Abstract
Despite moderate success with pharmacological and behavioral treatments, smoking relapse rates remain high, and many smokers report that smoking cues lead to relapse. Therefore, treatments that target cue reactivity are needed. One candidate for reducing craving is the neuropeptide oxytocin (OT). Here, we investigated the effects of intranasal OT on two types of craving for cigarettes: craving following overnight abstinence and craving elicited by smoking-related cues. In this within-subject, placebo-controlled pilot study, smokers (N = 17) abstained from smoking for 12 hours before attending two sessions randomized to intranasal OT or placebo (i.e., saline nasal spray). On each session, participants received two doses of OT (20 IU) or placebo at one-hour intervals, and rated craving before and after each dose. Spontaneous cigarette craving was assessed after the first spray, and cue-elicited craving was assessed following the second spray. OT did not reduce levels of spontaneous craving after the first spray, but significantly dampened cue-induced smoking craving. These results provide preliminary evidence that OT can reduce cue-induced smoking craving in smokers. These findings provide an important link between preclinical and clinical studies aimed at examining the effectiveness of OT as a novel treatment for drug craving.
Keywords: Oxytocin, smoking, craving, placebo-controlled trial, human
Introduction
Cigarette smoking is a major health concern worldwide. In the United States alone, approximately 70 million individuals over the age of 12 are current smokers (SAMHSA 2011), and cigarette smoking accounts for nearly 480,000 deaths annually (Rostron 2013). Despite moderate treatment success with both pharmacological and behavioral treatments, nicotine dependence remains a persistent, chronic problem for many individuals. Indeed, of the 50% of current smokers who attempt to quit smoking per year, only 6% succeed (CDC 2011), with most relapsing within a few days of attempted cessation.
Risk for relapse can remain high for years, even after acute withdrawal has passed (Wetter et al., 2004). This persistent risk may be due in part to the power of cues (sights, smells, or places associated with smoking), to evoke learned, or conditioned, drug-seeking responses (Ferguson and Shiffman, 2009). Through repeated associations, drug-related cues become conditioned to the reinforcing effects of the drug and acquire incentive motivational properties (Robinson and Berridge, 1993). Conditioned smoking cues increase negative mood, heart rate, skin conductance, and self-reported craving (Conklin and Tiffany, 2001; Drobes and Tiffany, 1997; LaRowe et al., 2007) as well as actual smoking and relapse (Glad and Adesso, 1976; Herman, 1974; Mucha et al., 1998; Payne et al., 1990, 1991; Surawy et al., 1985) Therefore, novel treatments for smoking that target cue-induced craving are needed.
Oxytocin (OT) has received increasing interest as a potential treatment for addiction (for reviews see (Kovács et al., 1998; McGregor and Bowen, 2010; Sarnyai, 2011). OT is a neuropeptide released from the posterior pituitary that evokes a range of peripheral and central effects on reproductive/sexual behavior, mood, emotions, and socialization (Gimpl and Fahrenholz, 2001). Indeed, exogenous administration of OT has been shown to increase trust, sociability, and bonding (Kosfeld et al., 2005; Lim and Young, 2006). In addition to these well-known “pro-social” effects, there is also mounting evidence that OT also plays a role in the processes involved in drug addiction (Sarnyai and Kovács, 1994). For instance, preclinical studies have shown that OT decreases drug self-administration, and drug-primed reinstatement of drug taking (Carson et al., 2010; Sarnyai et al., 1991) and facilitates extinction of drug-paired associations (Baracz et al., 2012; Kovács et al., 1998; Qi et al., 2009). OT, and drugs that increase OT such as lithium, also reduce the severity of drug withdrawal for opiates, ethanol, and cannabis in rats (Cui et al., 2001; Kovács et al., 1998; Szabó et al., 1987; You et al., 2001).
More recently, human work has also highlighted the benefits of OT as a potential treatment agent for drug abuse. OT has been shown to relieve negative affect and anxiety (de Oliveira et al., 2012; Heinrichs et al., 2003), which are cardinal symptoms of nicotine withdrawal (Baker et al., 2004; Hughes and Hatsukami, 1986). In the first study of the effects of OT on drug craving, McRae-Clark and colleagues (2013) showed that intranasal OT significantly attenuated stress-induced craving for marijuana in marijuana dependent individuals. However, despite this evidence that OT reduces craving in response to stress, there have been no other studies examining the relationship between OT and drug reward or drug craving in humans. Taken together, the evidence suggests that OT can dampen craving in humans, perhaps due to its anxiolytic effects, and can reduce drug reward in animals. Therefore, there is a possibility that OT might reduce craving to smoking-related cues, which have acquired incentive salience in smokers.
In the current experiment, we aimed to examine the effects of OT on craving in cigarette smokers. To date, there have been no studies assessing the effects of OT on: a) cigarette craving during short-term abstinence, or b) craving elicited by drug-related cues. In the current experiment, we tested the effect of intranasal OT (20 IU) on tonic craving following overnight abstinence and on craving following exposure to smoking-cues, in daily smokers. We hypothesized that OT would reduce both spontaneous craving and craving elicited by smoking cues.
Methods
Participants
Daily cigarette smokers (N=17; 6 female) aged 18 – 35 years participated in the study. Potential candidates completed an initial phone screen and an in-person interview, including a physical examination and electrocardiogram. Exclusion criteria were a current or past year diagnosis of a DSM-IV Axis I disorder (assessed using the non-patient version of the Structured Clinical Interview for DSM-IV), excluding nicotine dependence [assessed using the Fagerström Test for Nicotine Dependence (FTND; (Heatherton et al., 1991)], high blood pressure or a history of cardiovascular problems, less than a high school education, lack of fluency in English, and pregnancy or lactation in women. All participants provided informed consent prior to participating in the study.
Study Design
The study used a within-subjects design. Daily smokers abstained from smoking for 12h before attending two laboratory sessions, which was confirmed by assessing expired carbon monoxide (CO) levels. A criterion of 10 parts per million (ppm) or lower was used to confirm compliance with instructions (Jarvik et al., 2000; Jarvis et al., 1987; Leischow et al., 1997). Participants were randomized to receive two doses of intranasal OT (20 IU per dose) nasal spray on one testing session, and two placebo nasal sprays on the other. We chose the 20 IU dose per spray on the basis of previous studies utilizing intranasally administered oxytocin. This dose has been well tolerated by participants in our laboratory (see MacDonald et al., 2011 for a review of safety of intranasal oxytocin in humans). Participants rated their mood and smoking urge before and after the first spray. On the basis of estimates of plasma clearance time (Kirkpatrick et al, 2014) we expected that this first dose would peak after 20 min and return to near baseline levels by 75 minutes. One hour and fifteen minutes later, they received the second spray followed by exposure to smoking cues (smoking-related images followed by lighting and holding a lit cigarette) and they again rated mood and smoking urge.
Procedure
The protocol was approved by the University of Chicago Institutional Review Board. Experimental sessions were conducted in comfortably furnished rooms with a television/VCR, magazines and a computer for administering questionnaires and images. Sessions began at 12.00h and were conducted at least 72 h apart. For women not taking hormonal birth control, sessions were conducted during the follicular phase of their menstrual cycle (between days 3 and 10) to reduce variability in endogenous oxytocin levels that occur across the menstrual cycle (Salonia et al., 2005; Shukovski et al., 1989). Participants were required to abstain from smoking, starting at midnight the night before each session. Upon arrival, they provided breath and urine samples to detect recent drug and alcohol use, and pregnancy in women. Participants also provided expired air CO to assess compliance with instructions. After these measures, participants completed baseline mood and craving questionnaires (see below). At 12:30, they received a nasal spray containing either OT or placebo, followed by self-report mood and craving questionnaires at 10 – 20 minute intervals for 60 minutes. At 13.45, they received a second nasal spray. Thirty minutes later, they completed the 10-minute cue exposure which ended at approximately 14.30. They then provided ratings of craving and mood at subsequent 20–30 minute intervals over the next hour and a half. Once the last measures were collected, at approximately 16.00, individuals were allowed to leave. After participants had completed both testing sessions, they were debriefed and paid for their participation.
Smoking Cues
Cue exposure consisted of viewing a 5-minute set of pictures presented for 6 s each, with the pictures consisting of images of cigarettes, smoking paraphernalia, and individuals or groups of individuals smoking. These images were generated in our laboratory, and were rated by smokers, who reported the images produced a significant urge to smoke. Participants viewed a different set of images at each session. Immediately after viewing the images, participants were asked to light a cigarette provided by the researchers (Marlboro Light brand) and hold the cigarette for five minutes without smoking it.
Dependent Measures
Visual Analog Scales (VAS: 0 – 100 mm; not at all to extremely) consisted of adjectives describing OT-related effects (for a review see MacDonald et al., 2011) including Dizzy, Light-Headed, Stimulated, Focused, Dreamy, and Nauseous.
The Profile of Mood States (POMS; Johanson and Uhlenhuth, 1980) is a standardized questionnaire consisting of 72 adjectives used to describe momentary positive and negative mood states, including anxiety. Participants indicate how they feel at the moment in relation to each of the adjectives on a 5-point scale ranging from not at all (0) to extremely (4). In this study, we a priori chose to examine the Anxiety, Anger, and Depression POMS subscales.
The Short Tobacco Craving Questionnaire (S-TCQ: Heishman et al., 2008) is a 12-item validated questionnaire that measures subjective tobacco craving on a 7-point Likert scale and consists of four scales: Expectancy, Purposefulness, Emotionality, and Compulsivity.
The Brief Questionnaire of Smoking Urges (B-QSU: Cox et al., 2001) is a 10-item questionnaire that measures smoking urges, with each item scored on a 7-point scale. The B-QSU consists of two factor-derived subscales measuring a desire to smoke for the positive effects of a cigarette (Factor 1) and the anticipation of relief from withdrawal symptoms (Factor 2), as well as the total score (sum of the two factors; Total Composite Score).
Drug
Intranasal OT and placebo doses were prepared by the University of Chicago Hospitals investigational pharmacy. For active OT, two single doses of 20IU Pitocin (OT Injection USB; Monarch Pharmaceuticals; concentration 10 IU Pitocin/1 ml) were transferred into two, 1 ml clear syringes fitted with an atomizer to administer the liquid in spray form (MAD300 by LMA Inc., San Diego, CA). Placebo nasal sprays consisted of Ocean Spray Nasal Spray (Valeant Pharmaceuticals, Bridgewater, NJ). Each set of nasal sprays was administered in four, 0.25 ml doses to each nare over the course of 10 minutes (1 spray in alternating nares each minute).
Statistical Analyses
Analyses were conducted using SPSS version 16.0 for Windows. Missing cases (due to equipment malfunction or other data collection problems) were deleted list-wise. Two participants’ data were missing for the POMS following the first OT administration, leading to a smaller sample size for analyses using that measure. Independent 2 (dose) x 3 (time) repeated-measure analyses of variance (ANOVA) were used to assess the effects of OT on dependent measures (POMS, TCQ, and QSU) before and after cue exposure. Quadratic effects are reported following the second spray because we expected craving to rise and then fall following cue exposure. Simple-effects tests were used to compare dependent-measure ratings before and after cue exposure for both OT and placebo. For all analyses, differences were considered to be significant if p < 0.05. Effect sizes are reported throughout as partial eta-squared (ηp2) for ANOVAs (where 0.01, 0.06 and 0.16 represent small, medium and large effect sizes respectively). Initially, all analyses were conducted with gender and dose order as between-subjects factors. There were no significant main effects or interactions involving sex or dose order for any of the dependent measures. Therefore, all analyses presented were collapsed across sex and dose order.
Results
Demographics and smoking habits
Table 1 shows the demographics and smoking characteristics of the sample. Participants tended to be young, of Caucasian race and smoked an average of six cigarettes per day. They had an average FTND score of 2.2, indicating a relatively low level of physical nicotine dependence.
Table 1.
Demographics and baseline characteristics of participants, N = 17
| Sex (Men: Women) | 11:6 |
| Age (years) | 23.5 ± 4.2 |
| Education (years) | 14.4 ± 1.1 |
| Race N (%) | |
| Caucasian | 12 (70.6) |
| African-American | 2 (11.8) |
| Other | 3 (17.6) |
| Smoking Characteristics (TLFB) | |
| Average Daily Cigarettes | 6.6 ± 3.5 |
| Max Daily Cigarettes | 13.9 ± 6.3 |
| Minimum Daily Cigarettes | 2.5 ± 4.0 |
| Average Weekly Cigarettes | 46.3 ± 24.2 |
| FTND Score | 2.2 ± 1.6 |
| Current Drug Use (TLFB) | |
| Drinking occasions per week | 3.1 ± 1.4 |
| Average number of drinks per occasion | 4.4 ± 1.9 |
| Past month cannabis use (times used in past month) | 9.8 ± 12.9 |
FTND = Fagerström Test for Nicotine Dependence. Scores range from 0–10, and scores between 2 and 4 indicate low to minimal nicotine dependence.
TLFB – Timeline Follow-back calendar of past month drinking habits.
Effects of OT on self-reported craving and mood
There were no baseline differences in craving or mood between placebo and OT sessions prior to the first spray, ps > 0.9. After the first spray, OT (vs. placebo) did not alter craving (S-TCQ and B-QSU; dose x time Fs < 1.5, ps > 0.2), mood (POMS, dose x time Fs < 0.83, ps > 0.37), or subjective effects (VAS dose x time Fs < 3.9, ps > 0.6).
Effects of smoking cues and oxytocin on self-reported craving
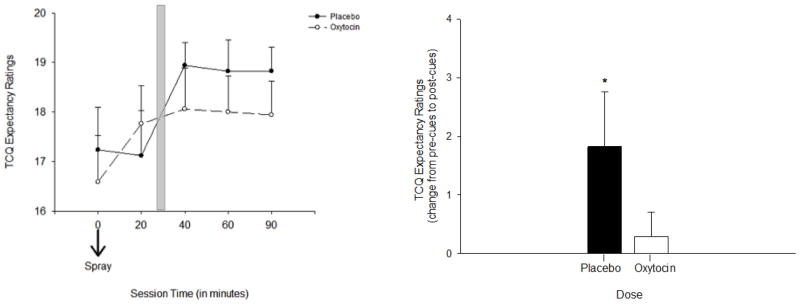
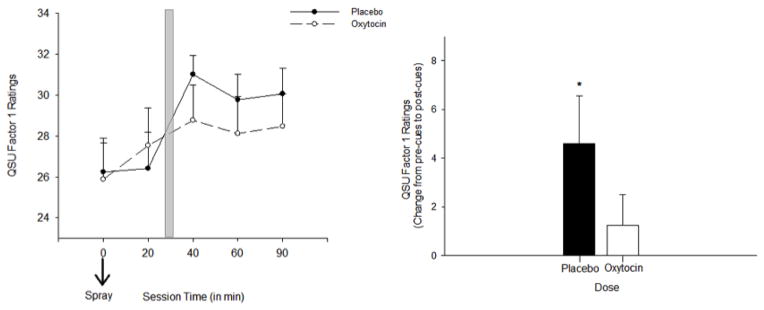
Craving significantly increased following exposure to the smoking cues in the placebo condition, supporting the effectiveness of the cue procedure. These increases were observed on the TCQ Expectancy scale and the QSU Total Composite and Factor 1 Subscales. OT dampened this increase in craving with significant dose x time interactions for S-TCQ Expectancy [F (1, 16) = 4.3, p < 0.05, ηp2= 0.2], the B-QSU Factor 1 subscale [F (1, 16) = 5.2, p < 0.05, ηp2 = 0.2], and the B-QSU Composite Score [F (1, 16) = 2.5, p = 0.06, ηp2= 0.14]. For both the S-TCQ Expectancy Scale and the B-QSU Factor 1 Subscale, simple-effects tests showed that there was a significant increase in ratings from pre-cue to post-cue exposure following placebo [pre-cue exposure (20 minutes post spray 2) versus post cue exposure (60 and 90 minutes post spray 2 on the S-TCQ Expectancy and 40, 60, and 90 minutes post Spray 2 on the B-QSU Factor 1), ps < 0.05], but not following OT for both measures (ps > 0.35) (Figures 1 and 2). There were no effects of cue or dose on ratings of B-QSU factor 2 (relief of negative affect) or the Emotionality, Compulsivity, or Purposefulness scales of the S-TCQ, ps >0.08).
Figure 1.
Smoking Craving on the S-TCQ Expectancy Scale in response to smoking cues following OT and placebo. Left panel: subjective craving on the S-TCQ Expectancy scale following the second OT and placebo administration (time 0 minutes). Shaded bar indicates cue exposure period. Right panel: change in ratings on the S-TCQ Expectancy before and after cues. Asterisk indicates a significant increase following placebo, p < 0.05. Capped bars indicate SEM.
Figure 2.
Smoking Craving on the B-QSU Factor 1 Scale in response to smoking cues following OT and placebo. Left panel:subjective craving on the B-QSU Factor 1 scale following the second OT and placebo administration (time 0 minutes). Shaded bar indicates cue exposure period. Right panel: change in ratings on the S-TCQ Expectancy before and after cues. Asterisk indicates a significant increase following placebo, p < 0.05. Capped bars indicate SEM.
Effects of OT and smoking cues on mood
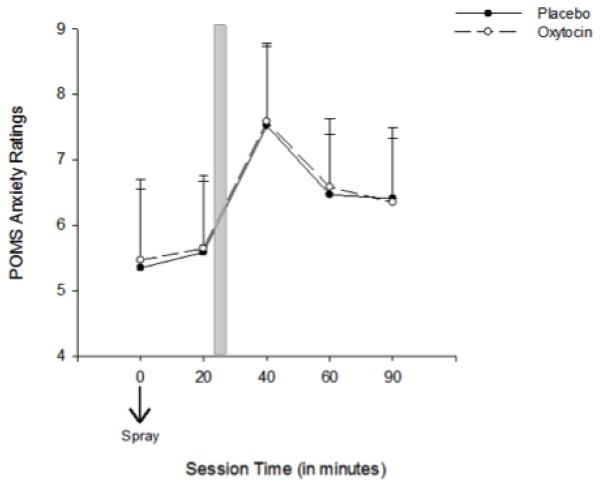
Smoking cues significantly increased ratings on the POMS Anxiety subscale [Time, F (1, 16) = 8.8, p < 0.01, ηp2 = 0.36], but there was no effect of OT on this measure [dose x time, F (1, 16) = 0.01, p = 0.9, ηp2 = 0.001] (Figure 3). There were significantly higher ratings of Depression on the POMS following OT [Dose, F (1, 16) = 4.5, p < 0.5, ηp2 = 0.22], however the effect of cues (main effect of time) and the dose x time interaction were not significant, ps > 0.07. There were no significant effects of cues or OT on the POMS Anger subscales (ps > 0.1).
Figure 3.
POMS Anxiety ratings in response to smoking cues following OT and placebo
POMS Anxiety ratings in response to smoking cues following the second OT and placebo dose administration (time 0 minutes). Shaded bar indicates cue exposure period. Capped bars indicate SEM.
Discussion
This study was the first to examine the effects of intranasal OT on cue-elicited craving in daily smokers. Although OT did not reduce tonic craving after the first dose administration, following overnight abstinence, it significantly dampened cigarette craving induced by smoking-related cues after the second dose administration 75 minutes later. Interestingly, the cues also increased self-reported anxiety, but OT did not dampen this effect, suggesting that the effect of OT was specific to self-reported cigarette craving and not due to a non-specific lowering of anxiety. These results extend findings in both animals and humans suggesting that OT may play a role in cue-induced craving.
One important finding in this study is that OT attenuated cigarette craving independent of any other effects on mood or affect. OT has been shown to have anxiolytic effects (de Oliveira et al., 2012; Heinrichs et al., 2003), which might make it beneficial for individuals who use drugs in response to stress. However, we found no evidence that OT altered anxiety elicited by smoking cues. Indeed, participants reported no subjective effects of OT at all, and when asked to identify what drug they had received at the end of the session, they guessed at chance level. Therefore, the effects of OT were specific to craving in this study, and not due to any calming effects that might have masked subjective craving.
Importantly, the effects of OT on craving were only seen in response to cue exposure, and not on tonic craving following the first OT administration. The participants abstained from smoking overnight before the sessions, and so their ratings of craving were moderately high at the time of the sessions. Whereas the first dose of OT did not affect these ‘tonic’ craving ratings, the second dose, administered 75 min later, decreased craving elicited by the cue. There are at least two possible explanations for this outcome. First, it is possible that different processes are involved in spontaneous craving and cue-induced craving, and that OT affects the latter but not the former. Second, it is possible that the sequential nature of the OT doses resulted in differential plasma levels or effects during the two administrations. That is, the first dose may have been subthreshold to reduce spontaneous craving, but carryover effects from that dose added to the effect of the second dose, resulting in significant cue-induced craving reduction during the later phase of the study. Although we cannot rule out this possibility, the finding that OT attenuated cue-induced craving is consistent with preclinical work demonstrating that OT reduces drug reward in animals. For instance, in rodents, OT has been shown to attenuate self-administration of cocaine (Sarnyai et al., 1992a; Sarnyai et al., 1992b), methamphetamine (Carson et al., 2010), and heroin (Kovacs and Van Ree, 1985), and to extinguish conditioned place preference (Qi et al., 2009).
The neurochemical mechanism by which OT reduces either drug reward or conditioned reward is not known, but there is mounting evidence that OT reduces the reinforcing properties of drugs by inhibiting drug-induced activity of dopamine in mesolimbic structures (Kovacs et al., 1990; McGregor et al., 2008; Qi et al., 2009). The mesolimbic dopamine system is strongly implicated in the rewarding effects of drugs (e.g., Di Chiara and Imperato, 1988; Koob and Le Moal, 1997; Robinson and Berridge, 1993; Wise, 1980). In drug-dependent humans, drug-related cues can increase dopamine activity in the dorsal (Volkow et al., 2006) and ventral (Heinz et al., 2004) striatum, perhaps related to their ability to increase incentive salience and activate neural networks involved in craving and reward. Thus, OT might reduce drug taking behavior by dampening the dopaminergic response, in this study by reducing the dopamine response to the smoking cue. Future studies should focus on identifying how OT alters drug and cue-induced dopamine activity in humans using appropriate imaging technologies such as positron emission tomography.
There are important limitations to the present study. First, the sample size was small, and comprised of young smokers who were not highly dependent on tobacco (i.e., smoking on average 6–7 cigarettes per day). It is not known if the effects would be more, or less, pronounced in heavier smokers. Perhaps related to their low tobacco dependence, the participants reported only modest craving, and did not report craving on factors assessing relief of negative affect or feeling as if they would be unable to stop if they started smoking. These are factors that reflect more significant nicotine dependence, and we cannot determine whether OT affects these aspects of tobacco addiction. Larger or more pervasive anti-craving effects may occur in heavier smokers. Second, we tested only one dose of OT at each of the two time points (i.e., 20 IU at the measure of spontaneous craving during the first measure and 20 IU at the measure of cue-induced craving during the second measure 75 minutes later). Ideally, each of the processes should be studied at several doses. Moreover, it is possible that the first dose affected responses to the second dose. Although plasma OT levels decline by 60 min after intranasal administration (Kirkpatrick et al., 2014), it is possible that OT from the first spray was still present at the time of the second spray, resulting in a higher dose during the second phase (cue-elicited craving). Studies examining OT levels in cerebral spinal fluid (CSF) indicate that it may take up to 75 minutes for OT to reach its peak, substantially longer than the plasma OT time course (Striepens et al., 2013). Thus, we cannot be sure that OT levels were sufficiently high during our test of spontaneous craving, or whether the effects on cue-elicited craving were a result of carryover effects from the first dose. Therefore, it will be important for future work to characterize the dose-response relationship and implement a longer measurement period to better define the dose dependency and time course of its effects on craving.
Taken together, these findings suggest that OT signaling pathways may be a target for reducing drug craving in humans. More specifically, OT may reduce subjective craving in response to smoking-related cues, possibly by reducing their incentive motivational properties. Future studies should further investigate whether OT affects other measures of craving, including physiological effects and actual smoking behavior.
Supplementary Material
Acknowledgments
Role of the funding source:
This research was supported by a Comprehensive Cancer Center Pilot Grant awarded by the University of Chicago (awarded to HdW, AK, and RL). The research was also funded by a grant from the National Institute of Mental Health (T32MH020065) (awarded to MAM).
Footnotes
Conflicts of interest: None declared
References
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological review. 2004;111:33. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behavioural brain research. 2012;228:185–193. doi: 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology. 2010;58:38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Experimental and Clinical Psychopharmacology. 2001;9:399. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Cui SS, Bowen RC, Gu GB, Hannesson DK, Peter HY, Zhang X. Prevention of cannabinoid withdrawal syndrome by lithium: involvement of oxytocinergic neuronal activation. The Journal of Neuroscience. 2001;21:9867–9876. doi: 10.1523/JNEUROSCI.21-24-09867.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira DC, Zuardi AW, Graeff FG, Queiroz RH, Crippa JA. Anxiolytic-like effect of oxytocin in the simulated public speaking test. Journal of Psychopharmacology. 2012;26:497–504. doi: 10.1177/0269881111400642. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106:15. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological Reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Glad W, Adesso VJ. The relative importance of socially induced tension and behavioral contagion for smoking behavior. Journal of Abnormal Psychology. 1976;85:119. doi: 10.1037//0021-843x.85.1.119. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser-Sinopoli SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation Between Dopamine D2 Receptors in the Ventral Striatum and Central Processing of Alcohol Cues and Craving. American Journal of Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Pickworth WB. Reliability and validity of a Short Form of the Tobacco Craving Questionnaire. Nicotine & Tobacco Research. 2008;10:643–651. doi: 10.1080/14622200801908174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman CP. External and internal cues as determinants of the smoking behavior of light and heavy smokers. Journal of Personality and Social Psychology. 1974;30:664. doi: 10.1037/h0037440. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacology Biochemistry and Behavior. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. American Journal of Public Health. 1987;77:1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson C, Uhlenhuth E. Drug preference and mood in humans: d-amphetamine. Psychopharmacology. 1980;71:275–279. doi: 10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Francis SM, Lee R, de Wit H, Jacob S. Plasma oxytocin concentrations following MDMA or intranasal oxytocin in humans. Psychoneuroendocrinology. 2014;46:23–31. doi: 10.1016/j.psyneuen.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kovács GL, Van Ree JM. Behaviorally active oxytocin fragments simultaneously attenuate heroin self-administration and tolerance in rats. Life Sciences. 1985;37:1895–1900. doi: 10.1016/0024-3205(85)90007-4. [DOI] [PubMed] [Google Scholar]
- Kovács G, Sarnyai Z, Babarczi E, Szabo G, Telegdy G. The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology. 1990;29:365–368. doi: 10.1016/0028-3908(90)90095-9. [DOI] [PubMed] [Google Scholar]
- Kovács G, Sarnyai Z, Szabó G. Oxytocin and addiction: a review. Psychoneuroendocrinology. 1998;23:945–962. doi: 10.1016/s0306-4530(98)00064-x. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addictive Behaviors. 2007;32:2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leischow SJ, Valente SN, Hill AL, Otte PS, Aickin M, Holden T, Kligman E, Cook G. Effects of nicotine dose and administration method on withdrawal symptoms and side effects during short-term smoking abstinence. Experimental and Clinical Psychopharmacology. 1997;5:54. doi: 10.1037//1064-1297.5.1.54. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Hormones and Behavior. 2010;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- McGregor I, Callaghan P, Hunt G. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? British Journal of Pharmacology. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Moran-Santa Maria M, Brady KT. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology. 2013;228:623–631. doi: 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha R, Pauli P, Angrilli A. Conditioned responses elicited by experimentally produced cues for smoking. Canadian journal of physiology and pharmacology. 1998;76:259–268. [PubMed] [Google Scholar]
- Payne TJ, Etscheidt M, Corrigan SA. Conditioning arbitrary stimuli to cigarette smoke intake: A preliminary study. Journal of Substance Abuse. 1990;2:113–119. doi: 10.1016/s0899-3289(05)80050-1. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Schare ML, Levis DJ, Colletti G. Exposure to smoking-relevant cues: Effects on desire to smoke and topographical components of smoking behavior. Addictive Behaviors. 1991;16:467–479. doi: 10.1016/0306-4603(91)90054-l. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56:856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rigatti P, Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Hormones and Behavior. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z. Oxytocin as a potential mediator and modulator of drug addiction. Addiction Biology. 2011;16:199–201. doi: 10.1111/j.1369-1600.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovács GL. Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology. 1994;19:85–117. doi: 10.1016/0306-4530(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Babarczy E, Krivan M, Szabo G, Kovacs G, Barth T, Telegdy G. Selective attenuation of cocaine-induced stereotyped behaviour by oxytocin: putative role of basal forebrain target sites. Neuropeptides. 1991;19:51–56. doi: 10.1016/0143-4179(91)90073-r. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Babarczy E, Vecsernyes M, Laczi F, Szabo G, Krivan M, Kovacs G, Telegdy G. Oxytocin modulates behavioural adaptation to repeated treatment with cocaine in rats. Neuropharmacology. 1992a;31:593–598. doi: 10.1016/0028-3908(92)90192-r. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Szabo G, Kovacs G, Telegdy G. Opposite actions of oxytocin and vasopressin in the development of cocaine-induced behavioral sensitization in mice. Pharmacology Biochemistry and Behavior. 1992b;43:491–494. doi: 10.1016/0091-3057(92)90182-f. [DOI] [PubMed] [Google Scholar]
- Shukovski L, Healy D, Findlay J. Circulating Immunoreactive Oxytocin During the Human Menstrual Cycle Comes From the Pituitary and Is Estradiol Dependent. The Journal of Clinical Endocrinology & Metabolism. 1989;68:455–460. doi: 10.1210/jcem-68-2-455. [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgra R, Wullner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surawy C, Stepney R, Cox T. Does watching others smoke increase smoking? British Journal of Addiction. 1985;80:207–210. doi: 10.1111/j.1360-0443.1985.tb03273.x. [DOI] [PubMed] [Google Scholar]
- Szabó G, Kovács G, Telegdy G. Effects of neurohypophyseal peptide hormones on alcohol dependence and withdrawal. Alcohol and Alcoholism. 1987;22:71–74. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. The Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, Cofta-Gunn L, Fouladi RT, Cinciripini PM, Sui D, Gritz ER. Late relapse/sustained abstinence among former smokers: a longitudinal study. Preventive Medicine. 2004;39:1156–1163. doi: 10.1016/j.ypmed.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Wise RA. Action of drugs of abuse on brain reward systems. Pharmacology Biochemistry and Behavior. 1980;13:213–223. doi: 10.1016/s0091-3057(80)80033-5. [DOI] [PubMed] [Google Scholar]
- You ZD, Li JH, Song CY, Lu CL, He C. Oxytocin mediates the inhibitory action of acute lithium on the morphine dependence in rats. Neuroscience Research. 2001;41:143–150. doi: 10.1016/s0168-0102(01)00272-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.