in MRSA colonization rates, which for this study population equates to an absolute risk reduction of 0.4% or 1 of 250 babies per month, would only be detected at 80% power with 7984 patients studied throughout their NICU stay.
Single-patient rooms might fail to prevent MRSA colonization and CLOS for several reasons. With single-patient rooms spread further apart in space, horizontal spread of infections would seem to decrease. If, however, hand-hygiene practices are universally followed and fomites carried by healthcare workers are limited, this may reduce the effect of spreading patients out geographically. Over the course of this study, there was high hand hygiene compliance, which may have diminished the impact of bed configuration, limiting generalizability for units in which hand hygiene compliance is low. In addition, non-horizontal spread of pathogens may contribute sufficient amounts of infectious “noise” to the data, decreasing the ability to detect the horizontal spread of infectious material. Such non-horizontal methods include vertical transmission at birth and contact with visitors including parents and family, which would not be expected to differ based on room configuration.
Our study indicates that high census periods are positively correlated with greater MRSA colonization only in single-patient room configurations. While bed occupancy rates have been shown to correlate positively with MRSA colonization,28 the mechanisms leading to this configuration-specific result remain to be determined.
This study has several limitations. First, the data were collected retrospectively. A large prospective randomized study however of this nature would be prohibitive given the constraints of patient staffing and physical space within an NICU environment. Second, while bed configuration might reduce horizontal spread of pathogens, such layouts might not mitigate other mechanisms of transfer, such as visitor-to-patient transmission or inter-host transfer29 which were not addressed. In a companion project conducted at the same time as this study, stools of three infants in open-unit rooms in the vicinity of two infants with GBS sepsis, and one infant in the vicinity of an infant with Serratia marcescens sepsis, contained the infecting strain, suggesting that inter-host spread is better detected by focusing on colonization than on culture-proven sepsis.29 Finally, our study was not designed to address culture negative sepsis. Isolation of bona fide bloodstream pathogens is a challenge in NICUs, because the volume of blood submitted for culture might be inadequate to confirm an etiologic agent.30 Identifying such cases in retrospect is difficult, and hence, we might have underestimated actionable events that occurred in either or both of the bed configurations. Using a broader definition than CLOS, however, could have overestimated sepsis events.
Considerations beyond infection have been put forth regarding choice of room configuration. Some studies demonstrate that single-patient rooms modestly improve breast feeding initiation, are quieter, and have better air quality,9 while others have highlighted the larger space requirement,31 resulting in larger NICUs, greater construction costs 32, 33 and longer distances travelled responding to emergencies, as well as issues with communication, patient monitoring,31, 34 and nurse isolation.35 While parent satisfaction scores, visitation rates, and noise levels favor single-patient rooms,31, 35–37 parental stress and neonatal language and motor development favor open units.38, 39
In conclusion, single-patient rooms did not provide protection against MRSA colonization, CLOS, and the combined outcome of CLOS or death in a NICU environment. While single-patient rooms may have other benefits, neonates in this bed configuration were as likely as those in open-units to acquire these infections and the morbidities and mortality that come with them. In this analysis, average census positively correlated with MRSA colonization only within the single-patient room configuration. Increased vigilance is required during periods of high census, with particular attention paid to hand hygiene, the only variable that affected MRSA colonization. Further studies are warranted to assess how facility design might reduce the burden of sepsis and MRSA colonization in this high-risk population.
Supplementary Material
Acknowledgments
This study was supported by NIH grant, UH3 AI083265. All authors have no financial relationships to disclose. All authors have no conflicts of interest to disclose. We thank Dr. Alexis Elward and the Infection Control Committee at St. Louis Children’s Hospital for their expertise, MRSA colonization data, and hand hygiene compliance data, Rachel Frobel for the training and technical expertise in repPCR, and the St. Louis Children’s Hospital Office of Planning and Business Development for the NICU billing and coding data used in this study. Finally, none of this would be possible without the cooperation of the staff of the NICU and the families of our patients at St. Louis Children’s Hospital.
References
- 1.Kochanek KDXJ, Murphy SL, et al. Deaths: Final Data for 2009. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2012;60(3):1–117. [PubMed] [Google Scholar]
- 2.Heron M. Deaths: leading causes for 2008. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2012;60(6):1–94. [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA : the journal of the American Medical Association. 2004;292(19):2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 4.Donovan EF, Sparling K, Lake MR, Narendran V, Schibler K, Haberman B, et al. The investment case for preventing NICU-associated infections. American journal of perinatology. 2013;30(3):179–84. doi: 10.1055/s-0032-1322516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lankford MG, Zembower TR, Trick WE, Hacek DM, Noskin GA, Peterson LR. Influence of role models and hospital design on hand hygiene of healthcare workers. Emerging infectious diseases. 2003;9(2):217–23. doi: 10.3201/eid0902.020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulrich R, Quan X, Zimring C, Joseph A, Choudhary R. The role of the Physical Environment In the Hospital of the 21st Century: A Once-in-a-Lifetime Opportunity. Concord, California: The Center for Health Design; 2004. [Google Scholar]
- 7.Guidelines for Design and Construction of Health Care Facilities. Washington, D.C: The American Institute of Architects; 2006. [Google Scholar]
- 8.Joseph A. The Impact of the Environment on Infections in Healthcare Facilities. The Center for Health Design; 2006. Jul, [Google Scholar]
- 9.Domanico R, Davis DK, Coleman F, Davis BO. Documenting the NICU design dilemma: comparative patient progress in open-ward and single family room units. Journal of perinatology : official journal of the California Perinatal Association. 2011;31(4):281–8. doi: 10.1038/jp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institutes of Health - Eunice Kennedy Shriver National Institute of Child Health and Human Development: Neonatal Research Network. cited Sept 20 2014; Available from: https://www.nichd.nih.gov/research/supported/Pages/nrn.aspx.
- 11.Washington University in St Louis Center for Biomedical Informatics. Clinical Investigation Data Exploration Repository. 2015. timestamp: 9/25/2014. doi:107936/B6CR0000. [Google Scholar]
- 12.Church DL, Chow BL, Lloyd T, Gregson DB. Comparison of automated repetitive-sequence-based polymerase chain reaction and spa typing versus pulsed-field gel electrophoresis for molecular typing of methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2011;69(1):30–7. doi: 10.1016/j.diagmicrobio.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Kang HP, Dunne WM. Stability of repetitive-sequence PCR patterns with respect to culture age and subculture frequency. Journal of clinical microbiology. 2003;41(6):2694–6. doi: 10.1128/JCM.41.6.2694-2696.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.bioMérieux. DiversiLab®: Healthcare Software. cited 06/11/2013; Available from: http://www.biomerieux-usa.com/servlet/srt/bio/usa/dynPage?open=USA_PRD_LST&doc=USA_PRD_LST_G_PRD_USA_9&pubparams.sform=3&lang=en.
- 15.Shane AL, Hansen NI, Stoll BJ, Bell EF, Sanchez PJ, Shankaran S, et al. Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics. 2012;129(4):e914–22. doi: 10.1542/peds.2011-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagliardi L, Cavazza A, Brunelli A, Battaglioli M, Merazzi D, Tandoi F, et al. Assessing mortality risk in very low birthweight infants: a comparison of CRIB, CRIB-II, and SNAPPE-II. Archives of disease in childhood Fetal and neonatal edition. 2004;89(5):F419–22. doi: 10.1136/adc.2003.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodha A, Sauve R, Chen S, Tang S, Christianson H. Clinical Risk Index for Babies score for the prediction of neurodevelopmental outcomes at 3 years of age in infants of very low birthweight. Developmental medicine and child neurology. 2009;51(11):895–900. doi: 10.1111/j.1469-8749.2009.03284.x. [DOI] [PubMed] [Google Scholar]
- 18.Parry G, Tucker J, Tarnow-Mordi W. CRIB II: an update of the clinical risk index for babies score. The Lancet. 2003;361(9371):1789–91. doi: 10.1016/S0140-6736(03)13397-1. [DOI] [PubMed] [Google Scholar]
- 19.Filippi L, la Marca G, Cavallaro G, Fiorini P, Favelli F, Malvagia S, et al. Phenobarbital for neonatal seizures in hypoxic ischemic encephalopathy: A pharmacokinetic study during whole body hypothermia. Epilepsia. 2011;52(4):794–801. doi: 10.1111/j.1528-1167.2011.02978.x. [DOI] [PubMed] [Google Scholar]
- 20.Vietri NJ, Dooley DP, Davis CE, Jr, Longfield JN, Meier PA, Whelen AC. The effect of moving to a new hospital facility on the prevalence of methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2004;32(5):262–7. doi: 10.1016/j.ajic.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Gregory ML, Eichenwald EC, Puopolo KM. Seven-year experience with a surveillance program to reduce methicillin-resistant Staphylococcus aureus colonization in a neonatal intensive care unit. Pediatrics. 2009;123(5):e790–6. doi: 10.1542/peds.2008-1526. [DOI] [PubMed] [Google Scholar]
- 22.Seybold U, Halvosa JS, White N, Voris V, Ray SM, Blumberg HM. Emergence of and risk factors for methicillin-resistant Staphylococcus aureus of community origin in intensive care nurseries. Pediatrics. 2008;122(5):1039–46. doi: 10.1542/peds.2007-3161. [DOI] [PubMed] [Google Scholar]
- 23.Song X, Perencevich E, Campos J, Short BL, Singh N. Clinical and economic impact of methicillin-resistant Staphylococcus aureus colonization or infection on neonates in intensive care units. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2010;31(2):177–82. doi: 10.1086/649797. [DOI] [PubMed] [Google Scholar]
- 24.Huang YC, Chou YH, Su LH, Lien RI, Lin TY. Methicillin-resistant Staphylococcus aureus colonization and its association with infection among infants hospitalized in neonatal intensive care units. Pediatrics. 2006;118(2):469–74. doi: 10.1542/peds.2006-0254. [DOI] [PubMed] [Google Scholar]
- 25.Sakaki H, Nishioka M, Kanda K, Takahashi Y. An investigation of the risk factors for infection with methicillin-resistant Staphylococcus aureus among patients in a neonatal intensive care unit. Am J Infect Control. 2009;37(7):580–6. doi: 10.1016/j.ajic.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Fritz SA, Garbutt J, Elward A, Shannon W, Storch GA. Prevalence of and risk factors for community-acquired methicillin-resistant and methicillin-sensitive staphylococcus aureus colonization in children seen in a practice-based research network. Pediatrics. 2008;121(6):1090–8. doi: 10.1542/peds.2007-2104. [DOI] [PubMed] [Google Scholar]
- 27.Geraci DM, Giuffre M, Bonura C, Matranga D, Aleo A, Saporito L, et al. Methicillin-resistant Staphylococcus aureus colonization: a three-year prospective study in a neonatal intensive care unit in Italy. PloS one. 2014;9(2):e87760. doi: 10.1371/journal.pone.0087760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaier K, Meyer E, Dettenkofer M, Frank U. Epidemiology meets econometrics: using time-series analysis to observe the impact of bed occupancy rates on the spread of multidrug-resistant bacteria. The Journal of hospital infection. 2010;76(2):108–13. doi: 10.1016/j.jhin.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Carl MA, Ndao IM, Springman AC, Manning SD, Johnson JR, Johnston BD, et al. Sepsis From the Gut: The Enteric Habitat of Bacteria That Cause Late-Onset Neonatal Bloodstream Infections. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piantino JH, Schreiber MD, Alexander K, Hageman J. Culture Negative Sepsis and Systemic Inflammatory Response Syndrome in Neonates. Neoreviews. 2013;14(6):e294–e305. [Google Scholar]
- 31.Shepley M, Harris D, White R, Steinberg F. Family behavior in a single-family room NICU. Washington, DC: The American Institute of Architects; 2006. [Google Scholar]
- 32.Chaudhury H, Mahmood A, Valente M. Coalition of Healthcare Environment Research. The use of single patient rooms versus multiple occupancy rooms in acute care environments. 2003 cited 12/03/2012; Available from: http://www.premierinc.com/quality-safety/tools-services/safety/topics/construction.
- 33.Bartley JM, Olmsted RN, Haas J. Current views of health care design and construction: practical implications for safer, cleaner environments. Am J Infect Control. 2010;38(5 Suppl 1):S1–12. doi: 10.1016/j.ajic.2010.04.195. [DOI] [PubMed] [Google Scholar]
- 34.Joseph A, Rashid M. The architecture of safety: hospital design. Current opinion in critical care. 2007;13(6):714–9. doi: 10.1097/MCC.0b013e3282f1be6e. [DOI] [PubMed] [Google Scholar]
- 35.Lester BM, Miller RJ, Hawes K, Salisbury A, Bigsby R, Sullivan MC, et al. Infant neurobehavioral development. Seminars in perinatology. 2011;35(1):8–19. doi: 10.1053/j.semperi.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White RD. Individual rooms in the NICU - an evolving concept. Journal of perinatology : official journal of the California Perinatal Association. 2003;23(Suppl 1):S22–4. doi: 10.1038/sj.jp.7210840. [DOI] [PubMed] [Google Scholar]
- 37.Lester BM, Hawes K, Abar B, Sullivan M, Miller R, Bigsby R, et al. Single-Family Room Care and Neurobehavioral and Medical Outcomes in Preterm Infants. Pediatrics. 2014 doi: 10.1542/peds.2013-4252. [DOI] [PubMed] [Google Scholar]
- 38.Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, et al. Alterations in Brain Structure and Neurodevelopmental Outcome in Preterm Infants Hospitalized in Different Neonatal Intensive Care Unit Environments. The Journal of pediatrics. 2013 doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pineda RG, Stransky KE, Rogers C, Duncan MH, Smith GC, Neil J, et al. The single-patient room in the NICU: maternal and family effects. Journal of perinatology : official journal of the California Perinatal Association. 2012;32(7):545–51. doi: 10.1038/jp.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.