Abstract
Aims/Introduction
Glycemic variability is known to induce oxidative stress. We investigated the relationships between glycemic variability and serum bilirubin levels, an endogenous anti‐oxidant, in patients with diabetes.
Materials and Methods
A cross‐sectional study was carried out with 77 patients with type 2 diabetes who had been recruited to two clinical studies from 2008 to 2014. There were no participants with diseases of the pancreas, liver, biliary tract and chronic renal insufficiency. Glycemic variation was calculated by a continuous glucose monitoring system, and correlation analyses were carried out to evaluate their association with bilirubin levels. Multiple linear regression was carried out to identify independent factors influencing bilirubin levels and glycemic variation.
Results
Among the participants, 42.3% were men. The mean (standard deviation) age was 61.5 years (10.4 years), body mass index was 24.2 kg/m2 (2.8 kg/m2), diabetes duration was 17.7 years (9.5 years), hemoglobin A1c was 60.7 mmol/mol (7.1 mmol/mol; 7.7 [0.7]%) and bilirubin was 11.8 μmol/L (4.10 μmol/L). Serum bilirubin levels were not different according to age, body mass index and hemoglobin A1c. However, the mean amplitude of glucose excursion was positively associated with bilirubin levels in women (r = 0.588, P < 0.001). After adjustment with duration of diabetes, serum albumin, liver enzymes, and mean glucose, the correlation between bilirubin and mean amplitude of glucose excursion remained significant (r = 0.566, P < 0.001). Multiple linear regression analyses showed that bilirubin was an independent determinant for the mean amplitude of glucose excursion in women. 1,5‐Anhydroglucitol was also associated with bilirubin levels in women.
Conclusions
Bilirubin level within the physiological range might be an independent predictor for glycemic variability in women with type 2 diabetes.
Keywords: Bilirubin, Continuous glucose monitoring, Glucose variability
Introduction
Because intermittent high glucose has more triggering effects on oxidative stress than chronic sustained hyperglycemia1, 2, and oxidative stress is suggested as one mechanism of complications of diabetes mellitus3, glycemic fluctuation has been examined with regard to diabetes mellitus complications4. However, there is no simple marker to assess glycemic variability (GV), despite the current technical progress in glucose monitoring systems. A continuous glucose monitoring system (CGMS) is used to evaluate glycemic excursion for a short period, but the cost and inconvenience preclude general use in clinical practice. Therefore, a surrogate marker that can reflect GV is required.
Bilirubin is known as an endogenous anti‐oxidant and anti‐inflammatory molecule5. Heme oxygenase‐1 (HO‐1) is an oxidative stress‐responsive enzyme, and degrades free heme to carbon monoxide, iron and biliverdin, which is converted to bilirubin6. It has become apparent that the HO‐1 system can act protectively in a variety of models of disease7. There are a couple of studies on the relationships between serum HO‐1 and diabetes: diabetes mellitus was found to be associated with high serum HO‐18, and attenuation of oxidative stress was associated with decreasing HO‐1 levels9. Therefore, we hypothesized that GV‐induced oxidative stress in diabetes activates the HO‐1 system and its product, bilirubin, in circulation. To examine it, we applied CGMS in patients with type 2 diabetes, calculated various indices of GV and analyzed relationships with serum bilirubin.
Methods
Population
The participants were adults with type 2 diabetes who had been recruited to two different clinical studies using CGMS (Medtronic Minimed, Northridge, CA, USA) carried out at Seoul National University Hospital, Seoul, Korea, from November 2008 to November 2014. Group 1 was composed of 60 persons under various treatment regimens (38 insulin users and 22 insulin non‐users)10. Group 2 was composed of 17 insulin users. Clinical characteristics of each group are presented in Table S1. We excluded participants with diseases of the pancreas, liver, biliary tract and chronic renal insufficiency, and Gilbert syndrome based on the presence of hyperbilirubinemia (serum bilirubin level >20.5 μmol/L) in the absence of hemolytic disease and/or hepatic dysfunction (Figure 1)11.
Figure 1.
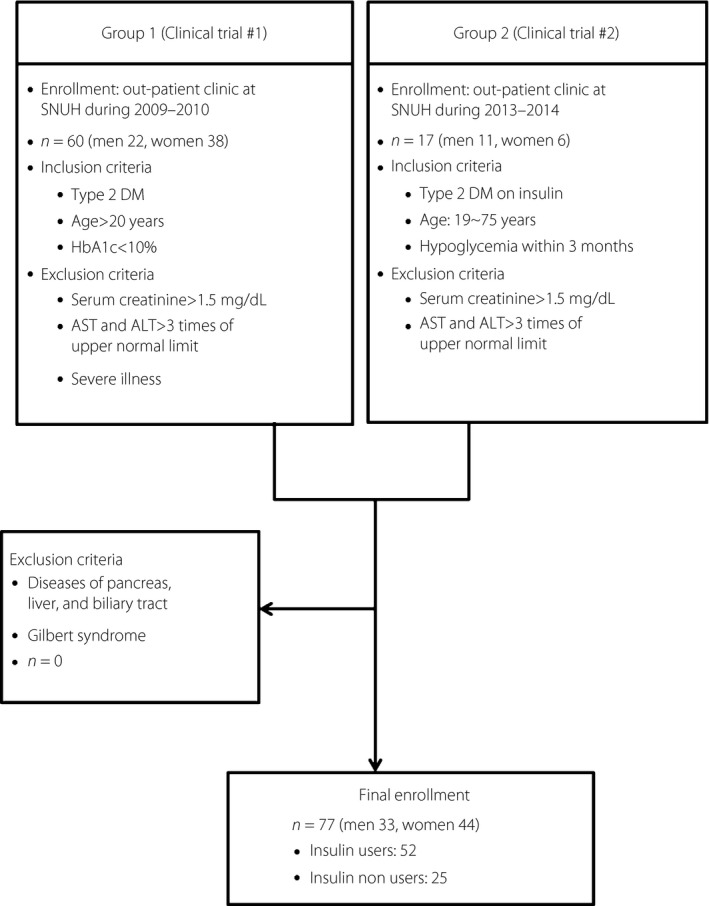
A flow chart for enrollment of patients. ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus; SNUH, Seoul National University Hospital.
Measurement
Medical history, concomitant drugs, bodyweight and height were investigated. After 12‐h overnight fasting, venous blood was collected, and the following were measured: hemoglobin (Hb), hemoglobin A1c (HbA1c; Sysmex XN‐9000 with Bio‐Rad Variant II Turbo; Bio‐Rad Laboratories, Hercules, CA, USA), glucose, C‐peptide, total cholesterol, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatinine levels (Toshiba‐200FR auto‐analyzer; Toshiba Medical Systems Corporation, Tokyo, Japan). Total serum bilirubin (Toshiba‐200FR, HITACHI 7170 Auto Analyzer; Hitachi, Ltd., Tokyo, Japan) was measured using the azobilirubin method (Daiichi Pure Chemical Co. Ltd., Tokyo, Japan). Intra‐assay coefficients of variance (CV) for bilirubin measurement was 1.9% and inter‐assay CV was 0.6%. Spot urine albumin and creatinine (Toshiba‐120FR auto‐analyzer; Toshiba Medical Systems Corporation) were also measured. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease Study equation method12.
To assess GV, CGMS was applied for 3 days10, and the first 48 h were used for the calculation of mean blood glucose (MBG) and the GV indices4, such as standard deviation (SD), mean amplitude of glucose excursion (MAGE), continuous overall net glycemic action calculated with 6‐h time intervals (CONGA‐6), M100 (a measure of the stability of the glucose excursions in comparison with glucose value of 5.55 mmol/L [100 mg/dL]), J‐index, mean post‐meal maximum glucose (MPMG) and area under the curve for glucose above 9.99 mmol/L (180 mg/dL; area under the curve for glucose above 180 mg/dL). MAGE is the mean of the absolute difference of peak‐to‐nadir or nadir‐to‐peak direction, in the glycemic excursions more than one SD during the 48 h. CONGA‐6 is the standard deviation of the differences between each glucose level and the corresponding glucose level measured 6 h earlier. M100 is a measure of the stability of the glucose excursions in comparison with glucose value of 5.55 mmol/L (100 mg/dL). J‐index takes account of both the mean glucose level and variability of glycemia.
Statistical analysis
Continuous variables are expressed as mean ± SD, and categorical variables as the number and percentage. A normality test was carried out for all continuous variables. A log transformation was carried out for skewed data before further analyses. Student's t‐test and chi square‐test were used for comparisons between sex or groups. Analysis of variance (anova) was applied for comparisons among the groups according to MAGE. The correlation coefficient was determined using Pearson's coefficient to determine the association between bilirubin levels and GV indices. Multiple linear regression using stepwise analyses were carried out to evaluate if MAGE was independently related with bilirubin levels. SPSS (version 18.0; SPSS, Chicago, IL, USA) was used for statistical analyses, and P < 0.05 was considered significant.
Results
Clinical characteristics and laboratory data were compared between the two study groups, and the fasting C‐peptide, albumin and MAGE were significantly different (shown in Table S1). However, according to general linear model analysis, all the relevance between bilirubin and C‐peptide, between bilirubin and albumin, and between bilirubin and MAGE did not differ according to the group. Therefore, we combined the two groups to examine the relationships between bilirubin and GV. The clinical characteristics of the total 77 participants are shown in Table1.
Table 1.
Clinical characteristics of the participants according to sex
| Total (n = 77) | Men (n = 33) | Women (n = 44) | P‐value men vs women | |
|---|---|---|---|---|
| Age (years) | 61.5 ± 10.4 | 63.8 ± 10.2 | 59.8 ± 10.3 | 0.101 |
| BMI (kg/m2) | 24.2 ± 2.8 | 23.9 ± 2.6 | 24.5 ± 2.9 | 0.321 |
| DM duration (years) | 24.2 ± 2.8 | 20.8 ± 10.0 | 15.4 ± 8.4 | 0.012 |
| HbA1c (mmol/mol) | 60.7 ± 7.1 | 61.7 ± 8.0 | 60.7 ± 7.1 | 0.304 |
| HbA1c (%) | 7.7 ± 0.7 | 7.8 ± 0.8 | 7.7 ± 0.7 | |
| 1.5‐AG (μmol/L) | 37.2 ± 17.7 | 37.1 ± 15.8 | 40.8 ± 17.7 | 0.020 |
| C‐peptide † (nmol/L) | 0.53 ± 0.36 | 0.39 ± 0.37 | 0.62 ± 0.33 | 0.008 |
| eGFR (mL/min/ 1.73 m2) | 68.1 ± 20.2 | 69.0 ± 23.1 | 67.5 ± 18.0 | 0.738 |
| Urine albumin/creatinine (mg/mmol) | 6.5 ± 19.8 | 3.7 ± 12.4 | 8.6 ± 23.8 | 0.282 |
| Retinopathy (%) (none/NPDR/PDR) | 50.0/37.1/12.9 | 40.0/43.3/16.7 | 57.5/32.5/10.0 | 0.336 |
| CVD (%) ‡ | 17.9 | 29.0 | 8.3 | 0.028 |
| Insulin users (%) | 71.4 | 75.8 | 68.2 | 0.466 |
| OAD (%) | 81.8 | 78.8 | 84.1 | 0.645 |
| MTF/SU/others § | 74.4/37.2/11.5 | 70.6/23.5/5.9 | 76.7/41.7/13.3 | |
| Total bilirubin and the range (μmol/L) | 11.80 ± 4.10 (1.71–23.95) | 13.17 ± 4.28 (4.13–23.95) | 10.95 ± 3.76 (1.71–20.52) | 0.018 |
| Hb (mmol/L) | 8.3 ± 0.9 | 8.8 ± 0.8 | 7.9 ± 0.7 | <0.001 |
| Albumin (g/L) | 43.0 ± 3.0 | 43.0 ± 3.0 | 43.0 ± 3.0 | 0.961 |
| AST (IU/L) | 25.9 ± 11.7 | 25.8 ± 13.1 | 26.0 ± 10.6 | 0.942 |
| ALT (IU/L) | 27.6 ± 17.5 | 27.1 ± 19.7 | 28.1 ± 15.8 | 0.800 |
| MAGE † (mmol/L) | 7.14 ± 2.75 | 8.06 ± 2.86 | 6.45 ± 2.48 | 0.010 |
| SD † (mmol/L) | 3.03 ± 1.08 | 3.26 ± 1.08 | 2.86 ± 1.05 | 0.086 |
| CONGA‐6 † (mmol/L) | 2.53 ± 0.90 | 2.74 ± 0.90 | 2.36 ± 0.87 | 0.045 |
| M100 † | 23.0 ± 15.8 | 25.7 ± 15.9 | 20.9 ± 15.5 | 0.070 |
| J‐index † | 50.0 ± 19.3 | 54.5 ± 19.3 | 46.7 ± 18.9 | 0.050 |
| MPMG † (mmol/L) | 13.11 ± 2.66 | 13.47 ± 2.39 | 12.84 ± 2.85 | 0.232 |
| AUC‐180 † (mmol/L day) | 1.12 ± 0.95 | 1.27 ± 0.96 | 1.00 ± 0.95 | 0.144 |
| MBG † (mmol/L) | 9.16 ± 1.72 | 9.51 ± 1.48 | 8.89 ± 1.85 | 0.077 |
Data are mean ± standard deviation for continuous variables. For frequency data, chi square‐test was applied. †Log‐transformed values were used for analysis. ‡Only available in 67 persons, 12 patients had cardiovascular disease. §Including α‐glucosidase inhibitors, thiazolidinediones, nateglinides, a dipeptidyl peptidase‐4 inhibitor and a glucagon‐like peptide‐1 (GLP‐1) receptor agonist. 1,5‐AG, 1,5‐anhydroglucitol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC‐180, area under the curve for glucose above 180 mg/dL; BMI, body mass index; CONGA‐6, continuous overlapping net glycemic action calculated with 6‐h time intervals; CVD, cardiovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; M100, a measure of the stability of the glucose excursions in comparison with glucose value of 5.55 mmol/L (100 mg/dL); MAGE, mean amplitude of glycemic excursions; MBG, mean blood glucose; MTF, metformin; MPMG, mean post‐meal maximum glucose; NPDR, non proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; SD, standard deviation; SU, sulfonylurea.
In the simple correlation analyses between bilirubin levels and the GV indices from CGMS, there were significant positive correlations between bilirubin and log(MAGE) (r = 0.305, P = 0.007), log(SD) (r = 0.252, P = 0.027), log(CONGA‐6) (r = 0.241, P = 0.034), log(J‐index) (r = 0.286, P = 0.012) and log(M100) (r = 0.277, P = 0.015). In the simple correlation analyses between bilirubin levels and GV‐related variables, there were significant positive correlations between bilirubin and log(MBG) (r = 0.262, P = 0.021) and diabetes mellitus duration (r = 0.266, P = 0.019). Neither fasting glucose nor HbA1c was correlated with bilirubin levels. Hb, which is a main source of bilirubin, was significantly associated with bilirubin (r = 0.300, P = 0.009), but AST, ALT and albumin levels were not correlated with bilirubin. Because there were significant sex differences in important variables, such as bilirubin, GV indices, diabetes duration, C‐peptide levels and Hb (Table 1), we carried out further analyses separately according to sex.
First, the participants were divided into three groups according to MAGE levels. Among the variables, body mass index was significantly different across the groups in the men participants (F = 4.56, P = 0.019), whereas bilirubin levels were significantly different across the groups in the female participants (F = 4.499, P = 0.017). Further correlation analyses showed that log(MAGE) was negatively correlated with body mass index in men (r = −0.429, P = 0.013), and positively correlated with bilirubin levels in women (r = 0.588, P < 0.001; Figure 2). Serum bilirubin was also positively associated with the duration of diabetes, serum albumin (which is an important transporter of unconjugated bilirubin in the circulation6), AST, ALT and log(MBG) in simple correlation analyses in women. Even after adjustment with these variables, the correlation between bilirubin and log(MAGE) remained significant, (r = 0.566, P < 0.001). In addition to MAGE, CONGA‐6, MPMG, J‐index and SD were also positively correlated with bilirubin levels in women (Table 2). In men, serum bilirubin levels were not correlated with log(MAGE) (Figure 2), regardless of adjustment with body mass index and other variables.
Figure 2.
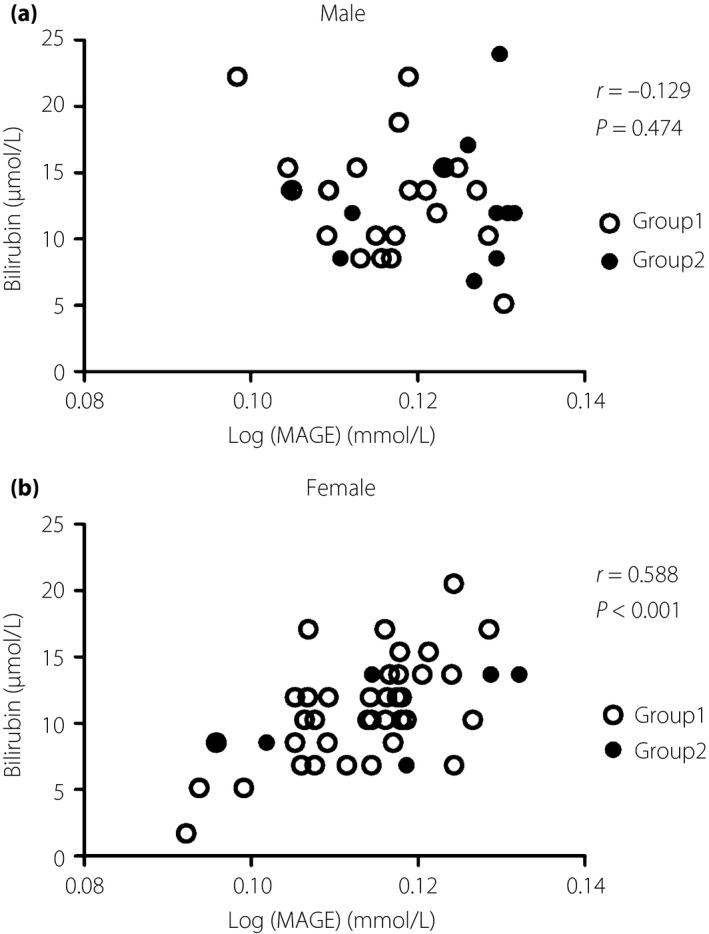
Correlation analyses between mean amplitude of glucose excursion (MAGE) and bilirubin levels according to sex. There was no correlation between serum bilirubin levels and log(MAGE) in (a) men, whereas there was a significant correlation in (b) women.
Table 2.
Partial correlation analyses between bilirubin concentrations and glycemic variability indices in women
| r | P‐value | ||
|---|---|---|---|
| Adjusted by DM duration, albumin, AST, ALT and log(MBG) | MAGE † | 0.566 | <0.001 |
| SD † | 0.416 | 0.008 | |
| CONGA‐6 † | 0.420 | 0.008 | |
| MPMG † | 0.441 | 0.006 | |
| J‐index † | 0.399 | 0.012 | |
| M100 † | 0.297 | 0.067 | |
| AUC‐180 † | 0.293 | 0.079 | |
†Log‐transformed values were used for analysis. 1,5‐AG, 1,5‐anhydroglucitol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC‐180, area under the curve for glucose above 180 mg/dL; CONGA‐6, continuous overlapping net glycemic action calculated with 6‐h time intervals; DM, diabetes mellitus; M100, a measure of the stability of the glucose excursions in comparison with glucose value of 5.55 mmol/L (100 mg/dL); MAGE, mean amplitude of glycemic excursions; MBG, mean blood glucose; MPMG, mean post‐meal maximum glucose; SD, standard deviation.
On the assumption that GV‐induced oxidative stress contributed to serum bilirubin levels, multiple linear regression analyses were carried out to evaluate independent effects of MAGE on bilirubin levels in women, using log(MAGE), log(MBG), duration of diabetes mellitus, albumin, AST, ALT and Hb as independent variables. Among the independent variables, log(MAGE), ALT, AST and DM duration were significant determinants (Table 3). In the case of men, only Hb was a significant determinant for bilirubin levels (β = 0.531. P = 0.019), although the F‐test was not significant (P = 0.259).
Table 3.
Multiple linear regression analyses for bilirubin levels in women
| Dependent variable | Bilirubin |
|---|---|
| Corrected r 2 | 0.480 |
| F | 6.414 |
| P | <0.0001 |
| β (P‐value) of independent variables | |
| Log(MAGE) | 0.578 (0.001) |
| AST | –0.011 (0.044) |
| ALT | 0.011 (0.004) |
| DM duration | 0.007 (0.031) |
| Log(MBG) | 0.339 (0.292) |
| Albumin | 0.094 (0.348) |
| Hb | 0.004 (0.885) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus; Hb, hemoglobin; MAGE, mean amplitude of glycemic excursions; MBG, mean blood glucose.
From a view of realistic significance in clinical practice, multiple linear regression analyses were carried out to identify independent factors determining GV, using log(MAGE) as a dependent variable, and bilirubin, log(C‐peptide), duration of diabetes mellitus and log(MBG) as independent variables, because they would represent insulin insufficiency and mean glucose, which were reported to be related to GV4. Interestingly, the well‐known factors, such as C‐peptide and mean glucose, were significant determinants of MAGE in men as expected, whereas bilirubin was the only factor determining MAGE in women (Table 4). Similar results were also found if the dependent variable was replaced by log(SD), another GV index (data not shown).
Table 4.
Multiple linear regression analyses for prediction of mean amplitude of glycemic excursions
| Men (n = 33) | Women (n = 44) | |
|---|---|---|
| Dependent variable | log(MAGE) | |
| Corrected r 2 | 0.345 | 0.309 |
| F | 5.074 | 5.817 |
| P | 0.004 | 0.001 |
| β (P‐value) of independent variables | ||
| Bilirubin | –0.110 (0.256) | 0.390 (0.002) |
| Log(MBG) | 1.104 (0.008) | 0.286 (0.297) |
| Log(C‐peptide) | –0.119 (0.043) | –0.003 (0.958) |
| DM duration | 0.002 (0.407) | 0.002 (0.558) |
DM, diabetes mellitus; MAGE, mean amplitude of glycemic excursions; MBG, mean blood glucose.
1,5‐Anhydroglucitol (1,5‐AG) is a candidate biomarker for GV, in the case of moderately‐controlled type 2 diabetes mellitus4. We explored the correlation between 1,5‐AG and bilirubin, and observed that there was a significant correlation between them regardless of adjustment with log(MBG), which is known to affect 1,5‐AG levels (Table 5). When we separated the participants by sex, the association between the biomarkers remained, although the statistical significance decreased in men.
Table 5.
Correlation analyses between 1,5‐anhydroglucitol and bilirubin levels
| Total participants (n = 77) | Men (n = 33) | Women (n = 44) | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Simple correlation | –0.353 | 0.002 | −0.231 | 0.195 | −0.381 | 0.011 |
| Adjusted by log(MBG) | –0.352 | 0.002 | −0.333 | 0.063 | −0.307 | 0.045 |
1,5‐AG, 1,5‐anhydroglucitol; MBG, mean blood glucose.
Discussion
In the present study, we found that in women, bilirubin was an independent factor for MAGE and SD, whereas insulin insufficiency and mean glucose significantly contributed to them in men. Another candidate biomarker for GV, 1,5‐AG, was well correlated with bilirubin, especially in women.
Bilirubin is produced from heme through HO‐16, and is a well‐known anti‐oxidant, as was described in the Introduction5. HO‐1 gene expression in the muscle of patients with type 2 diabetes was reported to be defective13, but the serum levels were found to be increased8, 9. In particular, relationships between HO‐1 activity and glucose variability have not been evaluated. If glucose variability including both hypoglycemia and hyperglycemia enhances oxidative stress by diabetes mellitus, the reactive increase of HO‐1 activity and its metabolites, such as bilirubin, is possible.
In addition, biliverdin reductase has been shown to have a role in glucose metabolism14. Biliverdin reductase (BVR) is not solely an enzyme converting biliverdin into bilirubin, but also a dual‐specificity kinase (Ser/Thr and Tyr), not related to its reductase activity. Kinase BVR was found to be involved in the regulation of multiple steps of insulin signaling. Therefore, it might play a role in the pathogenesis of diabetes. GV‐induced oxidative stress influences insulin signaling, too15, and therefore there can be interactions between GV and BVR activity. Furthermore, in the nucleus, as a basic‐leucine‐zipper deoxyribonucleic acid/chromatin‐binding transcription factor, BVR can act as a transcription factor, and has been shown to modulate HO‐1 expression, which would further affect bilirubin levels.
Not only the process of bilirubin production, but also that of bilirubin metabolism is related to oxidative stress. When unconjugated bilirubin produced from hemoproteins throughout the body is delivered into the hepatocytes, where it becomes conjugated forms, it is bound to various proteins including the glutathione‐S‐transferase superfamily, to prevent efflux back into the serum6. The glutathione‐S‐transferase superfamily is another anti‐oxidant detoxification enzyme that is associated with diabetes16. Therefore, oxidative stress might be able to regulate bilirubin levels through various pathways, which we cannot infer specifically with the current data.
We observed the close correlation between MAGE and bilirubin levels only in the women, but it is difficult to explain. We could presume that sex differences in response to oxidative stress caused by GV underlie it. It is known that men have insufficient anti‐oxidant defense mechanisms compared to women17, 18, and bilirubin might also be included in that kind of anti‐oxidant. In that case, bilirubin cannot reflect GV efficiently. Interestingly, in a cohort of 628 healthy participants aged 18~22 years, bilirubin levels were independently correlated with thiobarbituric acid‐reactive substances only in women19, suggesting bilirubin was a poor index for oxidative stress in men.
Our study had several limitations. First, owing to the cross‐sectional nature of the present study, we were not able to conclude a causal relationship. Second, the study population was relatively small to control various confounding factors for GV. However, comprehensive assessment of GV from CGMS in the participants combined from two different groups makes our observation between GV and bilirubin more reliable.
In conclusion, we found that serum bilirubin levels were positively associated with GV in women with type 2 diabetes mellitus, independently from albumin, liver enzymes and mean glucose. If it is consolidated by interventional studies in larger populations, bilirubin levels can be a simple marker for glycemic excursions in women.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1| Clinical characteristics of the participants according to the study group.
Acknowledgments
This study was supported by a grant from the Innovative Research Institute for Cell Therapy (A062260) by the Ministry of Health and Welfare, Korea.
J Diabetes Investig 2016; 7: 874–880
Reference
- 1. Ceriello A, Esposito K, Piconi L, et al Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008; 57: 1349–1354. [DOI] [PubMed] [Google Scholar]
- 2. Monnier L, Mas E, Ginet C, et al Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 3. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820. [DOI] [PubMed] [Google Scholar]
- 4. Jung HS. Clinical implications of glucose variability: chronic complications of diabetes. Endocrinol Metab (Seoul) 2015; 30: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stocker R, Yamamoto Y, McDonagh AF, et al Bilirubin is an antioxidant of possible physiological importance. Science 1987; 235: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 6. Longo D, Fauci A, Kasper D, Hauser S. Harrison's Principles of Internal Medicine, 19th edn New York: The McGraw‐Hill Companies, Inc., 2015. [Google Scholar]
- 7. Soares MP, Bach FH. Heme oxygenase‐1: from biology to therapeutic potential. Trends Mol Med 2009; 15: 50–58. [DOI] [PubMed] [Google Scholar]
- 8. Bao W, Song F, Li X, et al Plasma heme oxygenase‐1 concentration is elevated in individuals with type 2 diabetes mellitus. PLoS One 2010; 5: e12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rizzo M, Abate N, Chandalia M, et al Liraglutide reduces oxidative stress and restores heme oxygenase‐1 and ghrelin levels in patients with type 2 diabetes: a prospective pilot study. J Clin Endocrinol Metab 2015; 100: 603–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim MJ, Jung HS, Hwang‐Bo Y, et al Evaluation of 1,5‐anhydroglucitol as a marker for glycemic variability in patients with type 2 diabetes mellitus. Acta Diabetol 2013; 50: 505–510. [DOI] [PubMed] [Google Scholar]
- 11. Inoguchi T, Sasaki S, Kobayashi K, et al Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA 2007; 298: 1398–1400. [DOI] [PubMed] [Google Scholar]
- 12. Levey AS, Bosch JP, Lewis JB, et al A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 13. Bruce CR, Carey AL, Hawley JA, et al Intramuscular heat shock protein 72 and heme oxygenase‐1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 2003; 52: 2338–2345. [DOI] [PubMed] [Google Scholar]
- 14. Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci 2009; 30: 129–137. [DOI] [PubMed] [Google Scholar]
- 15. Evans JL, Goldfine ID, Maddux BA, et al Are oxidative stress‐activated signaling pathways mediators of insulin resistance and beta‐cell dysfunction? Diabetes 2003; 52: 1–8. [DOI] [PubMed] [Google Scholar]
- 16. Franco R, Schoneveld OJ, Pappa A, et al The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem 2007; 113: 234–258. [DOI] [PubMed] [Google Scholar]
- 17. Malorni W, Campesi I, Straface E, et al Redox features of the cell: a gender perspective. Antioxid Redox Signal 2007; 9: 1779–1801. [DOI] [PubMed] [Google Scholar]
- 18. Borras C, Sastre J, Garcia‐Sala D, et al Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med 2003; 34: 546–552. [DOI] [PubMed] [Google Scholar]
- 19. Stojanov M, Stefanovic A, Dzingalasevic G, et al Total bilirubin in young men and women: association with risk markers for cardiovascular diseases. Clin Biochem 2013; 46: 1516–1519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1| Clinical characteristics of the participants according to the study group.


