Abstract
Aims/Introduction
There is still a lack of simple methods and instruments for the early assessment of autonomic dysfunction in metabolic syndrome patients. Assessment of sudomotor function has been proposed to explore autonomic function, and could be used as an early biomarker for metabolic syndrome. In the present study, we use a quick and non‐invasive method to measure sudomotor function, and aimed to evaluate its efficacy to identify metabolic syndrome in a Chinese population.
Materials and Methods
Information on the 1,160 Chinese participants involved in the study, such as age, sex, blood pressure, waist circumference, body mass index, fasting plasma glucose and lipid profile, and SUDOSCAN, was recorded. During the sudomotor test, patients were asked to place their bare hands and feet on large electrodes. The test took 2 min to carry out, was painless and no participant preparation was required.
Results
A total of 567 participants were diagnosed with metabolic syndrome. The prevalence of metabolic syndrome correlated significantly with increasing SUDOSCAN cardiac risk score (P for trend <0.0001). Furthermore, an increase in cardiac risk score value was associated with an increase in the number of metabolic syndrome components (P for trend <0.0001). Compared with the no‐risk group (cardiac risk score <20), participants in the high‐risk group (cardiac risk score ≥30) had a 2.83‐fold increased risk of prevalent metabolic syndrome (P < 0.0001), and 1.51‐fold increased risk (P = 0.01) after adjustments.
Conclusions
Autonomic dysfunction is correlated to components of metabolic syndrome. The role of SUDOSCAN in the screening of at‐risk populations for metabolic syndrome has to be confirmed by further studies.
Keywords: Autonomic function, Metabolic syndrome, Sudomotor
Introduction
The metabolic syndrome (MetS) is a complex of interrelated risk factors for cardiovascular disease and diabetes. These factors include dysglycemia, raised blood pressure, elevated triglyceride levels, low high‐density lipoprotein cholesterol levels and obesity (particularly central adiposity)1. MetS is becoming increasingly common in China. Age standardized prevalence of MetS in Chinese populations was 9.8% in men and 17.8% in women in the year 20012. In urban residents, MetS prevalence is higher because of drastic lifestyle changes after China's rapid economic growth in recent decades; it was 35.1% in men and 32.5% in women during the Shanghai Zhabei Health 2020 survey (2009–2010)3. Therefore, more researchers are paying attention to MetS, and more studies on its mechanism are being carried out. MetS is associated with sympathetic activation4, 5, 6. A previous study found that MetS is a state of peripheral sympathetic nerve hyperactivity7. Autonomic dysfunction, therefore, can be considered one of the most critical pathogenic factors contributing to the development and progression of MetS8.
Autonomic dysfunction manifests as impaired sympathetic and parasympathetic function, resulting in hypertension, arrhythmia and sudomotor dysfunction. Examination of sudomotor function has been shown to be a highly sensitive detection method for small‐fiber neuropathy and autonomic fiber dysfunction9. Sudomotor function, therefore, could be used as an indicator of metabolic syndrome, allow for early detection of developing morbid autonomic abnormalities and result in appropriate early interventions. However, assessment of autonomic dysfunction until now has occurred late in its development, and has been limited to specialized centers as a result of the lack of simple, cost‐effective and rapid instruments.
In the present study, we use a quick and non‐invasive method to measure sudomotor function, and aim was to evaluate its efficacy to identify MetS in a Chinese population.
Methods
Ethics statement
The study protocol was approved by the ethics committee of Beijing Hospital, and was carried out in accordance with the principle of the Helsinki Declaration II. Written informed consent was obtained from each participant before data collection.
Study population
A total of 1,160 participants took part in the study from January 2013 to March 2014. Among them, 1,078 had a SUDOSCAN test, and completed information about age, sex, blood pressure, waist circumference, body mass index (BMI), fasting plasma glucose and lipid profile.
Data collection
A questionnaire was administered to collect information on health status, history of chronic diseases, medications and lifestyle risk factors. The definition of smoker and drinker were participants who smoked cigarettes or consumed alcohol regularly in the past 6 months. Anthropometric measurements were carried out by trained and certified clinical staff members using standard protocols and automated electronic devices. Height and weight were recorded to the nearest 0.1 cm and 0.1 kg while participants were wearing light indoor clothing without shoes. BMI was calculated as weight divided by height squared (kg/m2).
Waist circumference was measured to the nearest 0.1 cm at the midpoint between the lower margin of the least palpable rib and the top of the iliac crest using a stretch‐resistant tape when standing with feet close together, arms at the side and bodyweight evenly distributed. After at least 10 h of overnight fasting, venous blood samples were collected for the measurements of plasma glucose (automatic analyzer) and lipid profile. Measurement of triglycerides (TG), total cholesterol, high‐density lipoprotein cholesterol (HDL‐C) and low‐density lipoprotein cholesterol was carried out using an auto‐analyzer (automatic analyzer).
Definition of metabolic syndrome
The criteria for MetS were unified by a group of international organizations in 2009 in a Joint Interim Statement1. MetS is diagnosed by the presence of three or more of the following abnormal factors: (i) Serum TG concentration of ≥1.7 mmol/L or greater; (ii) HDL‐C < 1.0 mmol/L in men or <1.3 mmol/L in women; (iii) blood pressure of ≥130/85 mmHg; (iv) fasting plasma glucose greater than 5.6 mmol/L or receiving drug treatment for elevated glucose; and (v) waist circumference ≥85 cm in men and ≥80 cm in women. These adjusted waist circumference cut‐off points were recommended by the Joint Interim Statement for the Chinese population. The definition of diabetes followed the 1999 World Health Organization diagnostic criteria.
Assessment of sudomotor function
SUDOSCAN (Impeto Medical, Paris, France) is a simple, non‐invasive device designed to measure sudomotor function based on reverse iontophoresis and chronoamperometry. Four electrodes are placed on areas with the greatest density of sweat glands, namely the palms of the hands and the soles of the feet (Figure 1). A DC current at an incremental voltage of less than 4 V is applied, extracting chloride ions from the sweat and resulting in a current to the electrodes. The current produced is proportional to the chloride concentration that reacted with the electrodes, increasing with each increment of voltage applied. A time/ampere curve is recorded for each deviation and the mean value is estimated for each area. The electrochemical skin conductance (ESC; measured in μS), expressed as a ratio of the current generated and the constant DC stimulus, is calculated for hands (left and right) and feet (left and right). Low ESC indicates sudomotor function abnormality. In addition, an algorithm that integrates ESC with age and BMI has been developed to produce a score estimating the individual's current risk of cardiac autonomic neuropathy (cardiac risk score). This risk score has been validated in several clinical studies10, 11, 12, 13, 14.
Figure 1.
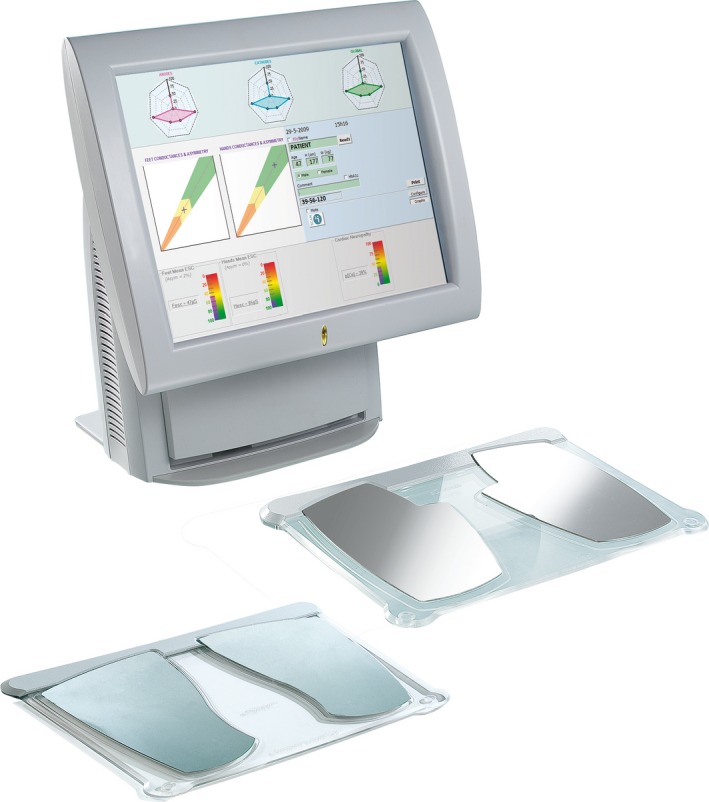
SUDOSCAN device with hand and foot electrodes. A typical presentation of results after the test is shown on the screen.
During the test, the patient places his or her hands and feet on the electrodes. The test takes 2 min to carry out, is painless and requires no participant preparation.
Statistical analysis
Statistical analysis was carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). For descriptive analysis, continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as percentages. Differences among groups of cardiac risk score were tested by one‐way anova for continuous variables and by χ2‐test for categorical variables. To evaluate the associations between SUDOSCAN value and demographic and clinical characteristics, we carried out Pearson's correlation coefficients and multivariate linear regression models. Unadjusted and multivariate adjusted logistic regression models were used to assess the relationship between MetS and values of cardiac risk score and conductance. In model 1, no covariate was adjusted; model 2 was adjusted for sex and age (only for the conductance), and model 3 was further adjusted for current smoking and drinking. Odds ratios (OR) and the corresponding 95% confidence intervals (95% CI) were calculated for an increase of each risk level. A two‐sided P‐value <0.05 was considered significant.
Results
Patient demographics and clinical characteristics of the total population are shown in Table 1. Among the 1,078 patients who had complete clinical information regarding MetS and SUDOSCAN, 567 had metabolic syndrome according to the specified criteria. In Table 2, patients are classified according to their cardiac risk score (no risk <19, moderate risk 20–29, high risk ≥30). The prevalence of MetS increased with the cardiac risk score level, with 42% for cardiac risk score 0–19, 60% for risk score 20–39 and 68% for risk score ≥30 (Figure 2). Pearson's correlation and linear regression analysis, adjusted for sex, showed that BMI, waist circumference, systolic blood pressure, diastolic blood pressure, TG, total cholesterol, low‐density lipoprotein cholesterol, HDL‐C, fasting plasma glucose, 2‐h post prandial glucose and glycated hemoglobin and feet conductance (feet ESC) were significantly correlated with cardiac risk score value (Table 3). Compared with the group classified as no risk according to cardiac risk score, univariate logistic regression analysis showed that participants in the moderate‐risk group and the high‐risk group, respectively, had a significant correlation with increased odds of the prevalence of MetS and each of its components, except HDL‐C (Table 4). However, only MetS and fasting plasma glucose remained significantly correlated after adjusting for sex, BMI, current smoking and current drinking. As shown in Figure 3, the cardiac risk score increased with the number of MetS components (P for trend <0.0001).
Table 1.
Characteristics of the study population
| Whole population | |
|---|---|
| n | 1,160 |
| Age (years) | 46.6 ± 8.7 |
| Male, n (%) | 816 (70.3) |
| BMI (kg/m²) | 25.4 ± 3.4 |
| Waist circumference (cm) | 88.0 ± 9.5 |
| SBP (mmHg) | 129.5 ± 17.0 |
| DBP (mmHg) | 81.1 ± 11.7 |
| TG (mmol/L) | 2.3 ± 2.3 |
| Total cholesterol (mmol/L) | 5.2 ± 1.0 |
| HDL‐C (mmol/L) | 1.3 ± 0.5 |
| LDL‐C (mmol/L) | 2.8 ± 0.7 |
| FPG (mmol/L) | 5.8 ± 1.5 |
| 2‐h postprandial glucose (mmol/L) | 7.9 ± 3.7 |
| HbA1c (%) | 5.8 ± 0.9 |
| Hands conductance (μS) | 73.9 ± 14.4 |
| Feet conductance (μS) | 73.3 ± 13.4 |
| Cardiac risk score | 19.6 ± 9.6 |
| Current smoking, n (%) | 355 (30.6) |
| Current drinking, n (%) | 573 (49.4) |
BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, high‐density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglyceride.
Table 2.
Characteristics of the study population classified according to the cardiac risk score
| Cardiac risk score | ||||
|---|---|---|---|---|
| 0–19 | 20–29 | ≥30 | P‐value | |
| n | 567 | 428 | 165 | |
| Age (years) | 41.3 ± 6.3 | 49.7 ± 6.3 | 56.7 ± 8.0 | <0.0001 |
| Male, n (%) | 403 (71.1) | 305 (71.3) | 108 (65.5) | 0.3313 |
| BMI (kg/m²) | 24.2 ± 2.8 | 26.0 ± 3.0 | 27.9 ± 4.5 | <0.0001 |
| Waist circumference (cm) | 85.3 ± 9.0 | 89.9 ± 9.1 | 92.3 ± 9.6 | <0.0001 |
| SBP (mmHg) | 126.3 ± 15.4 | 131.3 ± 17.7 | 135.6 ± 18.2 | <0.0001 |
| DBP (mmHg) | 80.0 ± 11.9 | 82.2 ± 11.1 | 82.3 ± 12.1 | 0.003 |
| TG (mmol/L) | 2.1 ± 2.2 | 2.4 ± 2.4 | 2.5 ± 2.3 | 0.0004 |
| Total cholesterol (mmol/L) | 5.1 ± 0.9 | 5.3 ± 1.0 | 5.4 ± 1.1 | 0.0008 |
| HDL‐C (mmol/L) | 1.3 ± 0.3 | 1.3 ± 0.6 | 1.3 ± 0.8 | 0.2367 |
| LDL‐C (mmol/L) | 2.7 ± 0.7 | 2.8 ± 0.7 | 2.9 ± 0.8 | 0.0186 |
| FPG (mmol/L) | 5.7 ± 1.6 | 5.8 ± 1.4 | 5.9 ± 1.3 | <0.0001 |
| 2‐h postprandial glucose (mmol/L) | 7.4 ± 3.7 | 8.3 ± 3.4 | 8.9 ± 3.9 | <0.0001 |
| HbA1c (%) | 5.7 ± 1.1 | 5.8 ± 0.9 | 5.8 ± 0.7 | <0.0001 |
| Current smoking, n (%) | 171 (30.2) | 140 (32.7) | 44 (26.8) | 0.3595 |
| Current drinking, n (%) | 292 (51.5) | 218 (50.9) | 63 (38.4) | 0.0095 |
| Hands conductance (μS) | 77.5 ± 12.5 | 72.1 ± 14.5 | 66.3 ± 16.5 | <0.0001 |
| Feet conductance (μS) | 78.1 ± 10.1 | 70.6 ± 13.0 | 63.2 ± 16.6 | <0.0001 |
Data are mean ± standard deviation for continuous variables and n (%) for categorical variables. P‐values were for anova or χ² analysis across the three groups. BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐ density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Figure 2.
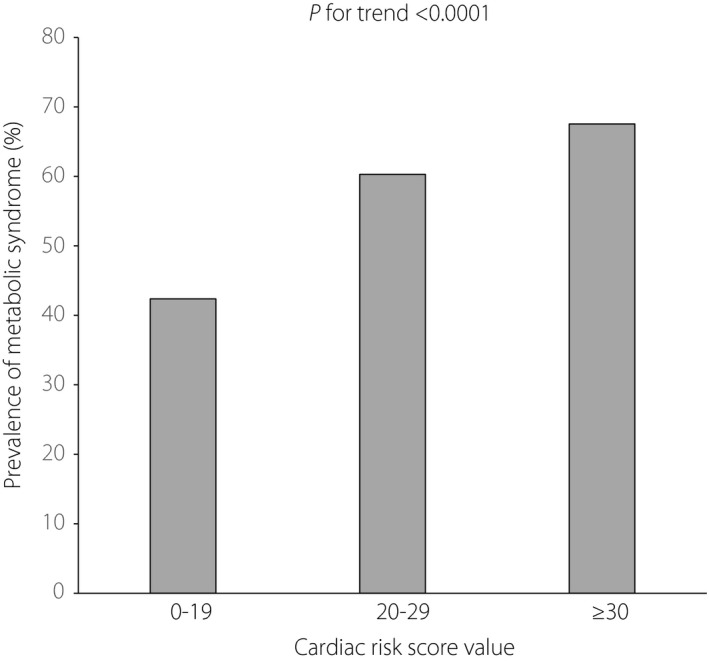
Prevalence of metabolic syndrome in different risk levels of the cardiac risk score. 0–19, n = 567; 20–29, n = 428; ≥30, n = 165.
Table 3.
Pearson's correlation and multiple regression analysis of risk factors associated with cardiac risk score value
| r | P‐value | β | P‐value | |
|---|---|---|---|---|
| BMI (kg/m²) | 0.44236 | <0.0001 | 1.34187 | <0.0001 |
| Waist circumference (cm) | 0.32425 | <0.0001 | 0.41899 | <0.0001 |
| SBP (mmHg) | 0.23887 | <0.0001 | 0.13878 | <0.0001 |
| DBP (mmHg) | 0.12201 | <0.0001 | 0.11235 | <0.0001 |
| TG (mmol/L) | 0.0665 | 0.0283 | 0.31211 | 0.014 |
| TC (mmol/L) | 0.12315 | <0.0001 | 1.16985 | <0.0001 |
| HDL‐C (mmol/L) | –0.00208 | 0.9455 | –0.24709 | 0.6573 |
| LDL‐C (mmol/L) | 0.09604 | 0.0015 | 1.29214 | 0.0012 |
| FPG (mmol/L) | 0.09575 | 0.0016 | 0.63985 | 0.0008 |
| 2‐h postprandial glucose | 0.1779 | <0.0001 | 0.46758 | <0.0001 |
| HBA1c (%) | 0.09069 | 0.0144 | 0.93755 | 0.0116 |
| Feet conductance (μS) | –0.44196 | <0.0001 | –0.32031 | <0.0001 |
Multiple regression analysis adjusted for sex. BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Table 4.
Odds ratio of having metabolic syndrome or abnormal values for the components of metabolic syndrome according to the cardiac risk score
| Cardiac risk score (0–19) | Cardiac risk score (20–29) | Cardiac risk score (≥30) | P for trend | ||
|---|---|---|---|---|---|
| Metabolic syndrome | Model 1 | 1 | 2.07 (1.59–2.69) | 2.83 (1.93–4.15) | <0.0001 |
| Model 2 | 1 | 1.48 (1.11–1.99) | 1.50 (0.97–2.32) | 0.0117 | |
| Model 3 | 1 | 1.47 (1.09–1.96) | 1.51 (0.98–2.34) | 0.0124 | |
| Waist circumference (cm) | Model 1 | 1 | 2.59 (1.91–3.52) | 4.29 (2.57–7.15) | <0.0001 |
| Model 2 | 1 | 1.43 (0.98–2.09) | 1.34 (0.72–2.50) | 0.1 | |
| Model 3 | 1 | 1.38 (0.94–2.02) | 1.32 (0.70–2.47) | 0.1384 | |
| HDL‐C (mmol/L) | Model 1 | 1 | 1.07 (0.79–1.46) | 1.45 (0.97–2.17) | 0.1015 |
| Model 2 | 1 | 0.91 (0.66–1.26) | 1.00 (0.64–1.57) | 0.8594 | |
| Model 3 | 1 | 0.91 (0.66–1.25) | 0.98 (0.63–1.54) | 0.7903 | |
| FPG (mmol/L) | Model 1 | 1 | 1.76 (1.35–2.29) | 1.98 (1.37–2.85) | <0.0001 |
| Model 2 | 1 | 1.57 (1.18–2.07) | 1.62 (1.08–2.43) | 0.0024 | |
| Model 3 | 1 | 1.57 (1.18–2.07) | 1.64 (1.10–2.46) | 0.0021 | |
| Triglycerides (mmol/L) | Model 1 | 1 | 1.35 (1.04–1.75) | 1.56 (1.08–2.24) | 0.0048 |
| Model 2 | 1 | 1.01 (0.76–1.34) | 0.93 (0.61–1.40) | 0.7936 | |
| Model 3 | 1 | 0.98 (0.74–1.31) | 0.93 (0.61–1.41) | 0.7453 | |
| Hypertension (mmHg) | Model 1 | 1 | 1.39 (1.07–1.81) | 1.94 (1.31–2.86) | 0.0002 |
| Model 2 | 1 | 1.18 (0.89–1.57) | 1.49 (0.97–2.28) | 0.0581 | |
| Model 3 | 1 | 1.18 (0.89–1.56) | 1.51 (0.98–2.32) | 0.0524 |
Data are odds ratios (95% confidence interval) compared with cardiac risk score (0–19) group. Participants with abnormal values for Waist circumference, high‐density lipoprotein cholesterol (HDL‐C), fasting plasma glucose (FPG), triglycerides and hypertension (as defined in the metabolic syndrome) are defined as 0, and with normal values as 1. Participants without metabolic syndrome are defined as 0 and with metabolic syndrome as 1. Model 1 is unadjusted. Model 2 is adjusted for sex and body mass index. Model 3 is adjusted for sex, body mass index, current smoking and current drinking.
Figure 3.
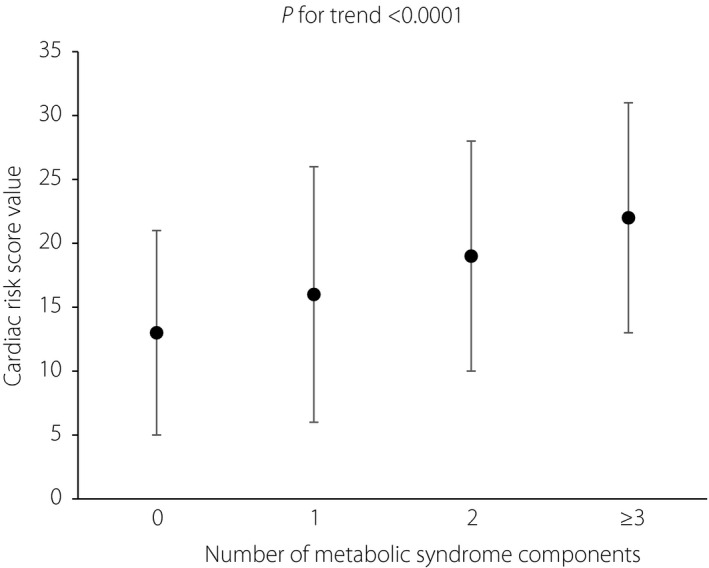
Cardiac risk score value in different numbers of metabolic syndrome components. 0, n = 91; 1, n = 177; 2, n = 243; ≥3, n = 567. Values are mean ± standard deviation.
Patients having feet ESC lower than 70 μS have an OR of 1.5 (P = 0.01) to have HDL‐C concentration of <1.0 mmol/L in men or <1.3 mmol/L in women, as compared with patients with feet ESC >70 μS. After adjustment for sex, BMI, current smoking and drinking status, it was 1.4 (P = 0.03). Patients with waist circumference ≥94.4 cm and TG ≥2.59 mmol/L had an OR of 3.4 (P = 0.007) and 4.7 (P = 0.0003), respectively, for having feet and hand ESC lower than 70 μS when compared with patients with waist circumference <81.8 cm and TG <1.15 mmol/L. Patients with waist circumference ≥80 cm for women and ≥85 cm for men, and TG ≥1.7 mmol/L had an OR of 2.1 (P = 0.02) for having feet ESC lower than 70 μS when compared with other patients.
Discussion
In the present study, we found that: (i) prevalence of MetS increased with the cardiac risk score level (P for trend <0.0001); (ii) cardiac risk score value was associated with an increase in the number of MetS components (P for trend <0.0001); (iii) compared with the no‐risk group (cardiac risk score <20), participants in the high‐risk group (cardiac risk score ≥30) had a 2.83‐fold increased risk of prevalent MetS (P < 0.0001) and 1.51‐fold (P = 0.01) after adjustment. Hands and feet ESC, direct quantitative measures of sudomotor function, were significantly correlated to components of MetS, but their predictive power for MetS requires further study.
MetS is highly prevalent in China. Diabetes, which in 2010 already affected 11.6% of the Chinese adult population15, is an important component of MetS, and a growing medical epidemic. Detection and diagnosis of MetS in the early stage will definitely extend people's life expectancy, and decrease the burden of the government. The sympathetic nervous system plays a central role in the body's metabolic balance16, 17, and regulates sweat gland function. An early marker of small fiber neuropathy is the abnormal sudomotor response in people who have sympathetic dysfunction18. Autonomic status, therefore, can be assessed by sudomotor function testing19, which can also be an efficient screening tool for autonomic‐related diseases20. Evaluating sudomotor dysfunction can be easily done using SUDOSCAN, and has been validated by many clinical studies in prediabetes and diabetes. A clinical study carried out by Casellini et al.10 showed that the SUDOSCAN test had a sensitivity of 78% and specificity of 92% for assessing peripheral neuropathy in people with diabetes. Similarly, Osaki et al.21 found that in Chinese people, this test has a good performance in the detection of diabetic kidney disease, with a sensitivity and specificity of 94% and 78%, respectively.
Early detection of complications associated with MetS is important, yet simple and sensitive techniques have been lacking. However, technological advances in autonomic function testing – and in particular sudomotor tests – are now allowing for precise evaluation at subclinical stages. Knowing the association between dysautonomia and MetS, these new techniques might become an innovative method for detecting and ascertaining the risk of MetS.
The SUDOSCAN test appears to have a number of advantages in the assessment of sudomotor function and its related metabolic disorders: (i) the test is practically automated, with an initial self‐calibration; (ii) the test is painless and non‐invasive; (iii) results are available immediately after the 2 min of testing; and (iv) neither special preparation of the participants nor medical training for the operator is necessary. There is no recognized gold standard sudomotor function test today against which to assess new technologies. Quantitative Sudomotor Axon Reflex Test is one of the most common tests for assessing autonomic nervous system disorders, peripheral neuropathies and certain pain syndromes. However, the test is lengthy and requires a highly skilled technician, limiting its application in large‐scale epidemiological studies or routine therapeutic monitoring22. Comparatively, the SUDOSCAN test is perfectly suited for general practitioners, pharmacists and neurologists for routine application. In addition, quantitative results obtained by SUDOSCAN can be used for the follow up of patients involved in prevention programs. Raisanen et al.14 showed that participants with low fitness levels had improvement of their cardiac risk score after 12 months of lifestyle intervention. This improvement was significantly higher in participants with the highest weekly activity as documented by pedometer, whereas no significant change was observed for weight and waist circumference.
There were several limitations to the present study. First, predicting MetS using an autonomic test, such as SUDOSCAN, alone might be relatively less sensitive and accurate compared with the clinical definition of MetS, which requires a number of abnormalities to be present. However, assessing MetS using multiple anthropometric measurements and a fasting blood test is invasive and time‐consuming, whereas a 2‐min SUDOSCAN test is rapid and convenient when needing to screen and monitor large populations at high risk for MetS. Second, the convenience and accuracy of SUDOSCAN make it highly suitable for screening rather than diagnosis; therefore, SUDOSCAN test results should be interpreted along with other factors, such as blood pressure, lipid profiles and BMI, for a proper evaluation of the individual's MetS risk. Third, the study was limited to an older Chinese population (age 40 years and older); additional studies will be required to evaluate the performance of the SUDOSCAN test in participants of different ages and races. Fourth, the thresholds for cardiac risk score and conductances (ESC) computed by the SUDOSCAN device will need to be validated for MetS assessment in larger populations. Finally, measuring the predictive power of autonomic function tests for MetS will require additional and specifically longitudinal studies.
In conclusion, sudomotor dysfunction as assessed using SUDOSCAN was found to be linked to components of MetS, but its place in the screening of at‐risk populations for MetS has to be confirmed by further studies.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This research study was supported by China Health Promotion Foundation Health Management Institute (foundation no. 2013‐1‐001). This study received grant support from Impeto Medical (Paris, France).
J Diabetes Investig 2016; 7: 901–907
References
- 1. Alberti KG, Eckel RH, Grundy SM, et al Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 2. Gu D, Reynolds K, Wu X, et al Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 2005; 365: 1398–1405. [DOI] [PubMed] [Google Scholar]
- 3. Wang GR, Li L, Pan YH, et al Prevalence of metabolic syndrome among urban community residents in China. BMC Public Health 2013; 13: 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grassi G, Seravalle G, Quarti‐Trevano F, et al Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension 2007; 49: 535–541. [DOI] [PubMed] [Google Scholar]
- 5. Grassi G, Dell'Oro R, Quarti‐Trevano F, et al Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia 2005; 48: 1359–1365. [DOI] [PubMed] [Google Scholar]
- 6. Straznicky NE, Lambert EA, Lambert GW, et al Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab 2005; 90: 5998–6005. [DOI] [PubMed] [Google Scholar]
- 7. Huggett RJ, Burns J, Mackintosh AF, et al Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension 2004; 44: 847–852. [DOI] [PubMed] [Google Scholar]
- 8. Grassi G, Seravalle G. Autonomic imbalance and metabolic syndrome: unravelling interactions, mechanisms and outcomes. J Hypertens 2006; 24: 47–49. [DOI] [PubMed] [Google Scholar]
- 9. Low VA, Sandroni P, Fealey RD, et al Detection of small‐fiber neuropathy by sudomotor testing. Muscle Nerve 2006; 34: 57–61. [DOI] [PubMed] [Google Scholar]
- 10. Casellini CM, Parson HK, Richardson MS, et al Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther 2013; 15: 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freedman BI, Bowden DW, Smith SC, et al Relationships between electrochemical skin conductance and kidney disease in type 2 diabetes. J Diabetes Complications 2014; 28: 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yajnik CS, Kantikar VV, Pande AJ, et al Quick and simple evaluation of sudomotor function for screening of diabetic neuropathy. ISRN Endocrinol 2012; 2012: 103714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith AG, Lessard M, Reyna S, et al The diagnostic utility of sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complications 2014; 28: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raisanen A, Eklund J, Calvet JH, et al Sudomotor function as a tool for cardiorespiratory fitness level evaluation: comparison with maximal exercise capacity. Int J Environ Res Public Health 2014; 11: 5839–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan JC, Zhang Y, Ning G. Diabetes in China: a societal solution for a personal challenge. Lancet Diabetes Endocrinol 2014; 2: 969–979. [DOI] [PubMed] [Google Scholar]
- 16. Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens 1999; 17: 719–734. [DOI] [PubMed] [Google Scholar]
- 17. Davy KP, Orr JS. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev 2009; 33: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheshire WP, Freeman R. Disorders of sweating. Semin Neurol 2003; 23: 399–406. [DOI] [PubMed] [Google Scholar]
- 19. Birklein F, Spitzer A, Riedl B. Assessment of sudomotor function as a diagnostic tool for autonomic disturbances: principles and methods. Fortschritte Der Neurologie Psychiatrie 1999; 67: 287–295. [DOI] [PubMed] [Google Scholar]
- 20. Kimpinski K, Iodice V, Sandroni P, et al Sudomotor dysfunction in autoimmune autonomic ganglionopathy. Neurology 2009; 73: 1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ozaki R, Cheung KK, Wu E, et al A new tool to detect kidney disease in Chinese type 2 diabetes patients: comparison of EZSCAN with standard screening methods. Diabetes Technol Ther 2011; 13: 937–943. [DOI] [PubMed] [Google Scholar]
- 22. Peltier A, Smith AG, Russell JW, et al Reliability of quantitative sudomotor axon reflex testing and quantitative sensory testing in neuropathy of impaired glucose regulation. Muscle Nerve 2009; 39: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]


