Abstract
Cystic fibrosis (CF) pathophysiology is hallmarked by excessive inflammation and the inability to efficiently resolve lung infections, contributing to major morbidity and eventually the mortality of patients with this disease. Macrophages (MΦs) are major players in lung homeostasis through their diverse contributions to both the innate and adaptive immune networks. The setting of MΦ function and activity in CF is multifaceted, encompassing the response to the unique environmental cues in the CF lung as well as the intrinsic changes resulting from CFTR dysfunction. The complexity is further enhanced with the identification of modifier genes, which modulate the CFTR contribution to disease, resulting in epigenetic and transcriptional shifts in MΦ phenotype. This review focuses on the contribution of MΦ to lung homeostasis, providing an overview of the diverse literature and various perspectives on the role of these immune guardians in CF.
Key Words: Cystic fibrosis, Macrophages, Plasticity, Infection, Inflammation
Role of Macrophages in Immunity
Tissue macrophages (MΦs) and blood-recruited monocytes have remarkable immune plasticity, with the ability to sense and adapt to the local milieu. MΦ phenotype is defined by the surrounding environment, which ultimately is controlled by the host. These environmental contributions to MΦ plasticity, in turn, contribute to the efficiency and potency of their involvement in inflammation and host homeostasis including host defense, the initiation and resolution of inflammation, tissue repair and the removal of dead cells and tissue debris. The plasticity and phenotypic response to the environment has led to the description of M1 MΦs and an entire series of M2 MΦs with variable surface expression of scavenger receptors, pathogen recognition receptors, autoregulatory machinery and secretion of inflammatory/anti-inflammatory molecules [1].
In the lung, 2 major MΦ populations have been described: alveolar (AMs), which are located in the airway lumen, and interstitial, which reside in the lung parenchyma. The AMs, distinguished by their unique expression pattern (CD11c-pos; CD11b-neg), are the sentinels of the lung. They maintain immunological and physiological homoeostasis (e.g. the removal of debris and the recycling of surfactant molecules) and provide a first line of host defense [1]. They are inherently suppressive, in order to protect the lung from inflammation due to environmental perturbations. Once the MΦs are activated, they rapidly amplify the host response via the secretion of antimicrobials and proinflammatory mediators, by recruiting specialized phagocytes (e.g. neutrophils) and by communicating with other lung cells (e.g. alveolar cells [1]) for a rapid amplified response. The interstitial MΦs (CD11c-low; CD11b-pos) are less characterized, being highly heterogeneous, with the potential for regulatory control. Indeed, it has been suggested that these MΦs are major contributors to the production of cytokines (IL-10) associated with the adaptive immune response. During infections, activation of the proinflammatory process and release of neutrophil granule proteins (e.g. azurocin, LL-37 and cathepsin G) will trigger the recruitment of monocytes from the circulation, aiding in the fight against infection, the removal of dead cells and, eventually, the resolution of the ensuing lung inflammatory response [2].
MΦs express a variety of receptors (plasma membrane or intracellular) and proteins which make them capable of sensing changes in the environment. These receptor interactions allow the MΦs to rapidly respond to the presence of microorganisms (e.g. bacteria, viruses and fungi), changes in physiology (e.g. changes in pH, hypoxia and osmolarity), metabolite concentrations (e.g. ATP, fatty acids, heme, etc.) and extracellular matrix alterations (e.g. collagen degradation products and hyaluronic acid). When tissue homeostasis is perturbed, these receptors are activated, leading to a specific signaling transduction and the expression of mediators that will allow cross-talk with neighboring cells and the recruitment of immune cells which, together, will cooperate in reestablishing the lung status quo [3].
MΦs sense microorganisms by recognizing pathogen-associated molecules or PAMPs, e.g. lipopolysaccharide (LPS), dsRNA and flagellin, through a repertoire of receptors called pattern-recognition receptors (PRRs). PRRs are expressed on immune and structural cells, including airway epithelium. They are divided into 4 major families: Toll-like receptors (TLRs), nucleotide oligomerization (NOD) receptors, c-type lectin receptors and retinoic acid-inducible gene 1 (RIG-I) receptors. Each of these receptor systems recognizes unique and diverse pathogen-associated molecules. PRRs are also activated by damaged cells that dump their cytoplasmic and nuclear components (e.g. HMGBI, ATP and adenosine) into the extracellular milieu; these are the ‘damage-associated molecular patterns’ (DAMPs) of the inflammatory response. Once activated, PRRs, through a tightly regulated signal transduction mechanism, initiate the inflammatory response by producing inflammatory mediators, e.g. proinflammatory cytokines, reactive oxygen species (ROS), nitric oxide, carbon monoxide and antimicrobials, which lead to pathogen elimination, inflammation resolution and, eventually, the reestablishment of tissue homeostasis. Abundance, location, turnover and signal transduction regulation of these receptors will determine the quality, intensity and duration of the immune response [4].
In addition to external effector functions, MΦs are also professional phagocytes, comprising the major mechanisms associated with the immune-regulated removal of pathogens, dead/dying cells and debris, and, in the case of AMs, are involved in surfactant homeostasis. Phagocytosis is a complex mechanism that involves an extraordinary cytoskeleton and plasma membrane reorganization, which allows MΦ chemotaxis and receptor-mediated binding to the inciting material to be phagocytized. Once internalized, elimination of the ingested material will be mediated by the fusion of phagosome with lysosomes. Thus, the MΦ ability to quickly remove microorganisms will depend on the activation of a complex transcriptional response, which encompasses cytoskeleton dynamics and vesicle trafficking/fusion, the production of lysosomal enzymes and the proton-pump acidification of lysosomal compartments. MΦ PRRs and other plasma membrane receptors, such as complement Fc and scavenger receptors, contribute to binding and responding to invading microorganisms [2].
The extensive role of MΦs in the pulmonary host response makes it an essential participant in lung homeostasis which, when dysfunctional, contributes to several human lung diseases including obstructive lung disease, asthma and allergic airways disease and fibrotic lung diseases [5].
Monocyte/MΦ Alterations in CF Are due to both Intrinsic and Acquired Factors
The hypothesis that monocytes/MΦs may contribute to CF lung disease was first proposed in 1982 [6]. CF MΦ dysfunction was associated with altered activation defined by metabolic hyperactivity, with elevated production of proinflammatory cytokines [7], elastase [8] and tissue-damage mediators (termed ciliary dyskinesia substances). These mediators impact airway epithelial-cell ciliary movements, contributing to the accumulation of mucus secretion [9]. Not until a few years later, with the discovery of the CFTR gene, was CFTR expression documented in MΦs [10].
Several descriptive studies using MΦs from CF patients have demonstrated that the MΦ phenotype changes during CF pathogenesis, as a result of plasticity. As already hypothesized 3 decades ago [11, 12], the CF lung environment (e.g. mucus, airway surface dehydration, increased protease activity, ROS, cytokines, etc.) plays a significant role in defining the phenotype of monocytes/MΦs, such that their ability to properly regulate the inflammatory response, clear bacteria and favor lung tissue repair is altered. Furthermore, it has been documented that there is an increase in the absolute numbers of MΦs in CF airways in the later stages of fetal development [13] and in young children with CF without detectable infection [14]. This increase has been correlated with elevated levels of the monocyte chemotactic protein 1 (MCP-1, also called CCL2) in the bronchoalveolar lavage fluid (BALF) and induced sputum from CF patients [14] and with lung exacerbations in patients with CF [15].
The remarkable ability of tissue MΦs to adapt to the environment and carry out different functions led to their broad classification as either classically activated M1 MΦs, implicated in initiatingand sustaining inflammation, or alternatively activated M2 MΦs, associatedwith anti-inflammatory, immunoregulatory and tissue-repair properties. M2 MΦs can also contribute to fibrotic pathology and allergic conditions [1]. In CF, the contribution of MΦ polarization to the lung disease is still unclear. In in vitro M2 cells, polarization is highly dependent on IL-4/1L-13 signaling and the production of high levels of arginase. Hartl et al. [16] reported that BALF from patients with CF infected with Pseudomonas aeruginosa had higher IL-4 and IL-13 concentrations and lower levels of IFN-γ (an environment that favors M2 MΦ polarization) compared with uninfected patients. Further, BALF levels of IL-4 and IL-13 correlated inversely with FEV1[16]. Arginase activity, which has been postulated to be an important mediator of airway remodeling and lung fibrosis, has been shown to be elevated in CF lungs [17], with a concurrent, increased expression of mannose receptor (CD206). These markers of lung fibrosis have been correlated with a decline in pulmonary function in P. aeruginosa-infected CF patients [18]. Increased arginase activity has also been identified in the lung and airways of Cftr-deficient mice that is further augmented by infection with P. aeruginosa [19]. The CF lung environment is highly complex, concurrently demonstrating a proinflammatory phenotype, with high levels of IL-8, IL-6 and TNF-α and low levels of IL-10, an environment that would favor M1 MΦ polarization instead [20, 21]. Furthermore, the expression of CD206 and other scavenger receptors (discussed later) has been found to be downregulated in sputum-derived MΦs from CF patients [22], suggesting differences in the milieu of the lung and sputum. Animal studies have suggested that alveolar and peritoneal MΦs from F508del CF mice exposed to LPS have elevated levels of both M1and M2 MΦs compared to control Cftr-sufficient animals [23]. Thus, defining the contribution of MΦ polarization in CF lung disease may be challenging, since these cells dynamically adapt to the tissue environment and can vary with age, the status of lung disease, the bacterial flora and the therapeutic regimen that are unique to each patient.
The plasticity of the MΦ has made it difficult to completely appreciate the mechanisms associated with the changes in CF MΦs [10]. The CFTR protein has been documented in murine [24, 25, 26], ferret [27] and human [28, 29] monocytes/MΦs and associated with CFTR-like Cl- conductance abnormalities [28, 30, 31]. Treatment of MΦs with specific CFTR inhibitors have been shown to change the MΦ phenotype to resembles CF MΦs, with an increased secretion of proinflammatory cytokines [24, 30]. LPS-induced hypersecretion of IL-8 has also been demonstrated utilizing peripheral blood (PB) monocytes isolated from subjects heterozygous for the F508del CFTR mutation compared to non-CF controls [32], further supported by the same observations with MΦs isolated from heterozygous Cftr mice [26]. Thus, a single allelic CFTR mutation is sufficient to augment proinflammatory activation in response to LPS in CF, implying CFTR-dependent defects in CF MΦs.
CF Monocytes/MΦs Are Hyperinflammatory
Cytokines such as TNF-α, IL-1β, IL-6 and IL-8 are elevated in the lungs of patients with CF compared with healthy controls, while the secretion of cytokines involved in resolution of inflammation, such as IL-10, is reduced [20, 21]; this correlates with the expression in AMs and MΦs. Further, treatments that improve clinical parameters in patients with CF, such as the antibiotic, azithromycin, have been found to reduce the proinflammatory phenotype in AMs [33], and PB monocytes respond to CFTR potentiator therapy (ivacaftor) in patients carrying the G551D mutation [34, 35, 36].
The proinflammatory behavior of MΦs may be directly associated with chronic bacterial infection, which is constitutive once established in the lung of CF patients. In addition, the altered CF lung environment, which is rich in inflammatory mediators such ROS, HMGB1, neutrophil proteases and cellular matrix proteolytic products (e.g. proline-glycine-proline and hyaluronan fragments) [37], may persistently activate the PRRs in MΦs (and other cell types in the lung) to optimize pathogen interaction and sensitivity as well as to stimulate proinflammatory pathways (e.g. NF-κB and MAPK). Mucus obstruction and changes in airway surface hydration [38], the hallmark of CF lung disease, may also affect the ability of MΦs to properly respond to inflammatory triggers or efficiently phagocytose the pathogen invader.
In recent studies, it has been demonstrated that the robust production of inflammatory mediators in human CF AMs may be due the activation of the inositol-requiring enzyme 1 (IRE1)-α-dependent X-box-binding protein-1 (XBP-1) arm of the unfolded protein response (UPR). These studies demonstrate higher mRNA levels of XBP-1 in CF when compared to non-CF AMs. This response seems to reflect an adaptation to the infectious/inflammatory environment of CF airways rather than the loss of CFTR in CF MΦs [39]. The factor in the CF lung milieu that contributes to this dysregulation is unknown.
Another observation, justified by a wealth of research, is that CF MΦs are intrinsically hyperinflammatory. In support of this hypothesis, studies have demonstrated that in vitro cultures of MΦs isolated from the PB [30, 32, 40, 41] of CF patients have an exaggerated inflammatory response to several inflammatory mediators. Consistent with the in vitro human data, MΦs from CF mice and ferrets have also been shown to be proinflammatory [23, 25, 26, 27, 30, 41, 42, 43], with an increased production of proinflammatory cytokines when exposed to bacteria such as P. aeruginosa [42] and Burkholderia cepacia [40, 44, 45] and PAMPs such as LPS [23, 25, 26] and flagellin [41]. Furthermore, studies in which bone marrow (BM) chimeras were made by transplanting wild-type and CF mice with either wild-type or CF BM, demonstrated the enhanced secretion of various proinflammatory cytokines after exposure to LPS. This demonstrates that the proinflammation may be related to MΦs lacking functional CFTR rather than on the resident epithelial cells [26]. These data were consistent with studies on a model of P. aeruginosa infection using myeloid-specific Cftr knockout mice [42] and in G551D-/G551D- CF mice [46]. Thus, studies on animal models suggest that a loss of CFTR in MΦs/monocytes contributes to lung hyperinflammation in CF.
Since the identification of CF differences in response to pathogens, the search for how CFTR alters MΦ function and activity has been ongoing. One potential hypothesis relates to the altered plasma membrane expression of immune receptors (i.e. TLR4 [30, 47] and TLR5 [48]) involved in immune signaling. In particular, the increased expression of TLR4, which is activated by LPS and also by DAMPs such as HMGB1, have been reported in murine [30] and human [30, 47] MΦs. Furthermore, increased TLR4 levels lead to the sustained signal transduction and activation of the NF-κB and MAPK pathways, which induces a robust transcription of proinflammatory cytokines. The changes in TLR4 presence on the plasma membrane of CF MΦs may be due to an abnormal internalization of the receptor in the endosomal compartment and the trafficking to the lysosomal compartment, where it is degraded, thus terminating signaling [30]. The endosomal defects contributing to the inefficient trafficking of TLRs may be related to observed abnormalities in the reduced expression of Rab proteins [30, 49] as well as the rate of microtubule formation [50], both of which contribute to vesicle trafficking, docking and maturation. CF cells, including MΦs, have altered levels of sphingolipids (e.g. ceramide) and cholesterol [51, 52, 53], which are key for shaping the signaling platform at the plasma membrane, called the lipid raft. CF MΦs challenged with LPS also have impaired expression/distribution of the plasma membrane scaffolding-protein caveolin-1 [43], which facilitates the cellular transport of cholesterol to the plasma membrane in MΦs [54]. Thus, low levels of caveolin-1 in CF MΦs may affect the organization of lipid rafts during activation.
All these dysfunctions may have a tremendous impact on the regulation of immune receptor signaling.
The mechanisms mediating negative feedback of immune receptor signaling are also altered in CF MΦs. CF MΦs have abnormal expression of nuclear receptors, such as peroxisome proliferator-activated receptors (PPARs) and liver X receptors (LXRs), which mediate fatty acid metabolism, a negative regulator of inflammation. MΦs isolated from Cftr−/− mice have low basal levels of PPARγ expression and attenuated LPS-driven induction of PPARδ and LXRα [25]. Cellular distribution of the heme-oxygenase 1 (HO-1) protein, a key stress response protein involved in balancing cellular redox status and inflammation (including TLR4-negative regulation) is also altered in murine and human CF MΦs exposed to LPS. In particular, HO-1 translocates to lipid rafts in a caveolin 1-dependent manner and catalyzes the local production of carbon monoxide (CO), which favors destabilization between TLR4 and its adaptor protein MyD88 (thus signaling termination), and this negative feedback mechanism is blunted in CF MΦs [43]. In addition, in response to TLR-MyD88 activation, CF MΦs display blunted PI3K/AKT signaling [41], which normally plays a key role in regulating immune function [55] and in downregulating levels of microRNAs that amplify the TLR4 signaling (e.g. miR-155, let7e, miR-125b and miR199a-5p) in MΦs.
Previous literature has suggested that both the mouse models of CF and the human disease have deficient autophagy mechanisms in their epithelial cells [56]. Autophagy is a conserved mechanism by which cells manage intracellular damage in order to maintain sustainability and efficiency. Furthermore, the process of autophagy allows for the recycling of membranes and cytosolic components for reuse. Deficiency in effective autophagy has been linked to a variety of diseases which have a part in the pathophysiology of chronic inflammation, including Crohn's disease and neutrodegeneration [57]. MΦ autophagy has been well established as a mechanism of sustaining homeostasis [58], and when homeostasis is defective, this is linked to ineffective MΦ function [57]. Importantly, a proper autophagy flux in activated MΦs has negative regulatory effects on TLR signaling, by controlling the signal transduction, trafficking and degradation of the receptor [57]. This autophagy dysfunction predisposes the CF MΦs to the elevated production of proinflammatory cytokines during B. cepacia infection [40].
Finally, a lack of CFTR in monocytes affects the Rho-small GTPase inside-out signaling trigged by monocyte/MΦ chemoattractant cytokines (e.g. MCP-1). This CFTR-dependent defect has been found to impair β-1 and β-2 integrin-mediated monocyte adhesion and chemotaxis. As a consequence, monocytes lacking CFTR accumulated in the lung parenchyma and displayed blunted transmigration into the BAL space of wild-type mice intranasally treated with MCP-1 [35]. This incapability of CF monocytes to localize appropriately in different lung compartments in response to chemoattractants may very well affect both the inflammatory response and the host defense.
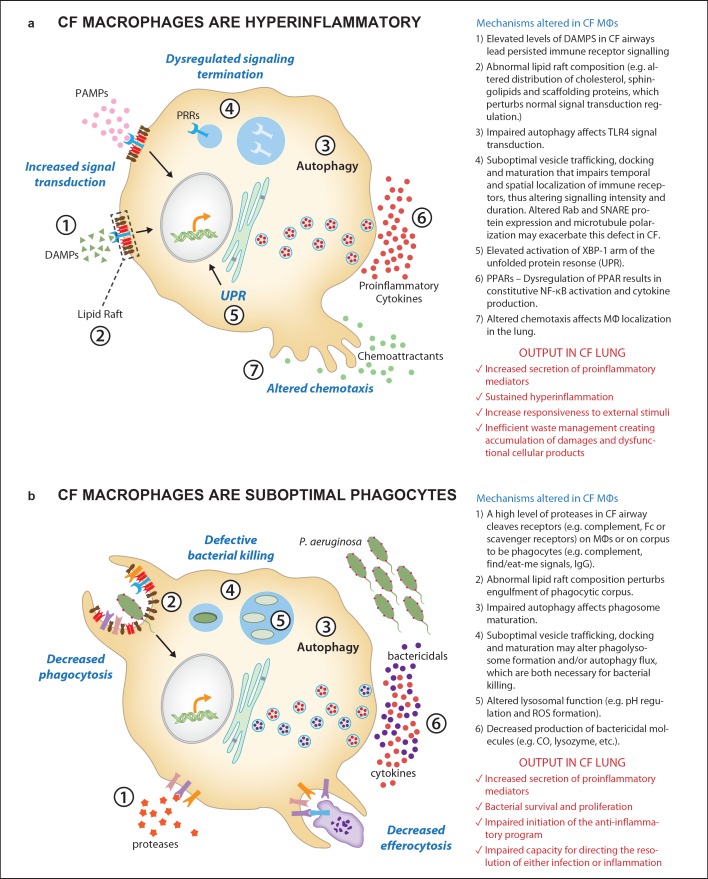
The mechanisms that could contribute to CF MΦs hyperinflammatory behavior are summarized in figure 1a.
Fig. 1.
A summary of abnormalities described in CF MΦs that lead to proinflammatory behavior (a) and suboptimal phagocytosis (b). Potential molecular mechanisms associated with these suboptimal performances are listed.
CF MΦs Display Inefficient Management of Infection
Monocytes from CF patients have relatively low expression of the complement receptors urokinase-type plasminogen-activator receptor (uPAR) and CD11b, both important in facilitating the binding and phagocytosis of opsonized P. aeruginosa [29]. CF lungs also have pronounced expansion of small MΦs with low expression of the scavenger receptors, CD206 and MARCO, involved in the binding and internalization of unopsonized particles as well as microbes [22]. Caveolin-1, which mediates P. aeruginosa internalization [59], is expressed at low levels in activated CF MΦs [41, 43]. These observations suggest that monocytes/MΦs in CF may have an impaired ability to properly phagocytose bacteria [29].
The CF environment also impacts the capability of CF MΦs to recognize and internalize bacteria [11] by altering signaling [38, 60] and cleaving the receptors necessary to orchestrate the adequate host-pathogen interaction. In particular, it has been found that elevated levels of neutrophil elastase in CF lungs cleave plasma membrane receptors/proteins such as complement proteins (e.g. C3, C5 and C3bi), complement receptors (e.g. CR1) and lymphocyte receptors (CD4 and CD8) [12]. Fick et al. [61], in 1981, suggested that specific P. aeruginosa IgG in the serum and sputum of CF patients functioned in an inhibitory fashion, decreasing the efficiency of P. aeruginosa phagocytosis and intracellular killing. These early studies suggested that the Fab and Fc portions of the CF immunoglobulin molecule are impaired in their attachment to the alveolar MΦ membrane Fc-γ receptors, thus decreasing internalization. The phagocytic function of the complement receptors has also been shown to be defective in CF, with active elastase cleaving CR3 off the surface of phagocytes, contributing to inefficient infection resolution [62].
MΦs isolated from CF animal models (mice and ferrets) and CF patients also display an impaired capability for killing internalized bacteria, including P. aeruginosa and Burkholderia cenocepacia. Consistent with early studies that suggested a potential role for CFTR in maintaining a differential pH in thetrans-Golgi, endosome and lysosome [63], Di et al. [24] proposed a mechanism by which a loss of CFTR in MΦs is associated with the alkalization of the phagosomal lumen, which impairs P. aeruginosa killing. The working hypothesis is that CFTR-mediated Cl- entry functions as counter-ion conductance to balance H+ influx through V-ATPase in the lysosome, thereby maintaining an acidified lysosomal environment. These data were corroborated in MΦs isolated from CF patients [64] and in AMs from CF ferrets [27]. More recently, vesicle alkalization also been correlated with the failure of CF MΦs to activate the acid sphingomyelinase enzyme, which is necessary for the formation of ceramide-enriched membrane platforms [52]. The ceramide facilitates gp91phox-mediated oxidative burst in response to P. aeruginosa infection, promoting killing [52]. An altered mechanism of vesicle acidification in CF MΦs was also proposed to favor intracellular survival of B. cenocepacia [44].
The major difficulty in all of these studies involving CFTR in lysosomal acidification has been the reproducibility of the observations, which has led to controversy about the theory of the dysfunctional lysosomal contribution of MΦs to CF pathophysiology. It is likely that the differences are not a matter of right or wrong, but more likely due to the complexity of MΦs and the exquisite sensitivity of the cell to minute changes in the environment of culture or purification [65, 66]. While this controversy has not yet been resolved, an accurate comparison of studies suggests that defective vesicle acidification in CF MΦs may depend on the complex cell signaling that is activated by MΦs in response to live bacteria, which cannot be fully recapitulated when phagocytosis is mimicked with opsonized beads [44]. In any case, whether or not CFTR directly controls lysosomal pH, a recent study suggests that drugs able to promote phagosome acidification, via potentiating the lysososmal activity of the transient receptor potential canonical-6 (TRPC6) calcium-permeable channel, can sufficiently restore microbicidal function in CF alveolar MΦs [67].
As discussed previously, autophagy, a mechanism first described to be defective in CF epithelia [56], has more recently been directly linked to the ineffective bactericidal function of MΦs in CF. Indeed, deficient autophagy prevents destruction of B. cenocepacia in murine CF MΦs [45, 68], and autophagy stimulation with rapamycin alleviates some of the dysfunction resulting in improved bacterial clearance of B. cenocepacia, P. aeruginosa and Staphylococcus aureus [45, 69, 70]. Unfortunately, rapamycin therapeutics are counter indicated for use in CF due to significant side effects, opening the door for therapeutic development targeting autophagy utilizing different drugs.
CF MΦs might be defective in releasing bactericidal mediators that contribute to the extraordinary role of keeping the lungs sterile. For example, as discussed in the previous section, the plasma membrane trafficking of HO-1 is blunted in activated CF MΦs [43]. HO-1 catabolic products have strong immune-modulatory effects, and, importantly, by producing CO, facilitate the killing of bacteria [71]. MΦs produce high levels of antimicrobial enzymes (e.g. lasozyme) and a loss of CFTR may also interfere with this basic MΦ function. The mechanisms that compromise the bactericidal function of CF MΦs are summarized in figure 1b.
CF MΦs Have Reduced Scavenger Ability
MΦ scavenger function is altered by the CF environment (e.g. mucus, increased proteases, ROS, etc.). Apoptosis and efferocytosis are in tandem in maintaining tissue homeostasis, with disruption in interactions contributing to inefficient inflammation resolution and tissue destruction [72]. Alveolar MΦs have been suggested to have deficient efferocytosis processes in a variety of diseases including CF [73]. The efficiency of the efferocytosis process is defined by MΦ phenotype, with the M1 phenotype having little or no capacity for the process while the M2 phenotype has a high capacity for efferocytosis [74]. Much of the regulator process of efferocytosis goes back to the inverse relationships between PPARγ and NF-κB again regulating the phenotypic characteristic of the contribution of MΦs to the inflammatory process. PPARγ drives the M2 phenotype whereas NF-κB drives the M1 phenotype [75]. The PPARs as retinoic acid-based lipid scavenger receptors are important in the process associated with surfactant and lipid metabolism [76]. Treatment of Cftr−/− AMs with endogenous PPARγ/α ligands, including rosiglitazone (PPARγ ligand) or WY14643 (PPARα ligand) decreases the LPS-induced TNF-α response [25, 77]. As PPARγ has been shown to be important for the maturation and phagocytic capacity of MΦs [78], these data would imply that the AM phenotype has the capacity to control proinflammatory cytokines via PPARγ in CF. The implication is that the change in the membrane lipids alters the functionality of the MΦs, which translates into inefficiency in both bacterial clearance and inflammation resolution. The role of these scavenger receptors is thought be important in the removal of foreign substances and waste materials that utilize extensive ligand specificity [79]. The absence of effective ‘clean-up’ and ‘removal’ systems can ultimately result in the accumulation of biologic waste, which interferes with homeostatic mechanisms. Therapeutic intervention aimed at improving scavenger receptor activity may provide support for the self-management of the unique milieu of the CF lung.
Removal of biologic waste in MΦs has been termed efferocytosis, which is the mechanism by which the MΦs recognize phagocytes and dispose of apoptotic cells from the airway. However, elevated proteases in the CF lung alter efferocytosis efficiency. Proteases such as active elastase cleave MΦ phosphatidylserine receptors; this impairs the capacity of MΦs in the recognition and phagocytosis of apoptotic cells in CF lungs [12, 80] (fig. 1b). Removal of dying apoptotic cells is fundamental for the resolution of the inflammatory response [81]. Neutrophil phagocytosis by MΦs triggers the production of mediators that curbs neutrophil migration and induces an anti-inflammatory transcriptional program in the MΦs (e.g. the expression of IL-10, lipoxins, resolvins, etc). In addition, it favors a high level of expression of scavenger receptors (e.g. CD206 and MARCO) that play a pivotal role in the binding and internalization of particles in the absence of opsonization, which reduces the responsiveness to TLR ligands [2]. In CF, small MΦs with defects in the expression of CD206 and MARCO have been shown to be present in the CF airways (BALF and induced sputum) [22, 82]. The lower expression of these receptors on MΦs might contribute to an inability to properly clear inflammatory glycoproteins, oxidized lipids and inhaled particles that may be abundant in the damaged CF lung, thus enhancing the inflammation and tissue damage that is observed in the CF airways.
The MΦ products of the inflammatory response not only manage the infection but often contribute to tissue damage due to the potency of the agents such as reactive oxygen radicals, matrix metalloproteinases, cytokines and other protein-modifying agents [83]. Many of these products are produced through redirecting MΦ phenotype. Further recent data suggest that many of these changes can alter the MΦ activation process, impacting inflammatory regulator pathways, activity and phenotype via epigenomic modification [75, 84]. Once the lung manages the intrusion, the healthy lung starts the reparative process, which includes reducing the inflammation by ‘ramping up’ anti-inflammatory molecules and protease-neutralizing agents as well as products potentially generated by the alveolar macrophage. Thus, MΦ abnormality in CF may perpetuate lung tissue scarring due to the inefficient activation of anti-inflammatory and repairing pathways.
The CF Phagocyte and Adaptive Communication
MΦs, which can also function as professional antigen-presenting cells (APC), have been suggested to participate in redirecting downstream adaptive responses in CF [85, 86]. In these studies, shifting costimulatory molecules like CD80 and CD86 are suggested to alter T-cell reactivity and, potentially, the management of both infection and inflammation in CF. In this case, although the primary defect in the epithelial cells establishes the abnormal airway surface environment and initiates the pathophysiology that leads to progressive lung disease in CF, changes in both adaptive and innate immunity exacerbate the disease pathophysiology associated with infection and inflammation resolution. The inability to resolve infection and attenuate inflammation downstream of infection plays a major role in the morbidity and mortality of the disease. Furthermore, improving the ability to manage exposure to pathogens and the relenting inflammatory process appears to be individualized, even within families that have the same CFTR mutation variant [87] or monozygotic twins [88]. The concept of genomic and environmental modifiers contributing to the complexity to CF pathophysiology provides a significant insight for therapeutic development and the understanding of the variability of the disease and its subsequent response to treatment.
Genome-wide association studies have begun to demonstrate that although the CFTR gene is the causative factor for the development of CF pathophysiology, there are certain associated genes that tend to correlate with the severity of CF disease [89, 90]. Table 1 highlights some genes that are particularly relevant, specifically for how MΦs may ultimately regulate the downstream adaptive control of inflammation and infection resolution. Alpha-1 antitrypsin plays a role in innate immunity and has been the focus of studies in CF [91]. Bioactive mediators such as IL-10, C3, MIF, TGF-β, IFN-γ and TNF-α have also been shown to correlate with some aspects of lung function or clinical exacerbations according to the comprehensive review by Weiler and Drumm [90].
Table 1.
Variability in CF disease presentation has been shown to include both the genotype as well as environmental influences
| Gene with polymorphism | Function | p value | Clinical correlation |
|---|---|---|---|
| 1MHC | antigen presentation | ≤0.04 onset of colonization | onset of pathogen colonization frequency of colonization |
| Alpha-1 antitrypsin | protease inhibition | 0.04 | FEV1% pred |
| ADRB2 | alternative vs. classical MΦs | ≤0.05 | FEV1% pred 5-year decline in pulmonary function |
| CD14 | pathogen interactions | no association | disease severity |
| DCTN4 | microtubule function | ≤0.05 | age at onset of chronic infection age at first infection |
| HLA | adaptive communication | ≤0.05 | FEV1 % pred age at onset of chronic infection |
| IFN-γ | MΦ activation | ≥0.09 | chronic infection: P. aeruginosa age at death age that FEV1 50% pred |
| HMOX1 | transcriptional regulation | 0.01 | FEV1% pred |
| IL-10 | immunoregulation | ≥0.02 | pulmonary function decline age of P. aeruginosa infection age at death |
| NOS | immunoregulation | ≥0.02 | age at colonization |
| decline in pulmonary function | |||
| TGF-β | immunoregulation | ≤0.04 | age that FEV1 ≤50% pred impairment of lung function |
| TLR4 | pathogen interactions | ≥0.10 | rate of change of FEV1 age at first infection |
| TNF-α | inflammatory response | ≥0.02 | mean FEV1 pred mean Shwachman score age at first infection |
Recently, significant progress in genome-wide association studies has generated considerable interest in understanding other genes that might either enhance the CFTR-deficient phenotype or improve outcomes. This table provides a summary of the genes associated with MΦ function and clinical outcomes in CF patients.
The control of pathogen response is based upon effective immune defenses. The immune system is composed of multiple cell types which, together, improve the resistance against infections. Communication between APC or phagocytes such as MΦs, their environment and downstream adaptive immunity is essential for an effective immune response. The studies of the CF genome-wide association project have shown that there is a significant correlation between MHC class II polymorphisms in the HLA-DR and HLA-DQ regions and overall disease severity [89, 92], implicating differences in the capacity to communicate to T cells and other adaptive immune cells. This is not unique to CF since there have been several instances of the association between specific MHC class II alleles and increased susceptibility or severity of inflammatory diseases, including allergic bronchopulmonary aspergillosis, multiple sclerosis, rheumatoid arthritis, sarcoidosis, asthma and diabetes [93, 94]. The interaction between T cells and MHC class II, along with the surrounding milieu, is crucial for defining the phenotype and success of the inflammatory response to infection. The host response to bacterial infection requires communication between APC and T cells [95]. This communication is relayed through MHC class II antigen presentation to helper T cells followed by adaptive T-cell or B-cell responses. Furthermore, the impact of HLA expression on MΦs has the capacity to change downstream adaptive antigen presentation-dependent events, and decreased expression of HLA-DQ and HLA-DR is described in CF monocytes/MΦs [96]. In traditional immunology, antigen presentation by APC, such as MΦs and dendritic cells, results in adaptive networks including T-cell immunity and B-cell activation [93]. Recently, it has become increasingly evident that B cells are not only responders to T-cell help, but, in exchange, are important programmers of the CD4 T-cell response including the priming and induction of T-cell memory [97]. In patients with CF, there is significant evidence supporting the concept of inefficient immune adaptive functions including the effector functions of NK cells [98], B cells [99] and T-cell abnormalities [86, 100] ultimately also contributing to the ineffective management of CF pathophysiology, which is directed by downstream communication circuits relayed through MΦs and dendritic cells.
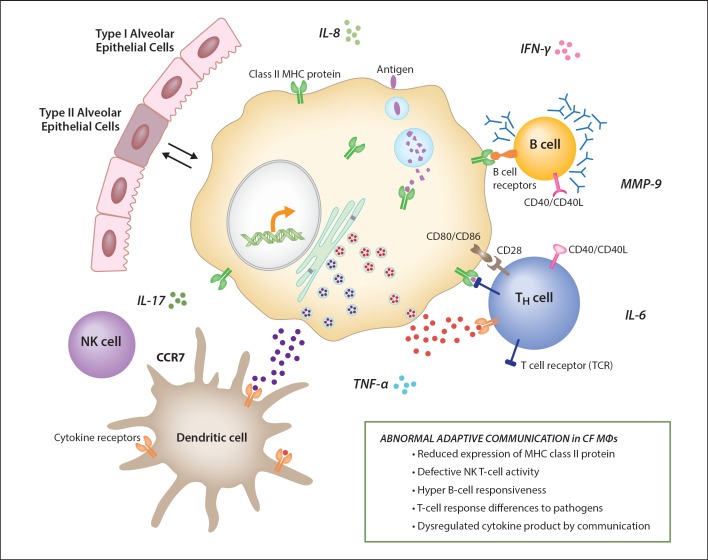
The interactions of MΦs with other cells of the immune system also implicate them in changing downstream immune events. Dysfunctional epithelial cell function, the hallmark of CF, and changes in MΦ phenotype, can be complicated by the cross-talk between MΦs and epithelial cells [1]. MΦs and respiratory epithelial cells can communicate via direct contact (the formation of gap junction channels), via cell surface receptors (e.g. CD200R and TGFBR) and via paracrine communication mediated by cytokines and microvesicles [1]. No knowledge is available regarding the potential dysregulation on MΦs and respiratory epithelium cross-talk in CF. However, as highlighted in this review, the combined effects of immune cross-talk and the cellular immune artillery amplifies the role of MΦs in the CF immune response to infection. Figure 2 demonstrates the hypothesis related to the diversity of the MΦ phenotype and the impact that this may have not only on MΦ function but also on the function of the adaptive immune cells, which ultimately will relay the MΦ message regarding the appropriate host response.
Fig. 2.
The figure emphasizes the ‘team’ function of MΦ contribution to immune function and the potential contributions to CF lung pathophysiology. MΦs, when dysfunctional, may not only alter their important role in healthy lung homeostasis, but also impact the surrounding immune community. T-cell communication networks through MΦ MHC class II (defined by genomic correlations), with the potential of contributing to inefficient B-cell, T-cell and NK-cell activity, suggesting downstream pathophysiology associated with MΦ dysfunction.
Concluding Remarks: MΦ Function and CF
In summary, the innate immune response is altered in CF lungs, and monocytes/MΦs are key contributors in orchestrating this process. The studies discussed in this review highlight that inherited (the loss of CFTR), as well as acquired factors (CF lung environment) affect the function of monocytes/MΦs, so that they do not properly handle inflammatory triggers, they struggle to resolve inflammation and they fail to clear bacterial infection. In addition, MΦs may improperly communicate with the other cells of the immune system, thus harming the adaptive immune response. Furthermore, the remarkable plasticity of MΦs and the different MΦ subpopulations that can coexist complicate how these cells participate in CF lung pathophysiology, and our understating of these mechanisms is still in its infancy. Altogether, these studies support the notion that MΦs contribute to CF lung pathology concomitantly with bronchial epithelium dysfunction.
Importantly, immune dysregulation represents a hallmark of the multiorgan manifestations in CF, such that hyperinflammation contributes to the destruction of the exocrine pancreas [101] and the tissue integrity of the gastrointestinal tract [102]. Thus, alterations in monocyte/MΦ function may contribute to CF manifestations beyond the lung disease.
As a prospective for the future, an effective, long-term therapy for CF should also modulate monocyte/MΦ function, as has also been suggested in studies on patients treated with ivacaftor [34, 35, 36].
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgements
We thank the American Cystic Fibrosis Foundation (BRUSCI14G0 and BRUSCI15P0 to E.M.B. and BONFIE1410, BONFIE15XX0 to T.L.B.) and NIH (DK027651 and NIH-HL104362 to T.L.B.) for their support. We acknowledge the vast number of clinicians and researchers who have contributed to the study of MΦs in CF. Our review did have a limitation on how many references could be used, so we apologize for not including any other manuscripts that could be perceived as supporting the review.
References
- 1.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 2.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 3.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2015;17:9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- 4.Pandey S, Kawai T, Akira S. Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harb Perspect Biol. 2015;7:a016246. doi: 10.1101/cshperspect.a016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne AJ, Mathie SA, Gregory LG, Lloyd CM. Pulmonary macrophages: key players in the innate defence of the airways. Thorax. 2015;70:1189–1196. doi: 10.1136/thoraxjnl-2015-207020. [DOI] [PubMed] [Google Scholar]
- 6.Wilson GB, Fudenberg HH. Does a primary host defense abnormality involving monocytes-macrophages underlie the pathogenesis of lung disease in cystic fibrosis? Med Hypotheses. 1982;8:527–542. doi: 10.1016/0306-9877(82)90014-7. [DOI] [PubMed] [Google Scholar]
- 7.Elborn JS, Norman D, Delamere FM, Shale DJ. In vitro tumor necrosis factor-alpha secretion by monocytes from patients with cystic fibrosis. Am J Respir Cell Mol Biol. 1992;6:207–211. doi: 10.1165/ajrcmb/6.2.207. [DOI] [PubMed] [Google Scholar]
- 8.Jones MM, Seilheimer DK, Pier GB, Rossen RD. Increased elastase secretion by peripheral blood monocytes in cystic fibrosis patients. Clin Exp Immunol. 1990;80:344–349. doi: 10.1111/j.1365-2249.1990.tb03290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson GB, Bahm VJ. Synthesis and secretion of cystic fibrosis ciliary dyskinesia substances by purified subpopulations of leukocytes. J Clin Invest. 1980;66:1010–1019. doi: 10.1172/JCI109929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimura K, Nakamura H, Trapnell BC, Chu CS, Dalemans W, Pavirani A, Lecocq JP, Crystal RG. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 1991;19:5417–5423. doi: 10.1093/nar/19.19.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomassen MJ, Demko CA, Wood RE, Sherman JM. Phagocytosis of Pseudomonas aeruginosa by polymorphonuclear leukocytes and monocytes: effect of cystic fibrosis serum. Infect Immun. 1982;38:802–805. doi: 10.1128/iai.38.2.802-805.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doring G. The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med. 1994;150:S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. [DOI] [PubMed] [Google Scholar]
- 13.Hubeau C, Puchelle E, Gaillard D. Distinct pattern of immune cell population in the lung of human fetuses with cystic fibrosis. J Allergy Clin Immunol. 2001;108:524–529. doi: 10.1067/mai.2001.118516. [DOI] [PubMed] [Google Scholar]
- 14.Brennan S, Sly PD, Gangell CL, Sturges N, Winfield K, Wikstrom M, Gard S, Upham JW, Arest CF. Alveolar macrophages and CC chemokines are increased in children with cystic fibrosis. Eur Respir J. 2009;34:655–661. doi: 10.1183/09031936.00178508. [DOI] [PubMed] [Google Scholar]
- 15.Regamey N, Tsartsali L, Hilliard TN, Fuchs O, Tan HL, Zhu J, Qiu YS, Alton EW, Jeffery PK, Bush A, Davies JC. Distinct patterns of inflammation in the airway lumen and bronchial mucosa of children with cystic fibrosis. Thorax. 2012;67:164–170. doi: 10.1136/thoraxjnl-2011-200585. [DOI] [PubMed] [Google Scholar]
- 16.Hartl D, Griese M, Kappler M, Zissel G, Reinhardt D, Rebhan C, Schendel DJ, Krauss-Etschmann S. Pulmonary T(h)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol. 2006;117:204–211. doi: 10.1016/j.jaci.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Grasemann H, Schwiertz R, Matthiesen S, Racke K, Ratjen F. Increased arginase activity in cystic fibrosis airways. Am J Respir Crit Care Med. 2005;172:1523–1528. doi: 10.1164/rccm.200502-253OC. [DOI] [PubMed] [Google Scholar]
- 18.Murphy BS, Bush HM, Sundareshan V, Davis C, Hagadone J, Cory TJ, Hoy H, Hayes D, Jr, Anstead MI, Feola DJ. Characterization of macrophage activation states in patients with cystic fibrosis. J Cyst Fibros. 2010;9:314–322. doi: 10.1016/j.jcf.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Jaecklin T, Duerr J, Huang H, Rafii M, Bear CE, Ratjen F, Pencharz P, Kavanagh BP, Mall MA, Grasemann H. Lung arginase expression and activity is increased in cystic fibrosis mouse models. J Appl Physiol (1985) 2014;117:284–288. doi: 10.1152/japplphysiol.00167.2014. [DOI] [PubMed] [Google Scholar]
- 20.Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–261. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 21.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 22.Wright AK, Rao S, Range S, Eder C, Hofer TP, Frankenberger M, Kobzik L, Brightling C, Grigg J, Ziegler-Heitbrock L. Pivotal advance: expansion of small sputum macrophages in CF: failure to express MARCO and mannose receptors. J Leukoc Biol. 2009;86:479–489. doi: 10.1189/jlb.1108699. [DOI] [PubMed] [Google Scholar]
- 23.Meyer M, Huaux F, Gavilanes X, van den Brule S, Lebecque P, Lo Re S, Lison D, Scholte B, Wallemacq P, Leal T. Azithromycin reduces exaggerated cytokine production by M1 alveolar macrophages in cystic fibrosis. Am J Respir Cell Mol Biol. 2009;41:590–602. doi: 10.1165/rcmb.2008-0155OC. [DOI] [PubMed] [Google Scholar]
- 24.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, Palfrey HC, Nelson DJ. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 25.Andersson C, Zaman MM, Jones AB, Freedman SD. Alterations in immune response and PPAR/LXR regulation in cystic fibrosis macrophages. J Cyst Fibros. 2008;7:68–78. doi: 10.1016/j.jcf.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Bruscia EM, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, Krause DS, Egan ME. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am J Respir Cell Mol Biol. 2009;40:295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keiser N, Gabdoulkhakova A, Riazanski V, Nelson D, Engelhardt J. Ferret alveolar macrophage function is dependent on CFTR. Pediatr Pulmonol. 2014;49:278–279. [Google Scholar]
- 28.Sorio C, Buffelli M, Angiari C, Ettorre M, Johansson J, Vezzalini M, Viviani L, Ricciardi M, Verze G, Assael BM, Melotti P. Defective CFTR expression and function are detectable in blood monocytes: development of a new blood test for cystic fibrosis. PLoS One. 2011;6:e22212. doi: 10.1371/journal.pone.0022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Weert-van Leeuwen PB, van Meegen MA, Speirs JJ, Pals DJ, Rooijakkers SH, Van der Ent CK, Terheggen-Lagro SW, Arets HG, Beekman JM. Optimal complement-mediated phagocytosis of Pseudomonas aeruginosa by monocytes is CFTR-dependent. Am J Respir Cell Mol Biol. 2013;49:463–470. doi: 10.1165/rcmb.2012-0502OC. [DOI] [PubMed] [Google Scholar]
- 30.Bruscia EM, Zhang PX, Satoh A, Caputo C, Medzhitov R, Shenoy A, Egan ME, Krause DS. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J Immunol. 2011;186:6990–6998. doi: 10.4049/jimmunol.1100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shenoy A, Kopic S, Murek M, Caputo C, Geibel JP, Egan ME. Calcium-modulated chloride pathways contribute to chloride flux in murine CF-affected macrophages. Pediatr Res. 2011;70:447–452. doi: 10.1203/PDR.0b013e31822f2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaman MM, Gelrud A, Junaidi O, Regan MM, Warny M, Shea JC, Kelly C, O'Sullivan BP, Freedman SD. Interleukin 8 secretion from monocytes of subjects heterozygous for the deltaF508 cystic fibrosis transmembrane conductance regulator gene mutation is altered. Clin Diagn Lab Immunol. 2004;11:819–824. doi: 10.1128/CDLI.11.5.819-824.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cory TJ, Birket SE, Murphy BS, Hayes D, Jr, Anstead MI, Kanga JF, Kuhn RJ, Bush HM, Feola DJ. Impact of azithromycin treatment on macrophage gene expression in subjects with cystic fibrosis. J Cyst Fibros. 2014;13:164–171. doi: 10.1016/j.jcf.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bratcher PE, Rowe SM, Reeves G, Roberts T, Szul T, Harris WT, Tirouvanziam R, Gaggar A. Alterations in blood leukocytes of G551D-bearing cystic fibrosis patients undergoing treatment with ivacaftor. J Cyst Fibros. 2016;15:67–73. doi: 10.1016/j.jcf.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorio C, Montresor A, Bolomini-Vittori M, Caldrer S, Rossi B, Dusi S, Angiari S, Johansson JE, Vezzalini M, Leal T, Calcaterra E, Assael BM, Melotti P, Laudanna C. Mutations of cystic fibrosis transmembrane conductance regulator (CFTR) gene cause a monocyte-selective adhesion deficiency. Am J Respir Crit Care Med. 2016;193:1123–1133. doi: 10.1164/rccm.201510-1922OC. [DOI] [PubMed] [Google Scholar]
- 36.Hisert KB, Schoenfelt KQ, Cooke G, Grogan B, Launspach JL, Gallagher CG, Donnelly SC, Welsh MJ, Singh PK, McKone EF, Becker L. Ivacaftor-induced proteomic changes suggest monocyte defects may contribute to the pathogenesis of cystic fibrosis. Am J Respir Cell Mol Biol. 2016;54:594–597. doi: 10.1165/rcmb.2015-0322LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros. 2015;14:419–430. doi: 10.1016/j.jcf.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Saini Y, Dang H, Livraghi-Butrico A, Kelly EJ, Jones LC, O'Neal WK, Boucher RC. Gene expression in whole lung and pulmonary macrophages reflects the dynamic pathology associated with airway surface dehydration. BMC Genomics. 2014;15:726. doi: 10.1186/1471-2164-15-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lubamba BA, Jones LC, O'Neal WK, Boucher RC, Ribeiro CM. X-box-binding protein 1 and innate immune responses of human cystic fibrosis alveolar macrophages. Am J Respir Crit Care Med. 2015;192:1449–1461. doi: 10.1164/rccm.201504-0657OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopp BT, Abdulrahman BA, Khweek AA, Kumar SB, Akhter A, Montione R, Tazi MF, Caution K, McCoy K, Amer AO. Exaggerated inflammatory responses mediated by Burkholderia cenocepacia in human macrophages derived from cystic fibrosis patients. Biochem Biophys Res Commun. 2012;424:221–227. doi: 10.1016/j.bbrc.2012.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang PX, Cheng J, Zou S, D'Souza AD, Koff JL, Lu J, Lee PJ, Krause DS, Egan ME, Bruscia EM. Pharmacological modulation of the AKT/ microRNA-199a-5p/CAV1 pathway ameliorates cystic fibrosis lung hyper-inflammation. Nat Commun. 2015;6:6221. doi: 10.1038/ncomms7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonfield TL, Hodges CA, Cotton CU, Drumm ML. Absence of the cystic fibrosis transmembrane regulator (CFTR) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol. 2012;92:1111–1122. doi: 10.1189/jlb.0412188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang PX, Murray TS, Villella VR, Ferrari E, Esposito S, D'Souza A, Raia V, Maiuri L, Krause DS, Egan ME, Bruscia EM. Reduced caveolin-1 promotes hyperinflammation due to abnormal heme oxygenase-1 localization in lipopolysaccharide-challenged macrophages with dysfunctional cystic fibrosis transmembrane conductance regulator. J Immunol. 2013;190:5196–5206. doi: 10.4049/jimmunol.1201607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamothe J, Valvano MA. Burkholderia cenocepacia-induced delay of acidification and phagolysosomal fusion in cystic fibrosis transmembrane conductance regulator (CFTR)-defective macrophages. Microbiology. 2008;154:3825–3834. doi: 10.1099/mic.0.2008/023200-0. [DOI] [PubMed] [Google Scholar]
- 45.Abdulrahman BA, Khweek AA, Akhter A, Caution K, Kotrange S, Abdelaziz DH, Newland C, Rosales-Reyes R, Kopp B, McCoy K, Montione R, Schlesinger LS, Gavrilin MA, Wewers MD, Valvano MA, Amer AO. Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy. 2011;7:1359–1370. doi: 10.4161/auto.7.11.17660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oceandy D, McMorran BJ, Smith SN, Schreiber R, Kunzelmann K, Alton EW, Hume DA, Wainwright BJ. Gene complementation of airway epithelium in the cystic fibrosis mouse is necessary and sufficient to correct the pathogen clearance and inflammatory abnormalities. Hum Mol Genet. 2002;11:1059–1067. doi: 10.1093/hmg/11.9.1059. [DOI] [PubMed] [Google Scholar]
- 47.Sturges NC, Wikstrom ME, Winfield KR, Gard SE, Brennan S, Sly PD, Upham JW. Monocytes from children with clinically stable cystic fibrosis show enhanced expression of Toll-like receptor 4. Pediatr Pulmonol. 2010;45:883–889. doi: 10.1002/ppul.21230. [DOI] [PubMed] [Google Scholar]
- 48.Simonin-Le Jeune K, Le Jeune A, Jouneau S, Belleguic C, Roux PF, Jaguin M, Dimanche-Boitre MT, Lecureur V, Leclercq C, Desrues B, Brinchault G, Gangneux JP, Martin-Chouly C. Impaired functions of macrophage from cystic fibrosis patients: CD11b, TLR-5 decrease and sCD14, inflammatory cytokines increase. PLoS One. 2013;8:e75667. doi: 10.1371/journal.pone.0075667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly C, Canning P, Buchanan PJ, Williams MT, Brown V, Gruenert DC, Elborn JS, Ennis M, Schock BC. Toll-like receptor 4 is not targeted to the lysosome in cystic fibrosis airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2013;304:L371–L382. doi: 10.1152/ajplung.00372.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rymut SM, Ivy T, Corey DA, Cotton CU, Burgess JD, Kelley TJ. Role of exchange protein activated by cAMP 1 in regulating rates of microtubule formation in cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol. 2015;53:853–862. doi: 10.1165/rcmb.2014-0462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamai H, Keyserman F, Quittell LM, Worgall TS. Defective CFTR increases synthesis and mass of sphingolipids that modulate membrane composition and lipid signaling. J Lipid Res. 2009;50:1101–1108. doi: 10.1194/jlr.M800427-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Li X, Grassme H, Doring G, Gulbins E. Alterations in ceramide concentration and pH determine the release of reactive oxygen species by CFTR-deficient macrophages on infection. J Immunol. 2010;184:5104–5111. doi: 10.4049/jimmunol.0902851. [DOI] [PubMed] [Google Scholar]
- 53.White NM, Jiang D, Burgess JD, Bederman IR, Previs SF, Kelley TJ. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;292:L476–L486. doi: 10.1152/ajplung.00262.2006. [DOI] [PubMed] [Google Scholar]
- 54.Gargalovic P, Dory L. Caveolins and macrophage lipid metabolism. J Lipid Res. 2003;44:11–21. doi: 10.1194/jlr.r200005-jlr200. [DOI] [PubMed] [Google Scholar]
- 55.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D'Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol. 2010;12:863–875. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- 57.Deretic V, Kimura T, Timmins G, Moseley P, Chauhan S, Mandell M. Immunologic manifestations of autophagy. J Clin Invest. 2015;125:75–84. doi: 10.1172/JCI73945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amer AO, Byrne BG, Swanson MS. Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes. Autophagy. 2005;1:53–58. doi: 10.4161/auto.1.1.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bajmoczi M, Gadjeva M, Alper SL, Pier GB, Golan DE. Cystic fibrosis transmembrane conductance regulator and caveolin-1 regulate epithelial cell internalization of Pseudomonas aeruginosa. Am J Physiol Cell Physiol. 2009;297:C263–C277. doi: 10.1152/ajpcell.00527.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bessich JL, Nymon AB, Moulton LA, Dorman D, Ashare A. Low levels of insulin-like growth factor-1 contribute to alveolar macrophage dysfunction in cystic fibrosis. J Immunol. 2013;191:378–385. doi: 10.4049/jimmunol.1300221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fick RB, Jr, Naegel GP, Matthay RA, Reynolds HY. Cystic fibrosis pseudomonas opsonins. Inhibitory nature in an in vitro phagocytic assay. J Clin Invest. 1981;68:899–914. doi: 10.1172/JCI110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tosi MF, Zakem H, Berger M. Neutrophil elastase cleaves C3bi on opsonized Pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest. 1990;86:300–308. doi: 10.1172/JCI114699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barasch J, Kiss B, Prince A, Saiman L, Gruenert D, al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991;352:70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- 64.Del Porto P, Cifani N, Guarnieri S, Di Domenico EG, Mariggio MA, Spadaro F, Guglietta S, Anile M, Venuta F, Quattrucci S, Ascenzioni F. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One. 2011;6:e19970. doi: 10.1371/journal.pone.0019970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haggie PM, Verkman AS. Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J Biol Chem. 2007;282:31422–31428. doi: 10.1074/jbc.M705296200. [DOI] [PubMed] [Google Scholar]
- 66.Barriere H, Bagdany M, Bossard F, Okiyoneda T, Wojewodka G, Gruenert D, Radzioch D, Lukacs GL. Revisiting the role of cystic fibrosis transmembrane conductance regulator and counterion permeability in the pH regulation of endocytic organelles. Mol Biol Cell. 2009;20:3125–3141. doi: 10.1091/mbc.E09-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riazanski V, Gabdoulkhakova AG, Boynton LS, Eguchi RR, Deriy LV, Hogarth DK, Loaec N, Oumata N, Galons H, Brown ME, Shevchenko P, Gallan AJ, Yoo SG, Naren AP, Villereal ML, Beacham DW, Bindokas VP, Birnbaumer L, Meijer L, Nelson DJ. TRPC6 channel translocation into phagosomal membrane augments phagosomal function. Proc Natl Acad Sci USA. 2015;112:E6486–E6495. doi: 10.1073/pnas.1518966112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdulrahman BA, Khweek AA, Akhter A, Caution K, Tazi M, Hassan H, Zhang Y, Rowland PD, Malhotra S, Aeffner F, Davis IC, Valvano MA, Amer AO. Depletion of the ubiquitin-binding adaptor molecule SQSTM1/p62 from macrophages harboring cftr ΔF508 mutation improves the delivery of Burkholderia cenocepacia to the autophagic machinery. J Biol Chem. 2013;288:2049–2058. doi: 10.1074/jbc.M112.411728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan K, Huang C, Fox J, Laturnus D, Carlson E, Zhang B, Yin Q, Gao H, Wu M. Autophagy plays an essential role in the clearance of Pseudomonas aeruginosa by alveolar macrophages. J Cell Sci. 2012;125:507–515. doi: 10.1242/jcs.094573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schnaith A, Kashkar H, Leggio SA, Addicks K, Kronke M, Krut O. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J Biol Chem. 2007;282:2695–2706. doi: 10.1074/jbc.M609784200. [DOI] [PubMed] [Google Scholar]
- 71.Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, Kaczmarek E, Lee PJ, Zuckerbraun BS, Flavell R, Soares MP, Otterbein LE. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest. 2014;124:4926–4940. doi: 10.1172/JCI72853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 73.Soleti R, Porro C, Martinez MC. Apoptotic process in cystic fibrosis cells. Apoptosis. 2013;18:1029–1038. doi: 10.1007/s10495-013-0874-y. [DOI] [PubMed] [Google Scholar]
- 74.McCubbrey AL, Curtis JL. Efferocytosis and lung disease. Chest. 2013;143:1750–1757. doi: 10.1378/chest.12-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Croasdell A, Duffney PF, Kim N, Lacy SH, Sime PJ, Phipps RP. PPARγ and the innate immune system mediate the resolution of inflammation. PPAR Res. 2015;2015:549691. doi: 10.1155/2015/549691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonfield TL, Farver CF, Barna BP, Malur A, Abraham S, Raychaudhuri B, Kavuru MS, Thomassen MJ. Peroxisome proliferator-activated receptor-gamma is deficient in alveolar macrophages from patients with alveolar proteinosis. Am J Respir Cell Mol Biol. 2003;29:677–682. doi: 10.1165/rcmb.2003-0148OC. [DOI] [PubMed] [Google Scholar]
- 77.Ollero M, Junaidi O, Zaman MM, Tzameli I, Ferrando AA, Andersson C, Blanco PG, Bialecki E, Freedman SD. Decreased expression of peroxisome proliferator activated receptor gamma in cftr−/− mice. J Cell Physiol. 2004;200:235–244. doi: 10.1002/jcp.20020. [DOI] [PubMed] [Google Scholar]
- 78.Majai G, Sarang Z, Csomos K, Zahuczky G, Fesus L. PPARγ-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur J Immunol. 2007;37:1343–1354. doi: 10.1002/eji.200636398. [DOI] [PubMed] [Google Scholar]
- 79.Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garratt LW, Wright AK, Ranganathan SC, Grigg J, Sly PD, behalf of AC Small macrophages are present in early childhood respiratory disease. J Cyst Fibros. 2012;11:201–208. doi: 10.1016/j.jcf.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 83.Haq IJ, Gray MA, Garnett JP, Ward C, Brodlie M. Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets. Thorax. 2016;71:284–287. doi: 10.1136/thoraxjnl-2015-207588. [DOI] [PubMed] [Google Scholar]
- 84.Bergougnoux A, Claustres M, de Sario A. Nasal epithelial cells: a tool to study DNA methylation in airway diseases. Epigenomics. 2015;7:119–126. doi: 10.2217/epi.14.65. [DOI] [PubMed] [Google Scholar]
- 85.Soltys J, Bonfield T, Chmiel J, Berger M. Functional IL-10 deficiency in the lung of cystic fibrosis (cftr−/−) and IL-10 knockout mice causes increased expression and function of B7 costimulatory molecules on alveolar macrophages. J Immunol. 2002;168:1903–1910. doi: 10.4049/jimmunol.168.4.1903. [DOI] [PubMed] [Google Scholar]
- 86.Mueller C, Braag SA, Keeler A, Hodges C, Drumm M, Flotte TR. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol. 2011;44:922–929. doi: 10.1165/rcmb.2010-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR. Quantification of the relative contribution of environmental and genetic factors to variation in cystic fibrosis lung function. J Pediatr. 2010;157:802–807. doi: 10.1016/j.jpeds.2010.05.018. e1-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cutting GR. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann NY Acad Sci. 2010;1214:57–69. doi: 10.1111/j.1749-6632.2010.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wright FA, Strug LJ, Doshi VK, Commander CW, Blackman SM, Sun L, Berthiaume Y, Cutler D, Cojocaru A, Collaco JM, Corey M, Dorfman R, Goddard K, Green D, Kent JW, Jr, Lange EM, Lee S, Li W, Luo J, Mayhew GM, Naughton KM, Pace RG, Pare P, Rommens JM, Sandford A, Stonebraker JR, Sun W, Taylor C, Vanscoy LL, Zou F, Blangero J, Zielenski J, O'Neal WK, Drumm ML, Durie PR, Knowles MR, Cutting GR. Genome-wide association and linkage identify modifier loci of lung disease severity in cystic fibrosis at 11p13 and 20q13.2. Nat Genet. 2011;43:539–546. doi: 10.1038/ng.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weiler CA, Drumm ML. Genetic influences on cystic fibrosis lung disease severity. Front Pharmacol. 2013;4:40. doi: 10.3389/fphar.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaggar A, Chen J, Chmiel JF, Dorkin HL, Flume PA, Griffin R, Nichols D, Donaldson SH. Inhaled alpha1-proteinase inhibitor therapy in patients with cystic fibrosis. J Cyst Fibros. 2016;15:227–233. doi: 10.1016/j.jcf.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chauhan B, Hutcheson PS, Slavin RG, Bellone CJ. MHC restriction in allergic bronchopulmonary aspergillosis. Front Biosci. 2003;8:s140–s148. doi: 10.2741/971. [DOI] [PubMed] [Google Scholar]
- 93.Jovanovic K, Siebeck M, Gropp R. The route to pathologies in chronic inflammatory diseases characterized by T helper type 2 immune cells. Clin Exp Immunol. 2014;178:201–211. doi: 10.1111/cei.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohashi W, Hattori K, Hattori Y. Control of macrophage dynamics as a potential therapeutic approach for clinical disorders involving chronic inflammation. J Pharmacol Exp Ther. 2015;354:240–250. doi: 10.1124/jpet.115.225540. [DOI] [PubMed] [Google Scholar]
- 95.Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P, Yao H, Cao X. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat Immunol. 2011;12:416–424. doi: 10.1038/ni.2015. [DOI] [PubMed] [Google Scholar]
- 96.Hofer TP, Frankenberger M, Heimbeck I, Burggraf D, Wjst M, Wright AK, Kerscher M, Nahrig S, Huber RM, Fischer R, Ziegler-Heitbrock L. Decreased expression of HLA-DQ and HLA-DR on cells of the monocytic lineage in cystic fibrosis. J Mol Med (Berl) 2014;92:1293–1304. doi: 10.1007/s00109-014-1200-z. [DOI] [PubMed] [Google Scholar]
- 97.Barr TA, Gray M, Gray D. B cells: programmers of CD4 T cell responses. Infect Disord Drug Targets. 2012;12:222–231. doi: 10.2174/187152612800564446. [DOI] [PubMed] [Google Scholar]
- 98.Siegmann N, Worbs D, Effinger F, Bormann T, Gebhardt M, Ulrich M, Wermeling F, Muller-Hermelink E, Biedermann T, Tighe M, Edwards MJ, Caldwell C, Leadbetter E, Karlsson MC, Becker KA, Gulbins E, Doring G. Invariant natural killer T (iNKT) cells prevent autoimmunity, but induce pulmonary inflammation in cystic fibrosis. Cell Physiol Biochem. 2014;34:56–70. doi: 10.1159/000362984. [DOI] [PubMed] [Google Scholar]
- 99.Bodas M, Vij N. The NF-kappaB signaling in cystic fibrosis lung disease: pathophysiology and therapeutic potential. Discov Med. 2010;9:346–356. [PMC free article] [PubMed] [Google Scholar]
- 100.Hector A, Schafer H, Poschel S, Fischer A, Fritzsching B, Ralhan A, Carevic M, Oz H, Zundel S, Hogardt M, Bakele M, Rieber N, Riethmueller J, Graepler-Mainka U, Stahl M, Bender A, Frick JS, Mall M, Hartl D. Regulatory T-cell impairment in cystic fibrosis patients with chronic Pseudomonas infection. Am J Respir Crit Care Med. 2015;191:914–923. doi: 10.1164/rccm.201407-1381OC. [DOI] [PubMed] [Google Scholar]
- 101.Abu-El-Haija M, Ramachandran S, Meyerholz DK, Abu-El-Haija M, Griffin M, Giriyappa RL, Stoltz DA, Welsh MJ, McCray PB, Jr, Uc A. Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways. Am J Pathol. 2012;181:499–507. doi: 10.1016/j.ajpath.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Munck A. Cystic fibrosis: evidence for gut inflammation. Int J Biochem Cell Biol. 2014;52:180–183. doi: 10.1016/j.biocel.2014.02.005. [DOI] [PubMed] [Google Scholar]