Abstract
Background
Advancing age is associated with a greater prevalence of coronary artery disease in heart failure (HF) with reduced ejection fraction and with a higher risk of complications following coronary artery bypass grafting (CABG). Whether the efficacy of CABG compared with medical therapy (MED) in patients with HF due to ischemic cardiomyopathy is the same in patients of different age is unknown.
Methods
1212 patients (median follow up 9.8 years) with ejection fraction ≤35% and coronary disease amenable to CABG were randomized to CABG or MED in the STICH trial.
Results
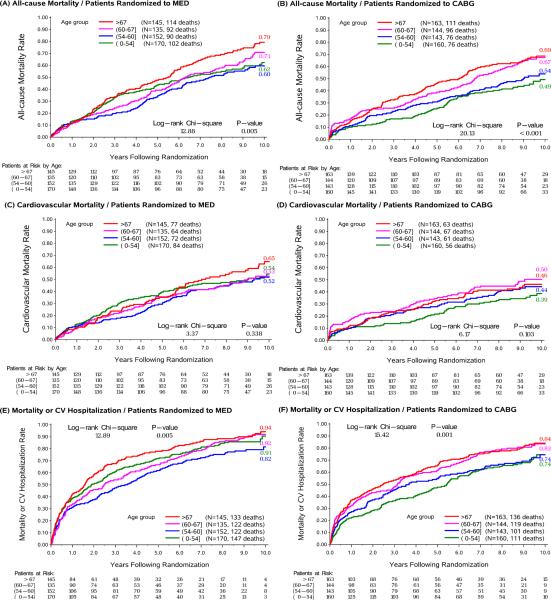
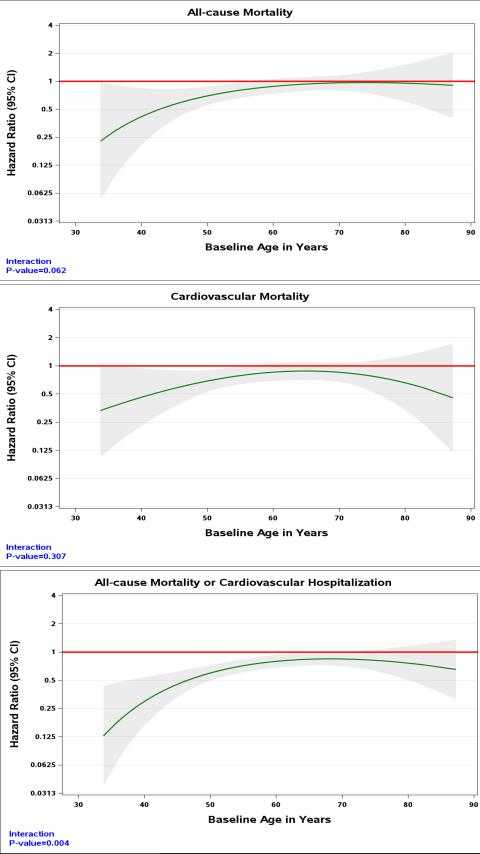
Mean age at trial entry was 60 years; 12% women; 36% non-white; baseline EF 28%. For the present analyses, patients were categorized by age quartiles: Q1 ≤54 years, Q2 >54 and ≤60 years, Q3 >60 and ≤67 years, Q4 >67 years. Older vs. younger patients had more comorbidities. All-cause mortality was higher in older compared with younger patients assigned to MED (79 vs. 60% for Q4 and Q1, respectively; log-rank p=0.005) and CABG (68 vs. 48% for Q4 and Q1, respectively; log-rank p<0.001). In contrast, CV mortality was not statistically significantly different across the spectrum of age in the MED group (53 vs. 49% for Q4 and Q1, respectively; log-rank p=0.388) or CABG group (39 vs 35% for Q4 and Q1, respectively; log-rank p=0.103). CV deaths accounted for a greater proportion of deaths in the youngest vs oldest quartile (79% vs 62%). The effect of CABG vs MED on all-cause mortality tended to diminish with increasing age (pinteraction=0.062), while the benefit of CABG on CV mortality was consistent over all ages (pinteraction =0.307). There was a greater reduction in all-cause mortality or CV hospitalization with CABG versus MED in younger compared with older patients (pinteraction = 0.004). In the CABG group, cardiopulmonary bypass time or days in intensive care did not differ for older vs. younger patients.
Conclusions
CABG added to MED has a more substantial benefit on all-cause mortality and all-cause mortality and CV hospitalization in younger compared to older patients. CABG added to MED has a consistent beneficial effect on CV mortality regardless of age.
Keywords: heart failure, ischemic cardiomyopathy, coronary artery bypass grafting, age
Introduction
Older patients with heart failure (HF) more commonly have coronary artery disease (CAD) as the cause of their HF than younger patients.1 With improving survival, the prevalence of patients living with both ischemic heart disease and HF who potentially require coronary revascularization has risen.2 Management of these patients is difficult; many have angina, evidence of ischemia or myocardial viability and are considered for coronary revascularization. As there have been no randomized trials of coronary percutaneous intervention in populations with HF, the benefits or harms of this approach are unknown. However, results from the Surgical Treatment for Ischemic Heart Failure (STICH) trial (including the extended follow up study)3,4 demonstrated improved clinical outcomes following Coronary Artery Bypass Grafting (CABG); over a median of 9.8 years, the risk of all-cause death, death from cardiovascular causes, and all cause death or hospitalization from cardiovascular causes was significantly lower in those randomized to receive CABG and guideline-directed medical therapy compared with medical therapy alone.4
Increasing age is associated with worse short and long-term outcomes following CABG in general populations of patients with CAD.5,6 As increasing age is associated with higher mortality in patients with HF 7, clinicians may be reluctant to recommend older patients for revascularization with CABG due to uncertainty of its benefits. We examined the effect of CABG and guideline-directed medical therapy compared with guideline-directed medical therapy alone according to age in the STICH trial.
Methods
The STICH trial 3 (ClinicalTrials.gov number, NCT00023595) and extended follow-up 4 have been described in detail previously. The median follow-up time was 9.8 years (interquartile range, 9.1 to 11.0 years). Patients ≥18 years old with CAD that was amenable to treatment with CABG and an ejection fraction of 35% or less, as determined at each enrolling site (measured by CMR ventriculogram, gated SPECT ventriculogram, echocardiography or contrast ventriculogram within 3 months of trial entry) were enrolled. Patients were randomized to CABG with guideline-directed medical therapy versus medical therapy alone. Trial sites were prompted by the STICH team to implement guideline recommended optimal medical therapy in both randomized arms. Patients were eligible for randomization only if they did not have a coronary stenosis of ≥50% of the diameter of the left main coronary artery and if they did not have Canadian Cardiovascular Society class III or IV angina while receiving medical therapy. The extended follow-up study was a pre-specified extension of the STICH trial with follow-up extended an additional 5 years. The study complied with the Declaration of Helsinki, and the locally appointed ethics committee approved the research protocol. Informed consent was obtained from the subjects or their legally authorized representative.
Outcomes
The primary outcome was all cause death and the 2 key secondary outcomes were cardiovascular (CV) death and a composite of all-cause death or CV hospitalizations. All deaths were classified by a blinded clinical events committee according to pre-specified criteria.
Statistical Analysis
The randomized population was divided according to age into quartiles: Q1 ≤54 years, Q2 >54 and ≤60 years, Q3 >60 and ≤67 years, Q4 >67 years. Baseline characteristics are presented by quartile of age. Continuous variables are presented as medians with 25th and 75th percentiles and categorical variables as counts with percentages. The distribution of continuous variables was tested using the Jonckheere-Terpstra trend test (Spearman correlation p values are presented in the Supplemental Materials) and categorical variables using the Cochran-Armitage trend test. Kaplan-Meier rates were computed for each age group by randomized treatment.8 The relationship between age as a continuous variable and outcomes was examined and graphed using the mfpi command in Stata as a fractional polynomial.9,10 The effect of randomized therapy (CABG with guideline-directed medical therapy versus medical therapy alone) by age was examined in a Cox proportional hazards model with an interaction term of randomized therapy and age as a continuous variable. All models were unadjusted and analyses conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata version 14 (College Station, TX, USA) with p<0.05 considered statistically significant.
Results
Baseline characteristics by age
The 1212 patients were split into 4 quartiles. Patients in the oldest quartile (age >67 years) tended to be more often women and of white race (Table 1 and Supplemental Material). Older patients had a higher prevalence of comorbidities, except for hyperlipidemia and depression. The proportion of patients with no or CCS class I angina was highest in the oldest age group. Older patients had a shorter 6-minute walk test distance. Systolic blood pressure was higher and heart rate was lower in the older group. Hemoglobin was lower and kidney function was worse in the older age groups. Within the oldest quartile, 75 (6%) were over the age of 75 years and 15 (1%) over the age of 80 years (Supplemental Material)
Table 1.
Baseline Characteristics by Age
| Variable | Baseline Age Quartiles | P-value for trend | |||
|---|---|---|---|---|---|
| Q1 (Age≤54 years) (n=330) | Q2 (54<Age≤60 years) (n=295) | Q3 (60<Age≤67 years) (n=279) | Q4 (Age>67 years) (n=308) | ||
| Age (year) | 50(47,53) | 57(56,58) | 64(62, 65) | 72(69,75) | |
| Women | 35(11%) | 26(9%) | 37(13%) | 50(16%) | 0.011 |
| White race | 187(57%) | 189(64%) | 200(72%) | 251(82%) | <0.001 |
| BMI (kg/m2) | 27(24,31) | 27(24,30) | 27(24,30) | 26(24,29) | 0.180 |
| Medical History: | |||||
| Diabetes | 103(31%) | 121(41%) | 124(44%) | 130(42%) | 0.003 |
| Hypertension | 178(54%) | 177(60%) | 159(57%) | 214(70%) | <0.001 |
| PVD | 36(11%) | 40(14%) | 42(15%) | 66(21%) | <0.001 |
| Renal insufficiency | 10(3%) | 16(5%) | 25(9%) | 43(14%) | <0.001 |
| Stroke | 23(7%) | 14(5%) | 21(8%) | 34(11%) | 0.028 |
| Atrial flutter/fibrillation | 19(6%) | 25(9%) | 42(15%) | 67(22%) | <0.001 |
| Previous MI | 250(76%) | 229(78%) | 208(75%) | 247(80%) | 0.320 |
| Hyperlipidemia | 190(58%) | 174(59%) | 181(65%) | 185(60%) | 0.286 |
| Depression | 24(7%) | 17(6%) | 15(5%) | 20(7%) | 0.646 |
| Current smoker | 104(32%) | 64(22%) | 50(18%) | 34(11%) | <0.001 |
| Previous PCI | 45(14%) | 38(13%) | 38(14%) | 35(11%) | 0.465 |
| Previous CABG | 8(2%) | 10(3%) | 11(4%) | 7(2%) | 0.974 |
| CCS angina class: | |||||
| No angina | 106(32%) | 97(33%) | 91(33%) | 148(48%) | <0.001 |
| I | 42(13%) | 44(15%) | 52(19%) | 49(16%) | 0.145 |
| II | 169(51%) | 141(48%) | 119(43%) | 96(31%) | <0.001 |
| III | 10(3%) | 12(4%) | 15(5%) | 11(4%) | 0.551 |
| IV | 3(1%) | 1(<1%) | 2(1%) | 4(1%) | 0.583 |
| NYHA class: | |||||
| I | 35(11%) | 50(17%) | 22(8%) | 32(10%) | 0.276 |
| II | 185(56%) | 134(45%) | 157(56%) | 150(49%) | 0.318 |
| III | 100(30%) | 106(36%) | 93 7(33%) | 113(37%) | 0.152 |
| IV | 10(3%) | 5(2%) | 7(3%) | 13(4%) | 0.315 |
| Median systolic BP (mmHg) | 120(110,130) | 120(110,130) | 120(110,130) | 122(110,136) | <0.001 |
| Median heart rate (bpm) | 76(68,84) | 75(68,82) | 74(66,82) | 71(63,80) | <0.001 |
| Median 6 minute walk distance (meter) | 352(259,434) | 360(273,415) | 340(270,400) | 321(250,385) | <0.001 |
| Lab measures: | |||||
| Hemoglobin (g/dL) | 14.3(13.2,15.4) | 13.9(12.7,14.9) | 13.7(12.6,14.8) | 13.6(12.3,14.6) | <0.001 |
| Creatinine (mg/dL) | 1.02(0.90,1.18) | 1.10(0.97,1.23) | 1.10(0.94,1.30) | 1.17(1.00,1.40) | <0.001 |
| Sodium (mEq/L) | 139(137,142) | 140 (137, 142) | 140(138,142) | 140(137,142) | 0.143 |
| BUN (mg/dL) | 22(15,37) | 21 (16, 34) | 21(16,36) | 24(18,39) | 0.031 |
Baseline medical and device therapy were similar across ages (Table 2) except for greater use of warfarin (due to more atrial fibrillation) and loop or thiazide diuretics in older patients. The proportion on guideline directed medical therapy fell in the older compared to younger patients over time (Supplemental Material). In each age quartile there was no difference in medical therapies in the CABG compared to medical therapy group (Supplemental Material).
Table 2.
Baseline Medical and Device Therapies by Age
| Variable N(%) | Baseline Age Quartiles | P-value for Trend | |||
|---|---|---|---|---|---|
| Q1 (Age≤54 years) (n=330) | Q2 (54<Age≤60 years) (n=295) | Q3 (60<Age≤67 years) (n=279) | Q4 (Age>67 years) (n=308) | ||
| Beta-blocker | 282(86%) | 247(84%) | 250(90%) | 257(83%) | 0.946 |
| ACE inhibitor | 267(81%) | 248(84%) | 233(84%) | 248(81%) | 0.879 |
| ARB | 27(8%) | 23(8%) | 23(8%) | 42(14%) | 0.023 |
| ACE or ARB | 288(87%) | 263(89%) | 252(90%) | 282(92%) | 0.068 |
| Statin | 271(82%) | 242(82%) | 230(82%) | 240(78%) | 0.216 |
| Digoxin | 68(21%) | 62(21%) | 55(20%) | 60(20%) | 0.651 |
| Aspirin | 273(83%) | 250(85%) | 232(83%) | 247(80%) | 0.348 |
| Warfarin | 25(8%) | 23(8%) | 35(13%) | 44(14%) | 0.001 |
| Clopidogrel | 57(17%) | 57(19%) | 47(17%) | 47(15%) | 0.387 |
| Diuretic | |||||
| Loop/thiazide | 200(61%) | 190(64%) | 184(66%) | 217(71%) | 0.008 |
| Potassium-sparing | 161(49%) | 137(46%) | 136(49%) | 122(40%) | 0.042 |
| Loop/thiazide or potassium sparing | 233(71%) | 222(75%) | 216(77%) | 241(78%) | 0.020 |
| Nitrate | 166(50%) | 154(52%) | 162(58%) | 164(53%) | 0.232 |
| Insulin | 42(13%) | 54(18%) | 49(18%) | 52(17%) | 0.191 |
| Oral antihyperglycemic agent | 62(19%) | 70(24%) | 84(30%) | 70(23%) | 0.089 |
| Antidepressant | 16(5%) | 17(6%) | 17(6%) | 15(5%) | 0.938 |
| Cardiac resynchronization therapy | 3(1%) | 0(0%) | 1(<1%) | 3(1%) | 0.871 |
| Pacemaker | 3(1%) | 3(1%) | 4(1%) | 8(3%) | 0.073 |
| ICD | 11(3%) | 6(2%) | 8(3%) | 4(1%) | 0.161 |
ACE – angiotensin converting enzyme inhibitor, ARB – angiotensin receptor blocker, ICD - implantable cardioverter defibrillator
Echocardiographic measures and coronary anatomy according to age
Left ventricular ejection fraction was similar over the age range, although end diastolic volume indexed to body surface area was lower in the oldest age group (Table 3). The E wave velocity and E/A ratio were lower in the older group than younger groups but there were no significant differences in the E/e’ ratio. The presence and severity of mitral regurgitation did not vary significantly. Older patients had more vessels with a coronary stenosis but less proximal left anterior descending artery stenosis. The Duke coronary artery disease severity index increased with age.
Table 3.
Baseline Left Ventricular Structure and Function and Coronary Anatomy by Age
| Variable | Baseline Age Quartiles | P-value for Trend | |||
|---|---|---|---|---|---|
| Q1 (Age≤54 years) (n=330) | Q2 (54<Age≤60 years) (n=295) | Q3 (60<Age≤67 years) (n=279) | Q4 (Age>67 years) (n=308) | ||
| Structure and function: | |||||
| LVEF (%) | 28(22,33) | 28(23,35) | 26(21,33) | 28(22,34) | 0.496 |
| ESVI | 81(62,103) | 81(61,98) | 77(60,105) | 77(61,98) | 0.179 |
| EDVI | 117(92,144) | 113(90,139) | 109(87,141) | 108(87,135) | 0.012 |
| E velocity (m/s) | 0.70(0.30,0.90) | 0.70(0.50,0.90) | 0.70(0.50,0.90) | 0.60(0.50,0.85) | <0.001 |
| A velocity (m/s) | 0.60(0.40,0.80) | 0.70(0.50,0.80) | 0.73(0.60,0.90) | 0.70(0.60,0.90) | <0.001 |
| E/A ratio | 1.00(0.75,2.25) | 1.00(0.71,1.78) | 0.80(0.63,1.57) | 0.75(0.57,1.33) | <0.001 |
| E/e' ratio (septal) | 14(11,20) | 17(12,23) | 15(12,24) | 17(11,23) | 0.183 |
| E/e' ratio (lateral) | 11(8,15) | 12(9,16) | 13(9,17) | 12(8,17) | 0.192 |
| Anterior akinesia or dyskinesia (%) | 43(30,57) | 43(20,50) | 43(29,57) | 40(14,57) | 0.155 |
| MR severity: | |||||
| None or trace | 123(37%) | 110(37%) | 106(38%) | 96(31%) | 0.145 |
| Mild | 149(45%) | 130(44%) | 128(46%) | 147(48%) | 0.456 |
| Moderate | 43(13%) | 47(16%) | 38(14%) | 53(17%) | 0.240 |
| Severe | 14(4%) | 8(3%) | 7(3%) | 10(3%) | 0.460 |
| Coronary anatomy: | |||||
| No of vessels with stenosis ≥ 50% | |||||
| 1 | 46(14%) | 24(8%) | 24(9%) | 18(6%) | <0.001 |
| 2 | 101(31%) | 94(32%) | 87(31%) | 84(27%) | 0.362 |
| 3 | 183(56%) | 177(60%) | 168(60%) | 205(67%) | 0.006 |
| Stenosis of proximal LAD ≥75% | 242(73%) | 200(68%) | 185(66%) | 199(65%) | 0.020 |
| Duke CAD severity index | 52(39,65) | 65(39,77) | 65(39,77) | 65(39,77) | 0.030 |
LVEF – left ventricular ejection fraction, ESVI – end systolicvolumeindexed, EDVI - end diastolic volume indexed, E - early diastolic fillingvelocity, A – atrial contraction induced diastolic filling velocity wave, e' - early diastolic myocardial velocity, MR – mitral regurgitation, LAD – left anterior descending , CAD – coronary artery disease
Procedural details and complications of CABG by age
In the CABG group, there was no difference in the number of conduits used by age but the older group was more likely to have more distal anastomoses performed (Table 4). There was no difference in time on bypass or length of stay in the intensive care unit by age. The proportion who had to return to the operating room, developed mediastinitis or intubation for pulmonary edema or who experienced a cardiac arrest was not different by age. New onset atrial fibrillation rose with increasing age as did the need for inotropes for low cardiac output.
Table 4.
Procedural Details and Perioperative Complications by Age
| Variable | Baseline Age Quartiles | P-value for Trend | |||
|---|---|---|---|---|---|
| Q1 (Age≤54 years) (n=149) | Q2 (54<Age≤60 years) (n=127) | Q3 (60<Age≤67 years) (n=131) | Q4 (Age>67 years) (n=148) | ||
| Number of conduits: | |||||
| 1 | 26(17%) | 10(8%) | 15(12%) | 18(12%) | 0.284 |
| 2 | 49(33%) | 37(29%) | 42(32%) | 47(32%) | 0.958 |
| 3 | 60(40%) | 60(47%) | 52(40%) | 64(43%) | 0.894 |
| ≥4 | 14(9%) | 20(16%) | 22(17%) | 19(13%) | 0.362 |
| Number of arterial conduits: | |||||
| 0 | 11(7%) | 9(7%) | 12(9%) | 18(12%) | 0.123 |
| 1 | 123(83%) | 104(82%) | 104(79%) | 115(78%) | 0.249 |
| ≥2 | 15(10%) | 14(11%) | 15(12%) | 15(10%) | 0.957 |
| Number of distal anastomoses: | |||||
| 0 | 2(1%) | 2(2%) | 2(2%) | 1(1%) | 0.631 |
| 1 | 23(15%) | 10(8%) | 14(11%) | 16(11%) | 0.319 |
| 2 | 41(28%) | 27(21%) | 30(23%) | 30(20%) | 0.185 |
| 3 | 57(38%) | 55(43%) | 50(39%) | 59(40%) | 0.982 |
| 4 | 22(15%) | 23(18%) | 22(17%) | 31(21%) | 0.211 |
| ≥5 | 4(3%) | 10(8%) | 12(9%) | 11(7%) | 0.090 |
| Off-pump surgery | 40(27%) | 24(19%) | 25(19%) | 27(18%) | 0.083 |
| Total minutes on cardiopulmonary bypass | 83(63,110) | 92(72,125) | 93(66,110) | 89(70,126) | 0.425 |
| Cross—clamp time in minutes | 50(33,67) | 55(41,79) | 54(35,72) | 56(39,80) | 0.203 |
| Intensive Care Unit length of stay in hours | 52(43,87) | 61(42,94) | 49(27,97) | 65(40,112) | 0.337 |
| Perioperative complications | |||||
| Return to operating room | 7(5%) | 9(7%) | 7(5%) | 12(8%) | 0.326 |
| Mediastinitis | 3(2%) | 4(3%) | 2(2%) | 2(1%) | 0.516 |
| Other infection | 9(6%) | 10(8%) | 8(6%) | 19(13%) | 0.061 |
| New onset Atrial Fibrillation | 10(7%) | 20(16%) | 22(17%) | 38(26%) | <0.001 |
| Worsening renal impairment | 2(1%) | 4(3%) | 12(9%) | 16(11%) | <0.001 |
| Intra-aortic balloon pump | 25(17%) | 22(17%) | 24(18%) | 18(12%) | 0.335 |
| Inotrope Use | 45(30%) | 44(35%) | 56(43%) | 71(48%) | <0.001 |
| Cardiac arrest requiring cardiopulmonary resuscitation | 3(2%) | 3(2%) | 10(8%) | 7(5%) | 0.079 |
| Pulmonary edema requiring intubation | 3(2%) | 3(2%) | 4(3%) | 4(3%) | 0.640 |
| Mortality within 30 days after CABG | 3(2%) | 5(4%) | 10(8%) | 8(5%) | 0.081 |
Effect of age on 10 Year outcomes
All-cause mortality increased with increasing age in both MED (60 vs. 79% for Q1 and Q4, respectively; log-rank p=0.005) and CABG (48 vs. 68% for Q1 and Q4, respectively; log-rank p<0.001) groups. CV mortality was higher in the older quartiles compared to the younger quartiles, but this difference was not statistically significant in either the MED group (49 vs. 53% in Q1 and Q4, respectively; log-rank p =0.338) or CABG group (35 vs 39% in Q1 and Q4, respectively; log-rank p=0.103) (Figure 1). CV deaths accounted for a greater proportion of all deaths in the young (79% in the youngest quartile vs 62% in the oldest quartile).
Figure1.
Kaplan-Meier rates of all cause death, cardiovascular death and all cause death or CV hospitalization as a function of time from randomization by quartiles of age in patients randomized to CABG and patients randomized to medical therapy. MED= medical therapy, CABG=coronary artery bypass grafting.
Effect of age on the impact of CABG
There was a trend towards a greater reduction in all-cause mortality with CABG compared to guideline-directed medical therapy in younger compared with older patients (HR in those age ≤54 years =0.66, 95%CI 0.49-0.89, HR in those age >67 years = 0.82, 95%CI 0.63-1.06, pinteraction=0.062). The efficacy of CABG in reducing CV mortality was consistent across all age groups (Figure 2 and Supplemental Material; HR in those age ≤54 years =0.61, 95%CI 0.43-0.85, HR in those >67 years = 0.70, 95%CI 0.50-0.97, pinteraction =0.307). CABG resulted in a greater reduction in all-cause death and CV hospitalizations compared with medical therapy alone, and the effect was greater in the young (HR in those age ≤54 years =0.55, 95%CI 0.43-0.71, HR in those age >67 years = 0.73, 95%CI 0.57-0.92, pinteraction=0.004). Non-CV deaths in the group randomized to CABG were not statistically different from the group randomized to medical therapy and did not vary by age (Table 5).
Figure 2.
Hazard ratio (solid line) and 95% confidence interval (grey area) for the effect of CABG vs medical therapy across the range of age.
Table 5.
All deaths, deaths due to cardiovascular, non-cardiovascular and unknown causes and all cause mortality or CV hospitalizations by quartiles of age
| Cause of death | Randomized Treatment | Baseline Age Quartiles | Total (n=1212) | P Value* | |||
|---|---|---|---|---|---|---|---|
| Q1 (Age≤54 years) (n=330) | Q2 (54<Age≤60 years) (n=295) | Q3 (60<Age≤67 years) (n=279) | Q4 (Age>67 years) (n=308) | ||||
| All cause | CABG | 76/160 (47.5%) | 76/143 (53.1%) | 96/144 (66.7%) | 111/163 (68.1%) | 359/610 (58.9%) | 0.004 |
| MED | 102/170 (60.0%) | 90/152 (59.2%) | 92/135 (68.1%) | 114/145 (78.6%) | 398/602 (66.1%) | ||
| Cardiovascular | CABG | 56/160 (35.0%) | 61/143 (42.7%) | 67/144 (46.5%) | 63/163 (38.7%) | 247/610 (40.5%) | 0.002 |
| MED | 84/170 (49.4%) | 72/152 (47.4%) | 64/135 (47.4%) | 77/145 (53.1%) | 297/602 (49.3%) | ||
| Non-cardiovascular | CABG | 10/160 (6.3%) | 8/143 (5.6%) | 21/144 (14.6%) | 32/163 (19.6%) | 71/610 (11.6%) | 0.714 |
| MED | 9/170 (5.3%) | 9/152 (5.9%) | 20/135 (14.8%) | 33/145 (22.8%) | 71/602 (11.8%) | ||
| Unknown | CABG | 10/160 (6.3%) | 7/143 (4.9%) | 8/144 (5.6%) | 16/163 (9.8%) | 41/610 (6.7%) | 0.205 |
| MED | 9/170 (5.3%) | 9/152 (5.9%) | 8/135 (5.9%) | 4/145 (2.8%) | 30/602 (5.0%) | ||
| All cause death or CV hospitalization | CABG | 111/160 (69.4%) | 101/143 (70.6%) | 119/144 (82.6%) | 136/163 (83.4%) | 467/610 (76.6%) | <0.001 |
| MED | 147/170 (86.5%) | 122/152 (80.3%) | 122/135 (90.4%) | 133/145 (91.7%) | 524/602 (87.0%) | ||
P values are from the Cochran-Mantel-Haenszel test which do not account for time to event
The numbers of patients crossing from medical therapy to CABG and from CABG to medical therapy was low and there was no difference in either by age (ptrend=0.25 and 0.62, respectively). The “as-treated” analysis demonstrated similar findings with perhaps an even greater impact of age on the effects of CABG v medical therapy on 10 year outcomes (ie greater benefit in younger patients and less benefit in older patients across all end points) when compared with as the intention to treat analysis (Supplemental Material).
Discussion
This analysis of the long-term follow-up of the STICH trial demonstrates that the benefit of CABG compared to guideline-directed medical therapy on all-cause mortality and the combination of all-cause mortality and CV hospitalizations is greater in younger compared with older patients. In contrast, the benefit of CABG on CV mortality is similarly seen across all age groups. The discrepancy between the effect of CABG across ages as it relates to CV mortality and all-cause mortality likely results from the greater proportion of non-CV deaths in older patients, deaths that are less likely to be avoided by CABG.
An understanding of the efficacy of CABG in patients of different ages is needed to help inform clinical decision making.11 In the STICH trial, older patients had higher all-cause mortality compared with younger patients, whether they were randomized to medical therapy or CABG. This result is consistent with recent HF trials12, and previous surgical trials in patients without severe left ventricular dysfunction11. It is not surprising, as in STICH older patients had more co-morbidities and were more likely to die of non-cardiovascular causes than younger patients.
In the present analyses, while CV mortality increased with age, it was not statistically significantly higher in the older compared with younger patients, suggesting that in patients such as those in STICH, with CAD, HF, and an ejection fraction ≤35%, the risk associated with their cardiovascular disease somewhat attenuates the risks associated with age, and the comorbidities that go along with age. The efficacy of CABG over medical therapy on CV mortality persisted across all ages despite more co-morbidities and slightly higher early post-operative mortality in older patients. A further explanation for the finding may be the excellent medical therapy received by STICH patients, regardless of age. Medical therapies used in the treatment of HF are similarly effective across the spectrum of age. 12,13 Use of guideline recommended medical therapies was lower in the older patients but not different between the randomized groups in any age group, and is unlikely to have biased our findings. The use of implantable cardioverter defibrillators (ICD) was low at baseline (the population was recruited from 2002-2007 and the benefit of primary prevention ICDs reported in 2004/5). Greater use of ICDs might have reduced the risk of CV death in STICH. As the rate of ICD use was similarly low across the age range and in both treatment groups, we do not believe under-use of ICDs biased our results. However, the rate of sudden death in our cohort may have been higher than in contemporary real world cohorts and therefore the potential benefit of CABG may be lower. As STICH is the only contemporary CABG trial of patients with HF and significant LV dysfunction, there are no trials with which to compare these findings.
Our finding that CABG had a consistent effect in all ages on the outcome that it is most likely to influence, CV death, is of clinical relevance. Cardiologists and surgeons can recommend surgical revascularization for patients with CAD amenable to CABG and HF knowing that a reduction in CV death is seen across the spectrum of age of those included in the STICH trial. The lack of effect of CABG on all-cause mortality in older patients is a consequence of two findings. First, CV deaths accounted for a greater proportion of all deaths in the younger compared to older patients (79% of deaths in the youngest quartile, but 62% of deaths in the older). Secondly, it may be unreasonable to expect CABG to reduce non-CV deaths. Of more concern in older patients was that CABG may in fact increase non-CV deaths through a greater burden of co-morbidities which in turn lead to a greater risk of post-operative complications and non-CV deaths. In this surgical trial, it was important to analyze all causes of death to ensure no harm. This is in contrast to trials of medical therapies where CV death is often the primary mortality endpoint, as there is less of a concern about increasing non-CV deaths. Although the numbers were small, we observed no difference in the numbers of non-CV deaths in the two treatment arms in the oldest quartile. Thus our finding that CABG did not reduce all-cause mortality in the older group was not entirely unexpected. It was reassuring that CABG on top of guideline-directed medical therapy did not result in an iatrogenic increase in the risk of all cause death.
This study has a number of limitations. Due to the relatively small numbers of women we were unable to examine potential interactions of sex with age and assigned strategy.14 This was a post-hoc, subgroup analysis, and was therefore not included in the power calculations for the original trial. Therefore, our findings should be considered exploratory rather than confirmatory. The patients and outcomes in the STICH trial may not be entirely representative of real world populations due to the selection bias that occurs when any trial is conducted. The outcomes may also have been better as sites were selected on the basis of their surgical expertise (they had to demonstrate a 30-day mortality of ≤5% for patients with a similar profile to those meeting the STICH inclusion criteria). There were few patients in the truly older age groups (75 (6%) were aged >75 of age and 15 (1%) over>=80 years of age). In older patients the true rate of complications and potential for long term benefit may be different.
In conclusion, the consistent benefit of CABG on CV mortality regardless of age supports the recommendation of surgical revascularization to reduce cardiovascular death in patients with severe LV dysfunction across all ages studied. As CV deaths accounted for more deaths in the younger age group, they tend to gain a greater reduction in all-cause mortality. Careful assessment of competing mortality risk is important prior to pursuing revascularization in older patients.
Supplementary Material
Clinical Perspective.
What is new?
The 10 year follow-up of the STICH trial demonstrated a reduction in all-cause mortality in patients with heart failure who received CABG added to guideline-directed medical therapy compared with medical therapy alone.
In the present analyses, we report that the reduction in all-cause mortality with CABG was most pronounced in younger patients. The impact of CABG on all-cause mortality and the combination of all-cause mortality and CV hospitalization is diminished in older patients.
The benefit of CABG on CV mortality is consistent across all ages in the trial.
What are the clinical implications?
Patients presenting with heart failure who are potential candidates for CABG should be investigated to establish if they have coronary heart disease amenable to surgical revascularization.
Cardiologists and cardiac surgeons can offer appropriate patients CABG, in addition to optimal medical therapy, with the knowledge that CV mortality is reduced across all age groups included in the trial.
When considering older patients for surgical revascularization, clinicians should be aware that the reductions in all-cause mortality and all-cause mortality and CV hospitalization seen in younger patients are diminished with increasing age.
Acknowledgments
We are extremely grateful to Vanessa Moore for her assistance in guiding this work to its final version.
Funding sources
This study was supported by the National Institutes of Health (NIH) grants U01-HL-69015, U01-HL-69013, and R01-HL-105853. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute (NHLBI) or NIH.
Footnotes
Clinical Trial Registration: ClinicalTrials.gov Identifier: NCT00023595
Disclosures
Drs. Petrie, Jhund, Adlbrecht, Doenst, Panza, Hill, Rouleau, Ali, Maddury, Lee, Carson, Miller, and Romanov have no conflicts to disclose.
Drs. Velazquez, Chrzanowski, White and She report grants from the NHLBI at the NHI.
Drs. Prior and Golba report grants from the Duke Clinical Research Institute during the conduct of the study.
Dr. Velazquez reports grants from Alnylam Pharmaceuticals, Inc. and Pfizer; grants and personal fees from Amgen and Novartis Pharmaceuticals Corp.; personal fees from Merck and Expert Exchange.
Dr. White reports grants from Sanofi Aventis, Eli Lilly and Company, Merck Sharpe & Dohm, GlaxoSmithKline, Omthera Pharmaceuticals, Pfizer New Zealand, Intarcia Therapeutics, Inc., Elsai Inc., DalGen Products, Daiichi Sankyo Pharma Development; grants and personal fees from AstraZeneca.
References
- 1.Wong CM, Hawkins NM, Petrie MC, Jhund PS, Gardner RS, Ariti CA, Poppe KK, Earle N, Whalley GA, Squire IB, Doughty RN, McMurray JJV. Heart failure in younger patients: the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). Eur Heart J. 2014;35:2714–2721. doi: 10.1093/eurheartj/ehu216. [DOI] [PubMed] [Google Scholar]
- 2.Shafazand M, Schaufelberger M, Lappas G, Swedberg K, Rosengren A. Survival trends in men and women with heart failure of ischaemic and non-ischaemic origin: data for the period 1987-2003 from the Swedish Hospital Discharge Registry. Eur Heart J. 2009;30:671–8. doi: 10.1093/eurheartj/ehn541. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O'Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau J-L. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne-Nickens P, Sopko G, Rouleau JL. STICHES Investigators. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med. 2016;374:1511–20. doi: 10.1056/NEJMoa1602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung PJ, Carter TI, Burack JH, Tam S, Alfonso A, Sugiyama G. Predicting the risk of death following coronary artery bypass graft made simple: a retrospective study using the American College of Surgeons National Surgical Quality Improvement Program database. J Cardiothorac Surg. 2015;10:62. doi: 10.1186/s13019-015-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Camacho FT, Wechsler AS, Lahey S, Culliford AT, Jordan D, Gold JP, Higgins RSD, Smith CR, Hannan EL. Risk score for predicting long-term mortality after coronary artery bypass graft surgery. Circulation. 2012;125:2423–30. doi: 10.1161/CIRCULATIONAHA.111.055939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN. Meta-Analysis Global Group in Chronic Heart Failure. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–13. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 10.Harrell FE., Jr . Regression modeling strategies:with application to linear models, logistic regression and survival anlysis. Springer; New York: 2001. [Google Scholar]
- 11.Dalen M, Ivert T, Holzmann MJ, Sartipy U. Coronary artery bypass grafting in patients 50 years or younger: a Swedish nationwide cohort study. Circulation. 2015;131:1748–1754. doi: 10.1161/CIRCULATIONAHA.114.014335. [DOI] [PubMed] [Google Scholar]
- 12.Jhund PS, Fu M, Bayram E, Chen C-H, Negrusz-Kawecka M, Rosenthal A, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, McMurray JJV, Packer M. PARADIGM-HF Investigators and Committees. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36:2576–84. doi: 10.1093/eurheartj/ehv330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen-Solal A, McMurray JJV, Swedberg K, Pfeffer MA, Puu M, Solomon SD, Michelson EL, Yusuf S, Granger CB, CHARM Investigators Benefits and safety of candesartan treatment in heart failure are independent of age: insights from the Candesartan in Heart failure--Assessment of Reduction in Mortality and morbidity programme. Eur Heart J. 2008;29:3022–8. doi: 10.1093/eurheartj/ehn476. [DOI] [PubMed] [Google Scholar]
- 14.Arif R, Farag M, Gertner V, Szabo G, Weymann A, Veres G, Ruhparwar A, Bekeredjian R, Bruckner T, Karck M, Kallenbach K, Beller CJ. Female Gender and Differences in Outcome after Isolated Coronary Artery Bypass Graft Surgery: Does Age Play a Role? PLoS One. 2016;11:e0145371. doi: 10.1371/journal.pone.0145371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.