Abstract
Aims/Introduction
The aim of the present study was to investigate the role of interleukin‐19 (IL‐19) in angiogenesis of type 2 diabetes mellitus with complications, and to assess the relationship of serum IL‐19 and angiopoietin‐2 (Ang‐2) in type 2 diabetes mellitus.
Materials and Methods
The group studied comprised of 240 patients with type 2 diabetes mellitus (132 men and 108 women), and included macrovascular complications, microvascular complications and type 2 diabetes mellitus without vascular complications. The control group consisted of 50 healthy blood donors. All participants were evaluated for: IL‐19, Ang‐2, fasting plasma glucose, fasting insulin and glycosylated hemoglobin.
Results
The serum IL‐19 levels of type 2 diabetes mellitus patients with angiopathy were found to be significantly higher compared with patients without angiopathy. IL‐19 levels were significantly positively correlated with Ang‐2, homeostasis model assessment for insulin resistance and glycosylated hemoglobin (r = 0.769, 0.523 and 0.491, respectively, P < 0.01). In multivariable logistic regression analysis, IL‐19 levels (P = 0.01) were found to be independently associated with patients with type 2 diabetes mellitus complications.
Conclusions
These data are the first to implicate the association between the IL‐19 and type 2 diabetes mellitus with vascular complications. IL‐19 is significantly positively correlated with Ang‐2. The potential role of IL‐19 and Ang‐2 in the pathogenesis of vascular complications in type 2 diabetes could warrant further study.
Keywords: Angiopoietin‐2, Interleukin‐19, Vascular complications
Introduction
Diabetes mellitus has become a global health problem, and its prevalence is expected to increase to 366 million worldwide by 20301. Diabetes mellitus, especially type 2 diabetes mellitus, is a metabolic disease characterized by chronic hyperglycemia, which mainly results from a deficiency in peripheral insulin effects (insulin resistance). However, morbidity and mortality from diabetes are mainly attributed to the development of both macrovascular and microvascular complications that rapidly leads to premature death2, 3. Vascular complications dominate the lifespan and the quality of life in type 2 diabetes mellitus patients, and it is important to prevent the development and progression of these complications4. Chronic inflammation, characterized by elevated circulating levels of inflammatory markers, appears to play a critical role in the pathogenesis of type 2 diabetes mellitus and its associated complications5, 6. Recent accumulating evidence shows that chemokines, adhesion molecules, growth factors, angiopoietins and interleukins are involved in the participation of immune‐mediated inflammatory processes in the pathophysiology of diabetes mellitus and its complications7, 8.
The angiopoietins are a family of seven secreted glycoprotein ligands, angiopoietin‐1 to ‐7, originally identified as important in blood vessel formation. The best characterizations of these ligands are angiopoietin‐1 (Ang‐1) and angiopoitein‐2 (Ang‐2). Ang‐2 is expressed by endothelial cells and acts as an antagonist for receptor Tie‐2. Ang‐2 has been reported to completely disrupt protective Tie‐2 signaling in numerous studies9, 10, 11.
Interleukin‐19 (IL‐19) is a newly discovered cytokine belonging to the IL‐10 family. This protein can induce the production of IL‐10 from human peripheral blood mononuclear cells12, 13. IL‐19 is a potent inducer of the T‐helper2 response, and has been implicated in a wide variety of allergies (i.e. asthma and atopic dermatitis)14, 15, 16, type 1 diabetes17 and cardiovascular disease18, 19.
Our previous study showed that Ang‐2 is closely related to vascular complications of type 2 diabetes mellitus20. However, how IL‐19 functions in type 2 diabetes mellitus, and whether Ang‐2 and IL‐19 play an important role in the pathogenesis of type 2 diabetes mellitus have been scarcely reported. The purpose of the present study was to extend those initial observations, and to determine the concentrations of IL‐19 in type 2 diabetes mellitus and its complications, and to investigate the relationship of serum Ang‐2 and IL‐19 in type 2 diabetes mellitus.
Material and Methods
Patient selection
A total of 240 patients with type 2 diabetes mellitus (132 men and 108 women) treated at Binhai County Hospital, Jiangsu Province, China, were recruited in the present study. The average patient age was 62 ± 11 years. The duration of diabetes development from 6 to 14 years. The patients were diagnosed according to World Health Organization criteria21. Primary diagnoses (by clinical, electrocardiogram and imaging diagnosis) included macrovascular complications (n = 76, including heart disease, cerebrovascular disease and peripheral arterial disease), microvascular complications (n = 110, including diabetic nephropathy [urinary albumin >30 mg/24 h], as well as diabetic retinopathy and diabetic peripheral neuropathy) and diabetic without vascular complications (n = 54). According to the number of macrovascular and microvascular injuries, respectively, patients with macrovascular or microvascular complications were divided into the following groups respectively: one kind of macrovascular complications group (n = 25) or microvascular complications group (n = 50), two kinds of macrovascular complications group (n = 30) or microvascular complications group (n = 35), and three kinds of macrovascular complications group (n = 21) or microvascular complications group (n = 25). The control group consisted of 30 men and 20 women, and the average age was 60 ± 15 years. The exclusion criteria included patients with evidence of neoplastic disease, acute or chronic infectious disease, and significant hepatic and renal disease. The study was approved by the Human Investigation Committee of BinHai County Hospital, and informed consent for all participants was obtained.
Laboratory analysis
Venous blood samples were obtained from all participants on hospital admission. All samples were collected in vacuum blood collection tubes with a clot activator, and immediately centrifuged at 1,500 g and 4°C for 10 min. Plasma was aliquoted and stored at −80°C until analysis.
Serum IL‐19 and Ang‐2 levels were determined by enzyme‐linked immunosorbent assay using commercial kits and reagents (R&D Systems, Minneapolis, MN, USA). Glycosylated hemoglobin (HbA1c) was measured by liquid chromatography (G8‐90SL; Tosoh, Japan). Fasting plasma glucose and fasting insulin were measured by routine techniques. The homeostasis model assessment of insulin resistance index (HOMA‐IR) was calculated by the following formula: HOMA‐IR = (fasting plasma glucose × fasting insulin)/22.5.
Statistical analysis
The values are expressed as mean ± standard error of the mean. Comparisons of means between two groups were carried out using Student's t‐test on test of normality and equality of variances. Correlations within each group were sought using Spearman's or Pearson's method. Multivariable logistic regression analyses were carried out to determine whether IL‐19 levels were independently associated with vascular complications of type 2 diabetes. P < 0.05 was considered statistical significance. All analyses were carried out using spss 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinical and laboratory measures
A total of 240 patients with type 2 diabetes and 50 normal healthy controls were recruited. These participants were age and sex ratio comparable. As expected, HbA1c, fasting insulin, HOMA‐IR, total cholesterol and triglyceride levels were higher, and high‐density lipoprotein cholesterol was lower in the diabetic patients. Serum IL‐19 levels was significantly higher in patients with type 2 diabetes mellitus than in healthy controls, and there were differences in the diabetic patient subgroups (Table1).
Table 1.
Clinical characteristics and research indexes in the study groups
| Controls | T2DM | T2DM with macrovascular complications | T2DM with microvascular complications | T2DM without vascular complications | P‐value | |
|---|---|---|---|---|---|---|
| n | 50 | 240 | 76 | 110 | 54 | — |
| Age (years) | 60 ± 15 | 62 ± 11 | 64 ± 5 | 69 ± 4 | 66 ± 5 | 0.125 |
| Sex(males/females) | 30/20 | 132/108 | 35/31 | 58/52 | 39/25 | 0.314 |
| Duration of diabetes (years) | — | 9 (6–14) | 10 (6–12) | 10 (7–14) | 8 (7–13) | 0.210 |
| BMI (kg/m2) | 25 ± 5 | 28 ± 6 | 29 ± 5 | 26 ± 4 | 25 ± 6 | 0.001 |
| SBP (mmHg) | 128 ± 16 | 139 ± 15 | 137 ± 16 | 138 ± 15 | 136 ± 15 | 0.060 |
| DBP (mmHg) | 77 ± 7 | 78 ± 11 | 75 ± 9 | 76 ± 8 | 75 ± 7 | 0.213 |
| TC (mmol/L) | 4.8 ± 1.0 | 5.7 ± 1.6 | 5.5 ± 1.5 | 5.9 ± 1.1 | 5.6 ± 1.2 | 0.911 |
| Triglycerides (mmol/L) | 1.5 ± 0.5 | 2.8 ± 1.6 | 2.0 ± 1.2 | 2.5 ± 1.6 | 2.4 ± 1.5 | 0.895 |
| HDL cholesterol (mmol/L) | 1.6 ± 0.5 | 1.2 ± 0.4 | 1.3 ± 0.2 | 1.1 ± 0.5 | 1.3 ± 0.3 | <0.001 |
| LDL cholesterol (mmol/L) | 3.2 ± 0.8 | 2.9 ± 0.8 | 2.5 ± 0.9 | 2.3 ± 0.7 | 2.6 ± 1.0 | 0.058 |
| Cystatin C (mg/L) | 0.6 ± 0.04 | 1.08 ± 0.07 | 1.32 ± 0.09 | 1.05 ± 0.07 | 0.87 ± 0.05 | 0.03 |
| IL‐19 (pg/mL) | 16.2 ± 8.5 | 41.9 ± 11.9 | 53.7 ± 14.3 | 40.5 ± 12.3 | 30.4 ± 9.2 | <0.001 |
| Ang‐2 (ng/mL) | 0.8 ± 0.2 | 2.2 ± 0.7 | 2.9 ± 0.7 | 2.2 ± 0.6 | 1.6 ± 0.4 | 0.001 |
| FBG (mmol/L) | 5.1 ± 0.5 | 9.4 ± 2.3 | 10.5 ± 2.8 | 9.2 ± 2.3 | 8.5 ± 1.7 | <0.001 |
| FINS (mU/L) | 8.0 ± 1.3 | 18.1 ± 2.7 | 18.7 ± 2.4 | 15.5 ± 2.1 | 20.1 ± 3.8 | <0.001 |
| HOMA‐IR | 1.8 ± 0.1 | 7.6 ± 0.2 | 8.7 ± 0.3 | 6.3 ± 0.2 | 7.6 ± 0.3 | <0.001 |
| HbA1c (%) | 4.8 ± 0.9 | 9.5 ± 2.1 | 11.3 ± 3.5 | 9.7 ± 2.7 | 8.9 ± 2.1 | <0.001 |
Data are presented as mean ± standard deviation. Ang‐2, angiopoietin‐2; BMI, body mass index; DBP, diastolic blood pressure; FINS, fasting insulin; FPG, fasting plasma glucose; TC, total cholesterol; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment for insulin resistance; IL‐19, interleukin‐19; LDL, low‐density lipoprotein; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus.
Relationship of IL‐19 and the number of microvascular or macrovascular Injuries in type 2 diabetes mellitus
In the type 2 diabetes mellitus cohort, 186 patients had suffered vascular complications. In type 2 diabetes mellitus patients, along with the increased numbers of microvascular or macrovascular complications, IL‐19 concentration also increased. IL‐19 levels were also significantly different (P < 0.05; Table2,3).
Table 2.
Results of the different numbers of microvascular injuries in type 2 diabetes mellitus
| n | No. microvascular complications | IL‐19 (pg/mL) |
|---|---|---|
| 50 | 1 | 33.1 ± 9.3 |
| 35 | 2 | 40.5 ± 12.7 |
| 25 | 3 | 48.1 ± 14.9 |
IL‐19, interleukin‐19.
Table 3.
Results of the different numbers of macrovascular injuries in type 2 diabetes mellitus
| n | No. macrovascular complications | IL‐19 (pg/mL) |
|---|---|---|
| 25 | 1 | 41.7 ± 10.3 |
| 30 | 2 | 52.5 ± 15.7 |
| 21 | 3 | 66.9 ± 16.9 |
IL‐19, interleukin‐19.
Correlations
Serum IL‐19 levels positively correlated with HOMA‐IR and HbA1c (r = 0.523 and 0.491, respectively, P < 0.01; Figure1).
Figure 1.
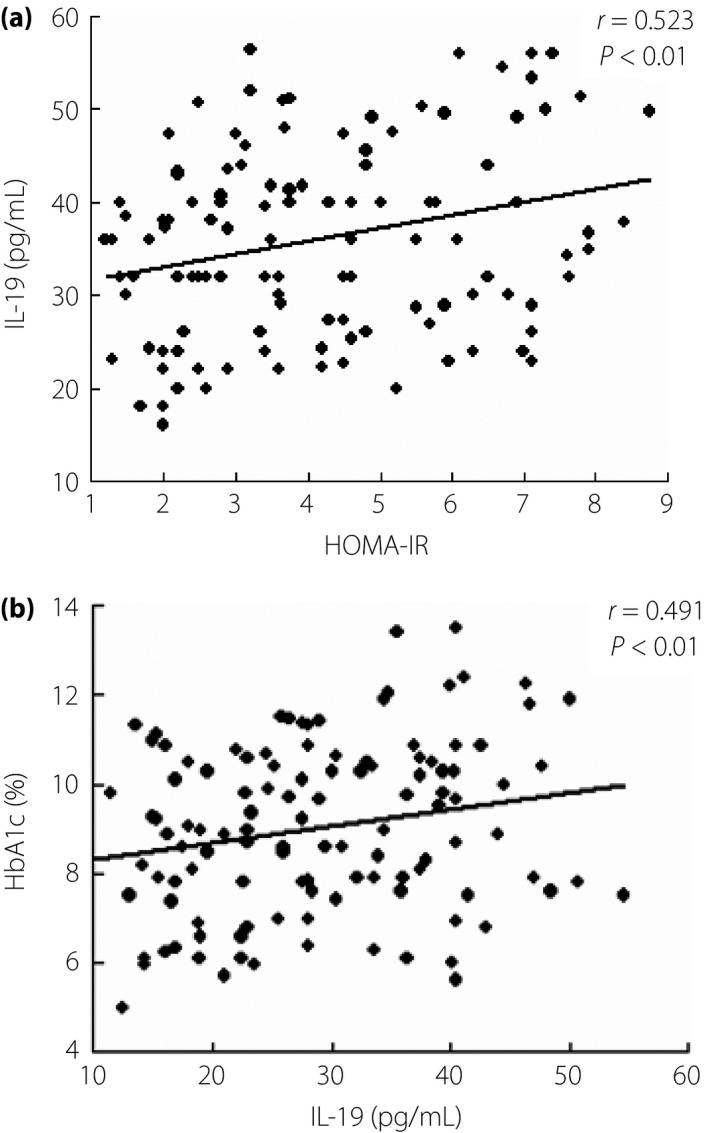
Serum (a) interleukin‐19 (IL‐19) levels and (b) Ang‐2 were positively correlated with homeostasis model assessment for insulin resistance (HOMA‐IR) and glycosylated hemoglobin (HbA1c), respectively.
Association between serum IL‐19 and vascular complications
In multivariable logistic regression analysis, IL‐19 levels (95% confidence interval 22.4–61.4; P = 0.01) were independently associated with type 2 diabetes mellitus patients with vascular complications. IL‐19 was positively correlated with microangiopathy and macroangiopathy numbers (r = 0.503 and 0.618, respectively; P < 0.05) after adjustment for age, sex, hypertension and blood fat.
Ang‐2 was associated with IL‐19 levels in type 2 diabetes mellitus
Previous studies20 observed that Ang‐2 concentrations were elevated in patients with type 2 diabetes. In the present study, we also observed a positive correlation between Ang‐2 and IL‐19 levels in type 2 diabetes mellitus patients (r = 0.769; P < 0.01).
Discussion
Diabetes is associated with a range of vascular diseases, including atherosclerosis, myocardial infarction and heart failure resulting from endothelial dysfunction22. Ang‐2 is a glycoprotein exclusively expressed by endothelial cells that acts as an antagonist for Tie‐2. Ang‐2 can inhibit Tie2 phosphorylation, damage blood vessels, promote blood vessel structure relaxation, and relieve inhibition effects on perivascular cells and extracellular matrix to the endothelium, causing vascular endothelial injury and pathological angiogenesis11, 23. In previous studies20, we have shown that serum Ang‐2 levels were positively associated with microvascular and macrovascular lesions in patients with type 2 diabetes mellitus.
IL‐19 was discovered as a member of the IL‐10 family, and it shares 20% amino acid identity with IL‐10, but does not engage the IL‐10 receptor. IL‐19 can be detected in human monocytes, and B and T lymphocytes, and IL‐19 expression can be upregulated in these cells by stimulation24, 25. IL‐19 expression is reported to be restricted to immune cells, and all of our knowledge regarding the function of this cytokine comes from experiments carried out in inflammatory cells.
Patients with diabetes are at risk for microvascular and macrovascular lesions26. Inflammatory events are thought to occur in all diabetic complications27. IL‐19 is considered by some investigators to have an anti‐inflammatory effect28, 29. However, IL‐19 is also capable of activating monocytes to release IL‐6, tumor necrosis factor TNF‐α, IL‐8 and reactive oxygen species, and has been implicated in the pathogenesis of sepsis‐induced organ injury30, 31. Cuneo et al.18 also reported that IL‐19 can be induced by inflammatory cytokines and inflammatory stimuli, and IL‐19 is expressed in injured and stimulated vascular smooth muscle cells. The present study found that IL‐19 was elevated in patients with diabetes, and it was positively associated with the number of concurrent microvascular and macrovascular lesions in patients with type 2 diabetes mellitus. The present results showed that IL‐19 play a critical role in the pathogenesis of vascular disease in diabetes mellitus.
Ang‐2 is stored in endothelial cell Weibel–Palade bodies, and high concentrations of Ang‐2 produce marked impairment of endothelial function. Ang‐2 is known to increase levels of pro‐inflammatory cytokines32. In the present study, serum IL‐19 levels were positively correlated with Ang‐2, and also a positive correlation between IL‐19 and HbA1c, HOMA‐IR was observed, which shows that IL‐19 was closely associated with glucose metabolism disorders and vascular lesions in patients with type 2 diabetes mellitus. The present results suggest that long‐term hyperglycemia increases IL‐19 expression through stimulating endothelial cells. Furthermore, insulin resistance and insufficient insulin signaling in patients with type 2 diabetes mellitus might also lead to the increased expression of IL‐19. Additionally, the increased expression of Ang‐2 in response to high glucose might result in increased expression of IL‐19. These data provide evidence to suggest that high glucose‐induced IL‐19 production might be mediated by the induction of Ang‐2 in vascular endothelial cells. However, the mechanism requires further investigation.
The high concentrations of glycation end‐products in diabetic patients can upregulate inflammatory cytokines expression by stimulating endothelial cells. Conversely, inflammatory cytokines can stimulate endothelial cell proliferation and induce local inflammation, accelerating the generation and development of vascular disease33, 34. In the present study, we found that IL‐19 levels gradually increased in the following order: type 2 diabetes mellitus patients without vascular complications group, the microangiopathy group and the macroangiopathy group. Importantly, IL‐19 was significantly higher among diabetic patients with macrovascular complications when compared with those without macrovascular complications. In the multivariable logistic regression analysis, IL‐19 levels were independently associated with type 2 diabetes mellitus patients with vascular complications. This showed that the extent of vascular damage might be closely linked to IL‐19 levels. Several hypotheses can be made on the role of IL‐19 levels in diabetic vasculopathy. First, IL‐19 alone might exert pro‐angiogenic activity. Second, high IL‐19 levels might result in local inflammation. Finally, IL‐19 expression contributes to Ang‐2‐induced impairment of endothelial responses. IL‐19 and Ang‐2 could emerge with a direct role in the acceleration of vascular injury, in addition to the pro‐inflammatory effect on this pathophysiology.
It has to be stressed that our current study was limited. The sample size of the present cross‐sectional study was relatively small. In addition, the correlation between IL‐19 and Ang‐2 in the present clinical study provides correlated evidence, but does not address the cause–effect relationship in type 2 diabetes.
In summary, the present data show that levels of serum IL‐19 and Ang‐2 were significantly elevated in patients with type 2 diabetes mellitus. Specifically, they were positively correlated with the numbers of vascular complications of type 2 diabetes mellitus. Therefore, these observations provide Ang‐2 and IL‐19 as further insights into the possible effectiveness of anti‐inflammatory therapy in the treatment and prevention of diabetic angiopathy.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The present study was supported by Yancheng Medical Science and Technology Development Project (no. YK2015066), KangDa Institute of Medicine and Health Development Foundation of Nanjing Medical University (no. NYKDKJ2015020) and Research Project of Binhai County People's Hospital (no. BYKJ2016001).
J Diabetes Investig 2016; 7: 895–900
References
- 1. Wild S, Roglic G, Green A, et al Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 2. Rasul S, Reiter MH, Ilhan A, et al Circulating angiopoietin‐2 and soluble Tie‐2 in type 2 diabetes mellitus: a cross‐sectional study. Cardiovasc Diabetol 2011; 10: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jaumdally RJ, Goon PK, Varma C, et al Effects of atorvastatin on circulating CD34 + /CD133 + /CD45‐ progenitor cells and indices of angiogenesis (vascular endothelial growth factor and the angiopoietins 1 and 2) in atherosclerotic vascular disease and diabetes mellitus. J Intern Med 2010; 267: 385–393. [DOI] [PubMed] [Google Scholar]
- 4. Fujita T, Ogihara N, Kamura Y, et al Interleukin‐18 contributes more closely to the progression of diabetic nephropathy than other diabetic complications. Acta Diabetol 2012; 49: 111–117. [DOI] [PubMed] [Google Scholar]
- 5. Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev 2004; 25: 971–1010. [DOI] [PubMed] [Google Scholar]
- 6. Navarro‐Gonzalez JF, Mora‐Fernandez C. The role of inflammatory cytokine in diabetic nephropathy. J Am Soc Nephrol 2008; 19: 433–442. [DOI] [PubMed] [Google Scholar]
- 7. Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diab Rep 2007; 7: 242–248. [DOI] [PubMed] [Google Scholar]
- 8. Navarro JF, Mora C. Role of inflammation in diabetic complications. Nephrol Dial Transplant 2005; 20: 2601–2604. [DOI] [PubMed] [Google Scholar]
- 9. Chen S, Guo L, Chen B, et al Association of serum angiopoietin‐1, angiopoietin‐2 and angiopoietin‐2 to angiopoietin‐1 ratio with heart failure in patients with acute myocardial infarction. Exp Ther Med 2013; 5: 937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas M, Augustin HG. The role of the angiopoietins in vascular morphogenesis. Angiogenesis 2009; 12: 125–137. [DOI] [PubMed] [Google Scholar]
- 11. Maisonpierre PC, Suri C, Jones PF, et al Angiopoietin‐2, anatural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277: 55–60. [DOI] [PubMed] [Google Scholar]
- 12. Gallagher G, Dickensheets H, Eskdale J, et al Cloning, expression and initial characterization of interleukin‐19 (IL‐19), a novel homologue of human interleukin‐10 (IL‐10). Genes Immun 2000; 1: 442–450. [DOI] [PubMed] [Google Scholar]
- 13. Jordan WJ, Eskdale J, Boniotto M, et al Human IL‐19 regulates immunity through auto‐induction of IL‐19 and production of IL‐10. Eur J Immunol 2005; 35: 1576–1582. [DOI] [PubMed] [Google Scholar]
- 14. Huang F, Wachi S, Thai P, et al Potentiation of IL‐19 expression in airway epithelia by IL‐17A and IL‐4/IL‐13: important implications in asthma. J Allergy Clin Immunol 2008; 121: 1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li HH, Lin YC, Chen PJ, et al Interleukin‐19 upregulates keratinocyte growth factor and is associated with psoriasis. Br J Dermatol 2005; 153: 591–595. [DOI] [PubMed] [Google Scholar]
- 16. Liao SC, Cheng YC, Wang YC, et al IL‐19 induced Th2 cytokines and was up‐regulated in asthma patients. J Immunol 2004; 173: 6712–6718. [DOI] [PubMed] [Google Scholar]
- 17. Barrett JC, Clayton DG, Concannon P, et al Genome‐wide association study and meta‐analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009; 41: 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuneo AA, Herrick D, Autieri MV. IL‐19 reduces VSMC activation by regulation of mRNA regulatory factor HuR and reduction of mRNA stability. J Mol Cell Cardiol 2010; 49: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jain S, Gabunia K, Kelemen SE, et al The anti‐inflammatory cytokine interleukin is expressed by and angiogenic for human endothelial cells. Arterioscler Thromb Vasc Biol 2011; 31: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li L, Qian L, Yu ZQ. Serum angiopoietin‐2 is associated with angiopathy in type 2 diabetes mellitus. J Diabetes Complications 2015; 29: 568–571. [DOI] [PubMed] [Google Scholar]
- 21. Geneva S. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Diabet Med: World Health Organisation, 1999. [Google Scholar]
- 22. Szarvas T, Jäger T, Tötsch M, et al Angiogenic switch of angiopietins‐Tie2 system and its prognostic value in bladder cancer. Clin Cancer Res 2008; 14: 8253–8262. [DOI] [PubMed] [Google Scholar]
- 23. Gomolak JR, Didion SP. AngiotensinII‐induced endothelial dysfunction is temporally linked with increases in interleukin‐6 and vascular macrophage accumulation. Front Physiol 2014; 5: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parrish‐Novak J, Xu W, Brender T, et al Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor‐ligand interactions mediate unique biological function. J Biol Chem 2002; 277: 47517–47523. [DOI] [PubMed] [Google Scholar]
- 25. Dumoutier L, Leemans C, Lejeune D, et al Cutting edge: STAT activation by IL‐19, IL‐20 and mda‐7 through IL‐20 receptor complexes of two types. J Immunol 2001; 167: 3545–3549. [DOI] [PubMed] [Google Scholar]
- 26. Felmeden DC, Spencer CG, Belgore FM, et al Endothelial damage and angiogenesis in hypertensive patients: relationship to cardiovascular risk factors and risk factor management. Am J Hypertens 2003; 16: 11–20. [DOI] [PubMed] [Google Scholar]
- 27. Calderari S, Chougnet C, Clemessy M, et al Angiopoietin 2 alters pancreatic vascularization in diabetic conditions. PLoS One 2012; 7: e29438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Azuma YT, Matsuo Y, Nakajima H, et al Interleukin‐19 is a negative regulator of innate immunity and critical for colonic protection. J Pharmacol Sci 2010; 115: 105–111. [DOI] [PubMed] [Google Scholar]
- 29. Sabat R, Wallace E, Endesfelder S, et al IL‐19 and IL‐20: two novel cytokines with importance in inflammatory diseases. Expert Opin Ther Targets 2007; 11: 601–612. [DOI] [PubMed] [Google Scholar]
- 30. Liao YC, Liang WG, Chen FW, et al IL‐19 induces production of IL‐6 and TNF‐alpha and results in cell apoptosis through TNF‐alpha. J Immunol 2002; 169: 4288–4297. [DOI] [PubMed] [Google Scholar]
- 31. Hsing CH, Chiu CJ, Chang LY, et al IL‐19 is involved in the pathogenesis of endotoxic shock. Shock 2008; 29: 7–15. [DOI] [PubMed] [Google Scholar]
- 32. Luo C, Li T, Zhang C, et al Therapeutic effect of alprostadil in diabetic nephropathy: possible roles of angiopoietin‐2 and IL‐18. Cell Physiol Biochem 2014; 34: 916–928. [DOI] [PubMed] [Google Scholar]
- 33. Fisman EZ, Adler Y, Tenenbaum A. Biomarkers in cardiovascular diabetology: interleukins and matrixins. Adv Cardiol 2008; 45: 44–64. [DOI] [PubMed] [Google Scholar]
- 34. Zorena K, Raczyńska D, Raczyńska K. Biomarkers in diabetic retinopathy and the therapeutic implications. Mediators Inflamm 2013; 2013: 193604. [DOI] [PMC free article] [PubMed] [Google Scholar]


