Abstract
Aims/Introduction
Type 2 diabetes is a worldwide disease that is associated with increased rates of obesity and reduced physical activity. Obesity‐associated insulin resistance in type 2 diabetes is a disorder in the balance between pro‐inflammatory and anti‐inflammatory signals. T cell immunoglobulin and mucin domain‐containing molecule 3 (Tim‐3) has been reported as an important regulatory inflammation molecule, and plays a pivotal role in several inflammation‐related diseases.
Materials and Methods
Peripheral blood mononuclear cells were obtained from type 2 diabetes patients (n = 31) and healthy donors (n = 18), and Tim‐3 expression on peripheral blood mononuclear cells was evaluated by flow cytometry.
Results
We showed the downregulated expression of Tim‐3 on CD14+ monocytes from type 2 diabetes patients. In addition, the upregulated expression of Tim‐3 on peripheral CD4+ T cells and CD8+ T cells was observed in the present study. The correlation analysis between Tim‐3 expression on CD14+ monocytes and diabetes duration showed the longer diabetes duration time, the lower Tim‐3 expression on CD14 monocytes.
Conclusions
The present results suggest that Tim‐3 might participate in the progression of type 2 diabetes by its negative regulation on these immune cells, and Tim‐3 on CD14+ monocytes serves as a novel biological marker for diabetes duration in type 2 diabetes patients.
Keywords: Biological marker, T cell immunoglobulin and mucin domain‐containing molecule 3, Type 2 diabetes
Introduction
Type 2 diabetes, which is largely associated with increased rates of obesity and reduced physical activity, has become a global epidemic1. Approximately 3.4 million deaths are attributable to type 2 diabetes according to an investigation by the World Health Organization. Type 2 diabetes is characterized by hyperglycemia in the context of insulin resistance (IR) with microvascular and macrovascular complications2. Several studies have focused on the inflammation issue in type 2 diabetes, as obesity‐associated IR in type 2 diabetes is a disorder in the balance between pro‐inflammatory and anti‐inflammatory signals3. On one hand, the pro‐inflammatory cytokines can impede insulin signaling in obesity and diabetes, such as tumor necrosis factor‐α, interleukin (IL)‐1β and interferon (IFN)‐γ4, 5, 6, 7. On the other hand, anti‐inflammatory cytokines, such as IL‐10 and IL‐4, can help maintain insulin sensitivity8, 9. In addition, CD8+ and CD4+ T cells in adaptive immunity10, and natural killer cells11 and macrophages12 in innate immunity are also involved in type 2 diabetes‐related inflammation. Therefore, now the key task for researchers is to find new inflammatory molecules involved in the pathogenesis of type 2 diabetes. In addition, diabetes duration in type 2 diabetes patients might affect effective treatment, and most type 2 diabetes patients cannot clearly determine the disease onset. Therefore, it is important to find a novel biological marker for diabetes duration calculation in type 2 diabetes patients.
T cell immunoglobulin and mucin domain‐containing molecule 3 (Tim‐3) is a membrane protein initially identified as a negative regulator of Th1 immunity13, 14. Recently, Tim‐3 was also shown to play important roles in activated T helper 1715, cytotoxic T cell 116, macrophages/monocytes17, 18, dendritic cells19 and natural killer cells20. A strong correlation between Tim‐3 expression and some chronic diseases has been recently confirmed, such as atherosclerosis21, 22 and chronic viral infection23. Recent data showed that Tim‐3 has a potential relationship with type 2 diabetes. Tim‐3 is involved in the therapeutic function in type 1 diabetes24, and its ligand, galectin‐9, is elevated in patients with type 2 diabetes25. Here, we will focus on the expression of Tim‐3 on the peripheral blood mononuclear cells in type 2 diabetes patients, and provide a novel biological marker on CD14+ monocytes for diabetes duration in type 2 diabetes patients.
Materials and Methods
Blood samples
Peripheral blood samples obtained from 31 patients with type 2 diabetes at the Endocrinology Department in Qilu Hospital, Jinan, Shandong, China, were involved for the isolation of peripheral mononuclear cells. Type 2 diabetes was diagnosed on the basis of fasting blood glucose ≥7.0 mmol/L and/or random blood glucose ≥11.1 mmol/L. None of the individuals used antihypertensive agents or statin agents in the diabetes duration. Diabetes duration means the timespan from the first diagnosis to when the blood samples were obtained. Blood samples of 18 healthy donors were obtained from a clinical laboratory at Qilu Hospital. None of the individuals were positive for hepatitis B virus, hepatitis V virus or HIV, consumed excessive alcohol, or other special cases before sampling. The study was approved by the medical ethics committee of Shandong University, and informed consent was acquired from each participant. All the data of the human participants are summarized in Table 1.
Table 1.
Clinical characteristics of participants
| Characteristics | T2D patients (n = 31) | Healthy donors (n = 18) |
|---|---|---|
| Female, n (%) | 18 (58.1) | 10 (55.6) |
| Age, years (range) | 57 (37–75) | 52 (30–68) |
| BMI (kg/m2) | 24.96 ± 0.65 | 22.34 ± 0.81 |
| HbA1c (%) | 8.92 ± 0.31 | 5.25 ± 0.98 |
| FPG (mmol/L) | 9.80 ± 0.56 | 4.18 ± 0.78 |
| Insulin (μIU/mL) | 30.31 ± 8.04 | 10.82 ± 5.09 |
| Diabetes duration (years) | 13.45 ± 1.66 | – |
| Total lymphocytes (%) | 32.91 ± 1.90 | 35.01 ± 2.30 |
| Total monocytes (%) | 5.25 ± 0.33 | 4.99 ± 0.36 |
| CD4+ T cells (%) | 35.92 ± 2.35 | 33.95 ± 2.44 |
| CD8+ T cells (%) | 32.10 ± 1.45 | 34.26 ± 2.38 |
| CD14+ monocytes (%) | 72.21 ± 2.37 | 73.86 ± 3.50 |
Data are expressed as median ± standard error of the mean. BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; T2D, type 2 diabetes.
Peripheral blood mononuclear cells isolation and flow cytometry
Human peripheral blood mononuclear cells were isolated by centrifuging the whole blood with an EZ‐Sep™ (Dakewe, Shenzhen, China) lymphocyte separation tube. The peripheral blood mononuclear cells were stained with anti‐human Tim‐3‐PE (Biolegend, San Diego, CA, USA), anti‐human CD3‐APC (Biolegend), anti‐human CD4‐FITC (Biolegend), anti‐human CD8‐FITC (Biolegend), anti‐human CD14‐FITC for 30 min. At least 10,000 cells were analyzed by a FACSAria II (BD, Franklin Lakes, NJ, USA), and the respective isotype control immunoglobulin G was involved as the control. Cells were gated based on their forward and side scatter properties. The cells in the Figure 1a were gated by the strategy in Figure S1a, and the cells in Figures 2a and 3a were gated by the strategy in Figure S1b.
Figure 1.
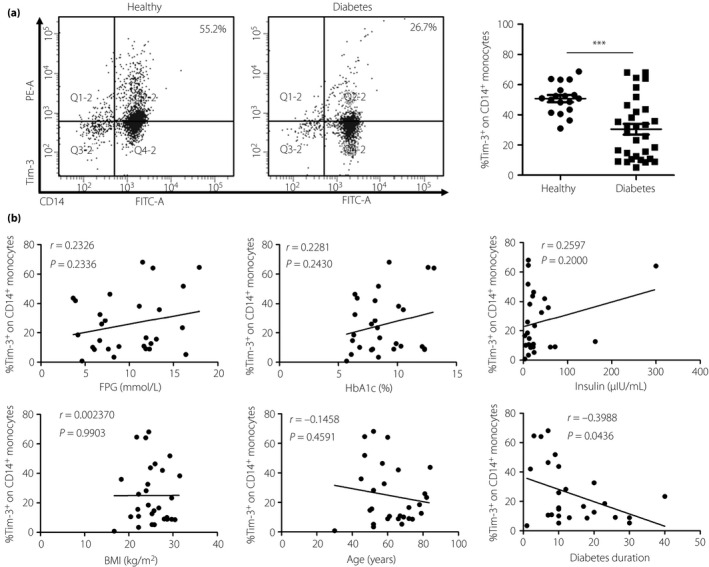
T cell immunoglobulin and mucin domain‐containing molecule 3 (Tim‐3) expression on CD14+ T cells in type 2 diabetes patients is significantly decreased. Peripheral blood mononuclear cells were isolated from healthy donors (n = 18) and patients with type 2 diabetes (n = 31). (a) Flow cytometry analysis of Tim‐3 expression on CD14+ T cells (left) and the statistical graph is shown (right) for type 2 diabetes patients (n = 31, 30.43 ± 3.58%) and healthy donors (n = 18, 50.78 ± 2.36%). (b) Correlation analysis of Tim‐3 expression on CD14+ T cells and type 2 diabetes patients’ fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), insulin, body mass index (BMI), age and diabetes duration. ***P < 0.001.
Figure 2.
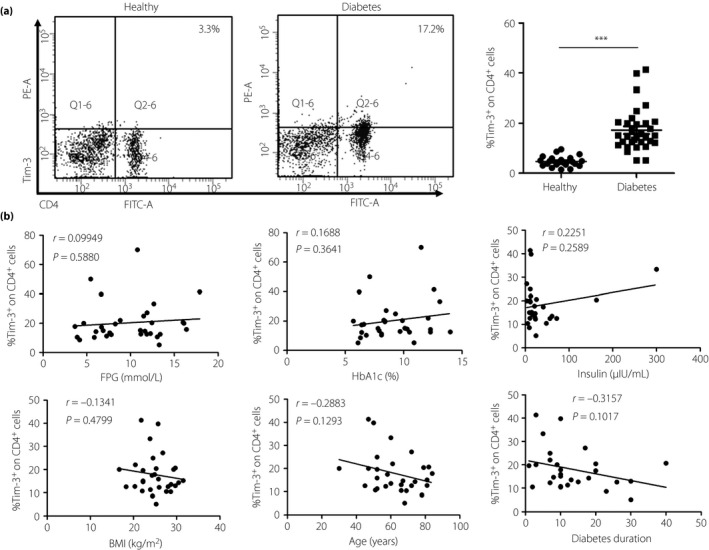
T cell immunoglobulin and mucin domain‐containing molecule 3 (Tim‐3) expression on CD4+ T cells in type 2 diabetes patients is significantly increased. Peripheral blood mononuclear cells were isolated from healthy donors (n = 18) and patients with type 2 diabetes (n = 31). (a) Flow cytometry analysis of Tim‐3 expression on CD4+ T cells (left) and the statistical graph are shown (right) of type 2 diabetes patients (n = 31, 17.26 ± 1.51%) and healthy donors (n = 18, 4.69 ± 0.45%). (b) Correlation analysis of Tim‐3 expression on CD4+ T cells and type 2 diabetes patients’ fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), insulin, body mass index (BMI), age and diabetes duration. ***P < 0.001.
Figure 3.
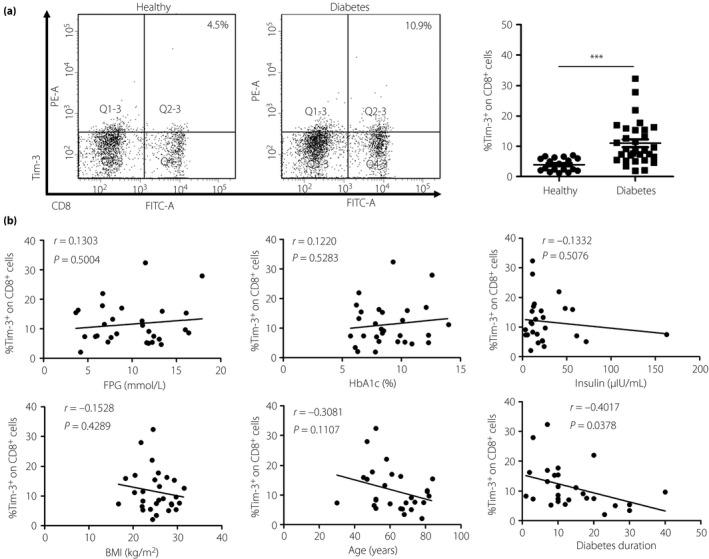
T cell immunoglobulin and mucin domain‐containing molecule 3 (Tim‐3) expression on CD8+ T cells in type 2 diabetes patients is significantly increased. Peripheral blood mononuclear cells were isolated from healthy donors (n = 18) and patients with type 2 diabetes (n = 31). (a) Flow cytometry analysis of Tim‐3 expression on CD8+ T cells (left) and the statistical graph is shown (right) of type 2 diabetes patients (n = 31, 11.01 ± 1.29%) and healthy donors (n = 18, 3.93 ± 0.51%). (b) Correlation analysis of Tim‐3 expression on CD8+ T cells and type 2 diabetes patients’, fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), insulin, body mass index (BMI), age and diabetes duration. ***P < 0.001.
Statistical analysis
All the data were analyzed by GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). Unpaired t‐test was used for comparison between groups. Pearson's correlation analysis was used to calculate the correlation coefficient. P < 0.05 was considered a significant difference.
Results
Tim‐3 expression is decreased on peripheral CD14+ monocytes in patients with type 2 diabetes
Monocytes and macrophages are a heterogeneous population of immune cells, and have been proven to function in type 2 diabetes development12, 26. We detected the expression of Tim‐3 on peripheral CD14+ monocytes in both the healthy donors and the type 2 diabetes patients by flow cytometry. The results showed that monocytes from type 2 diabetes patients (n = 31, 30.43 ± 3.58%) express less Tim‐3 than that from healthy donors (n = 18, 50.78 ± 2.36%; Figure 1a). Tim‐3 has been confirmed to be the key molecule in macrophages M1–M2 polarization27. Just opposite to the M1, the M2 phenotype carries out tissue surveillance and remodeling functions, and is associated with maintaining insulin sensitivity26. The aforementioned results show that the circulating monocytes polarize toward M1 macrophages and damage the insulin sensitivity.
Tim‐3 expression is increased on peripheral CD4+ in patients with type 2 diabetes
Based on the evidence that the circulating CD4+ T cells play important roles in type 2 diabetes28, we first analyzed the expression of Tim‐3 on peripheral CD4+ T cells in both the healthy donors and the type 2 diabetes patients by flow cytometry. As shown in Figure 2a, the type 2 diabetes patients (n = 31, 17.26 ± 1.51%) have a much higher level of Tim‐3 on CD4+ T cells than healthy donors (n = 18, 4.69 ± 0.45%). This result shows that Tim‐3 expression is increased on peripheral CD4+ T cells in patients with type 2 diabetes. Given that Tim‐3 is a negative regulatory molecule on CD4+ T cells, CD4+ T cells of type 2 diabetes patients might stay in a more suppressed state than healthy controls.
Tim‐3 expression is increased on peripheral CD8+ in patients with type 2 diabetes
As obesity‐associated CD8+ T cells could secrete IFN‐γ, which could activate macrophages and induce obesity‐related inflammation6, we analyzed the expression of Tim‐3 on peripheral CD8+ T cells in both the healthy donors and the type 2 diabetes patients flow cytometry. Tim‐3 expression on CD8+ T cells from type 2 diabetes patients (n = 31, 11.01 ± 1.29%) was significantly higher than that from healthy donors (n = 18, 3.93 ± 0.51%; Figure 3a). This result showed that CD8+ T cells from type 2 diabetes patients display much more Tim‐3 than that from healthy donors.
Correlation analysis of Tim‐3 expression on CD4+ T cells CD8+ T cells and type 2 diabetes indicators
As fasting plasma glucose, glycated hemoglobin, insulin, body mass index, age and diabetes duration are significant indicators in type 2 diabetes, we correlated Tim‐3 expression on CD4+ and CD8+ T cells with these indicators. As Figure 2b shows, no correlation was found between Tim‐3 expression on CD4+ T cells and these indicators. It might be that more complicated factors affect the expression of Tim‐3 on CD4+ T cells. An inverse correlation was found between Tim‐3 expression on CD8+ T cells and diabetes duration (P = 0.0378; Figure 3b). This result was inconsistent with the increased expression of Tim‐3 in Figure 3a. In addition to diabetes duration, there was no correlation was found between Tim‐3 expression on CD8+ T cells and other factors. Maybe the transient high glucose upregulates the Tim‐3 expression on CD8+ T cells, and the long diabetes duration restores CD8+ T cells function by downregulating the Tim‐3 expression.
Correlation analysis of Tim‐3 expression on CD14+ monocytes and type 2 diabetes indicators
In the correlation analysis of Tim‐3 expression on CD14+ monocytes and type 2 diabetes indicators, the same inverse correlation with CD8+ T cells was found between Tim‐3 expression on CD14+ monocytes and diabetes duration (P = 0.0436; Figure 1b). The other indicators had no association with Tim‐3 expression on CD14+ monocytes. The aforementioned results show that Tim‐3 on CD14+ monocytes could serve as novel biological markers for diabetes duration in type 2 diabetes patients, which means that the longer the hyperglycemia time the lower Tim‐3 expression on CD14+ monocytes.
Discussion
Tim‐3, a regulator of immune regulation and immune tolerance, is expressed in both innate and adaptive immune cells. Increased evidence has shown that dysregulation of Tim‐3 expression on peripheral CD4+ T cells, CD8+ T cells and monocytes is closely related to many autoimmune diseases, viral infections, and cancer15, 16, 29. However, it is still unclear whether Tim‐3 is involved in the pathogenesis of type 2 diabetes, which is characterized by hyperglycemia in the context of insulin resistance. In the present case–control study, we compared the Tim‐3 expression on various immune cells between patients with type 2 diabetes and healthy donors. We did not, however, find significant correlations between Tim‐3 expression on various immune cells and indicators of type 2 diabetes (fasting plasma glucose, glycated hemoglobin, insulin, body mass index, age and diabetes duration).
As type 2 diabetes is characterized by obesity‐associated chronic low‐grade inflammation, accumulating evidence shows multiple immune cells are involved in the pathogenetic process of type 2 diabetes. In 2012, Morinaga et al.30 reported that circulating immune cells infiltrate the expanding adipose tissue in response to high‐fat feeding. Circulating CD4+ T cells frequency consistently correlating positively with increased body mass index or adiposity in human subjects31 indicates that CD4+ T cells might function in type 2 diabetes. Tim‐3 is a negative regulatory molecule on CD4+ T cells. Tim‐3 overexpression on CD4+ T cells results in a low level of IL‐2 and IFN‐γ production32. In the present study, augmented Tim‐3 expression observed on CD4+ T cells in type 2 diabetes patients (Figure 2a) showed that CD4+ T cells of type 2 diabetes patients were in a more suppressed state than healthy controls. CD8+ T cells are the main T cells responsible for the eradication of altered or foreign cells by secreting perforin and granzyme33, 34. Previous reports claimed that CD8+ T cells were involved in the type 2 diabetes‐related inflammation. Obesity‐associated CD8+ T cells could secrete IFN‐γ, an important pro‐inflammatory cytokine for macrophage activation and obesity‐induced inflammation6, 35. CD8+ T cells deletion results in a relative lack of inflammatory macrophages in obese adipose tissue and, importantly, systemic insulin sensitivity35. Tim‐3 is a negative regulator of CD8+ T cells function, and Tim‐3 blockade can restore proliferation and cytokine production of CD8+ T cells36, 37. Upregulated expression of Tim‐3 on CD8+ T cells was observed in our current research (Figure 3a). The results suggest that CD8+ T cells in type 2 diabetes patients show a dysfunctional state. However, there is no related correlation between Tim‐3 expression on CD4+ and CD8+ T cells, and type 2 diabetes indicators except the inverse correlation between Tim‐3 expression on CD8+ T cells and diabetes duration (Figures 2b and 3b). This inverse correlation is inconsistent with the increased Tim‐3 expression on CD8+ T cells in type 2 diabetes patients. The exact reason why Tim‐3 was upregulated on CD4+ and CD8+ T cells of type 2 diabetes patients, the inverse correlation between Tim‐3 expression on CD8+ T cells and diabetes duration, still requires further research.
Compared with CD4+ and CD8+ T cells, monocytes and macrophages play a more important role in type 2 diabetes‐related inflammation. Adipose tissue macrophages in type 2 diabetes can be distinguished as the anti‐inflammatory phenotype and pro‐inflammatory phenotype. The anti‐inflammatory phenotypes are referred to as M2‐like or alternatively activated macrophages, and pro‐inflammatory phenotypes are referred to as M1‐like or classically activated macrophages38. M2 macrophages carry out tissue surveillance and remodeling functions, and are associated with maintaining insulin sensitivity39. Tim‐3 promotes the macrophages to polarize to the M2 phenotype28. The results of the present study showed that type 2 diabetes patients have a much lower level of Tim‐3 on monocytes (Figure 1a). This shows that type 2 diabetes patients display chaotic insulin sensitivity. In the correlation analysis (Figure 1b), the inverse correlation between Tim‐3 expression on CD14+ monocytes and diabetes duration was observed (P = 0.0436). This indicates that the longer the hyperglycemia time, the lower Tim‐3 expression on CD14 monocytes.
In conclusion, the expression of Tim‐3 is downregulated on CD14+ monocytes and upregulated on CD4+ T cells and CD8+ T cells in type 2 diabetes patients. The correlation analysis between Tim‐3 expression on CD14+ monocytes and diabetes duration showed the longer hyperglycemia time, the lower Tim‐3 expression on CD14 monocytes. Our data suggest that Tim‐3 might participate in the progression of type 2 diabetes by its negative regulation on these immune cells, and Tim‐3 on CD14+ monocytes serves as a novel biological marker for diabetes duration in type 2 diabetes patients. However, the exact regulative mechanism of Tim‐3 expression on immune cells and the exact role of Tim‐3 in type 2 diabetes still need to be further elucidated.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1| Gated strategy of FCM analysis. (a) Gate strategy of CD14+ monocytes (Figure 1a). (b) Gate strategy of CD4+ (Figure 2a) and CD8+ (Figure 3a) T cells.
Acknowledgments
We thank the Endocrinology Department at Qilu Hospital for the collection of blood from type 2 diabetes patients. We are grateful to the clinical laboratory for helping us with the healthy control blood samples.
J Diabetes Investig 2016; 7: 867–873
References
- 1. Casey G. The sugar disease – understanding type 2 diabetes mellitus. Kai Tiaki Nur N Z 2011; 17: 16–21. [PubMed] [Google Scholar]
- 2. Smushkin G, Vella A. What is type 2 diabetes? Medicine 2010; 38: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 2014; 13: 465–476. [DOI] [PubMed] [Google Scholar]
- 4. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor‐alpha: direct role in obesity‐linked insulin resistance. Science 1993; 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 5. Vandanmagsar B, Youm YH, Ravussin A, et al The NLRP3 inflammasome instigates obesity‐induced inflammation and insulin resistance. Nat Med 2011; 17: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rocha VZ, Folco EJ, Sukhova G, et al Interferon‐γ, a Th1 Cytokine, Regulates Fat Inflammation: a Role for adaptive immunity in obesity. Circ Res 2008; 103: 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguyen MTA, Favelyukis S, Nguyen AK, et al A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll‐like receptors 2 and 4 and JNK‐dependent pathways. J Biol Chem 2007; 282: 35279–35292. [DOI] [PubMed] [Google Scholar]
- 8. Hong EG, Ko HJ, Cho YR, et al Interleukin‐10 prevents diet‐induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 2009; 58: 2525–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ricardo‐Gonzalez RR, Eagle AR, Odegaard JI, et al IL‐4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci 2010; 107: 22617–22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chng MHY, Alonso MN, Barnes SE, et al Adaptive immunity and antigen‐specific activation in obesity‐associated insulin resistance. Mediators Inflamm 2015; 2015:593075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar NP, Sridhar R, Nair D, et al Type 2 diabetes mellitus is associated with altered CD8+ T and natural killer cell function in pulmonary tuberculosis. Immunology 2015; 144: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity 2014; 41: 36–48. [DOI] [PubMed] [Google Scholar]
- 13. Sabatos CA, Chakravarti S, Cha E, et al Interaction of Tim‐3 and Tim‐3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol 2003; 4: 1102–1110. [DOI] [PubMed] [Google Scholar]
- 14. Sánchez‐Fueyo A, Tian J, Picarella D, et al Tim‐3 inhibits T helper type 1‐mediated auto‐and alloimmune responses and promotes immunological tolerance. Nat Immunol 2003; 4: 1093–1101. [DOI] [PubMed] [Google Scholar]
- 15. Liberal R, Grant CR, Holder BS, et al The impaired immune regulation of autoimmune hepatitis is linked to a defective galectin‐9/tim‐3 pathway. Hepatology 2012; 56: 677–686. [DOI] [PubMed] [Google Scholar]
- 16. Wu W, Shi Y, Li S, et al Blockade of Tim‐3 signaling restores the virus‐specific CD8+ T‐cell response in patients with chronic hepatitis B. Eur J Immunol 2012; 42: 1180–1191. [DOI] [PubMed] [Google Scholar]
- 17. Monney L, Sabatos CA, Gaglia JL, et al Th1‐specific cell surface protein Tim‐3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002; 415: 536–541. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Ma CJ, Wang JM, et al Tim‐3 negatively regulates IL‐12 expression by monocytes in HCV infection. PLoS One 2011; 6: e19664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiba S, Baghdadi M, Akiba H, et al Tumor‐infiltrating DCs suppress nucleic acid‐mediated innate immune responses through interactions between the receptor TIM‐3 and the alarmin HMGB1. Nat Immunol 2012; 13: 832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ndhlovu LC, Lopez‐Vergès S, Barbour JD, et al Tim‐3 marks human natural killer cell maturation and suppresses cell‐mediated cytotoxicity. Blood 2012; 119: 3734–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiu MK, Wang SC, Dai YX, et al PD‐1 and Tim‐3 pathways regulate CD8+ T cells function in atherosclerosis. PLoS One 2015; 10: e0128523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foks AC, Ran IA, Wasserman L, et al T‐cell immunoglobulin and mucin domain 3 acts as a negative regulator of atherosclerosis. Arterioscle Thromb Vasc Biol 2013; 33: 2558–2565. [DOI] [PubMed] [Google Scholar]
- 23. Cheng Y Q, Ren J P, Zhao J, et al MicroRNA‐155 regulates interferon‐γ production in natural killer cells via Tim‐3 signalling in chronic hepatitis C virus infection. Immunology 2015; 145: 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kanzaki M, Wada J, Sugiyama K, et al Galectin‐9 and T cell immunoglobulin mucin‐3 pathway is a therapeutic target for type 1 diabetes. Endocrinology 2011; 153: 612–620. [DOI] [PubMed] [Google Scholar]
- 25. Kurose Y, Wada J, Kanzaki M, et al Serum galectin‐9 levels are elevated in the patients with type 2 diabetes and chronic kidney disease. BMC Nephrol 2013; 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kraakman M J, Murphy AJ, Jandeleit‐Dahm K, et al Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol 2014; 5: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yan W, Liu X, Ma H, et al Tim‐3 fosters HCC development by enhancing TGF‐β‐mediated alternative activation of macrophages. Gut 2015; 64: 1593–1604. [DOI] [PubMed] [Google Scholar]
- 28. Wang Q, Zhai X, Chen X, et al Dysregulation of circulating CD4+ CXCR5+ T cells in type 2 diabetes mellitus. APMIS 2015; 123: 146–151. [DOI] [PubMed] [Google Scholar]
- 29. Anderson AC. Tim‐3, a negative regulator of anti‐tumor immunity. Curr Opin Immunol 2012; 24: 213–216. [DOI] [PubMed] [Google Scholar]
- 30. Morinaga H, Talukdar S, Bae EJ, et al Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012; 61: 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pecht T, Gutman‐Tirosh A, Bashan N, et al Peripheral blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes Rev 2014; 15: 322–337. [DOI] [PubMed] [Google Scholar]
- 32. Tang F, Wang F, An L, et al Upregulation of Tim‐3 on CD4+ T cells is associated with Th1/Th2 imbalance in patients with allergic asthma. Int J Clin Exp Med 2015; 8: 3809–3816. [PMC free article] [PubMed] [Google Scholar]
- 33. Kägi D, Ledermann B, Bürki K, et al Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin‐deficient mice. Nature 1994; 369: 31–37. [DOI] [PubMed] [Google Scholar]
- 34. Heusel JW, Wesselschmidt RL, Shresta S, et al Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 1994; 76: 977–987. [DOI] [PubMed] [Google Scholar]
- 35. Nishimura S, Manabe I, Nagasaki M, et al CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15: 914–920. [DOI] [PubMed] [Google Scholar]
- 36. Golden‐Mason L, Palmer BE, Kassam N, et al Negative immune regulator Tim‐3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol 2009; 83: 9122–9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jones RB, Ndhlovu LC, Barbour JD, et al Tim‐3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV‐1 infection. J Exp Med 2008; 205: 2763–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72: 219–246. [DOI] [PubMed] [Google Scholar]
- 39. Odegaard JI, Ricardo‐Gonzalez RR, Goforth MH, et al Macrophage‐specific PPARgamma; controls alternative activation and improves insulin resistance. Nature 2007; 447: 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1| Gated strategy of FCM analysis. (a) Gate strategy of CD14+ monocytes (Figure 1a). (b) Gate strategy of CD4+ (Figure 2a) and CD8+ (Figure 3a) T cells.


