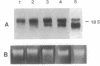
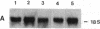Abstract
Cardiac fibroblasts are mainly responsible for the synthesis of major extracellular matrix proteins in the heart, including fibrillar collagen types I and III and fibronectin. In this report we show that these cells, when stimulated by transforming growth factor beta 1 (TGF-beta 1), acquire certain myocyte-specific properties. Cultured cardiac fibroblasts from adult rabbit heart were treated with TGF-beta 1 (10-15 ng/ml) for different periods of time. Northern hybridization analysis of total RNA showed that cells treated with TGF-beta 1 for 24 hr expressed mRNA corresponding to sarcomeric actin mRNA. Immunofluorescence staining and light microscopy showed that cultured cardiac fibroblasts treated with TGF-beta 1 became stained with a monoclonal antibody to muscle-specific actin. After treatment of quiescent cells with TGF-beta 1, cell proliferation (as measured by [3H]thymidine incorporation) was moderately increased (1.5-fold, P less than 0.001). NIH 3T3 cells and human skin fibroblasts, treated with TGF-beta 1, did not express sarcomeric actin mRNA. Treatment of cardiac fibroblasts with the mitogenic agent phorbol 12-myristate 13-acetate or with norepinephrine, angiotensin II, or interleukin 1 beta did not induce myocyte-specific actin mRNA. Cultured cardiac fibroblasts at the subconfluent stage, when exposed to TGF-beta 1 in the presence of 10% fetal bovine serum, gave rise to a second generation of slowly growing cells that expressed muscle-specific actin filaments. Our findings demonstrate that cardiac fibroblasts can be made to differentiate into cells that display many characteristics of cardiac myocytes. TGF-beta 1 seems to be a specific inducer of such conversion.
Full text
PDF

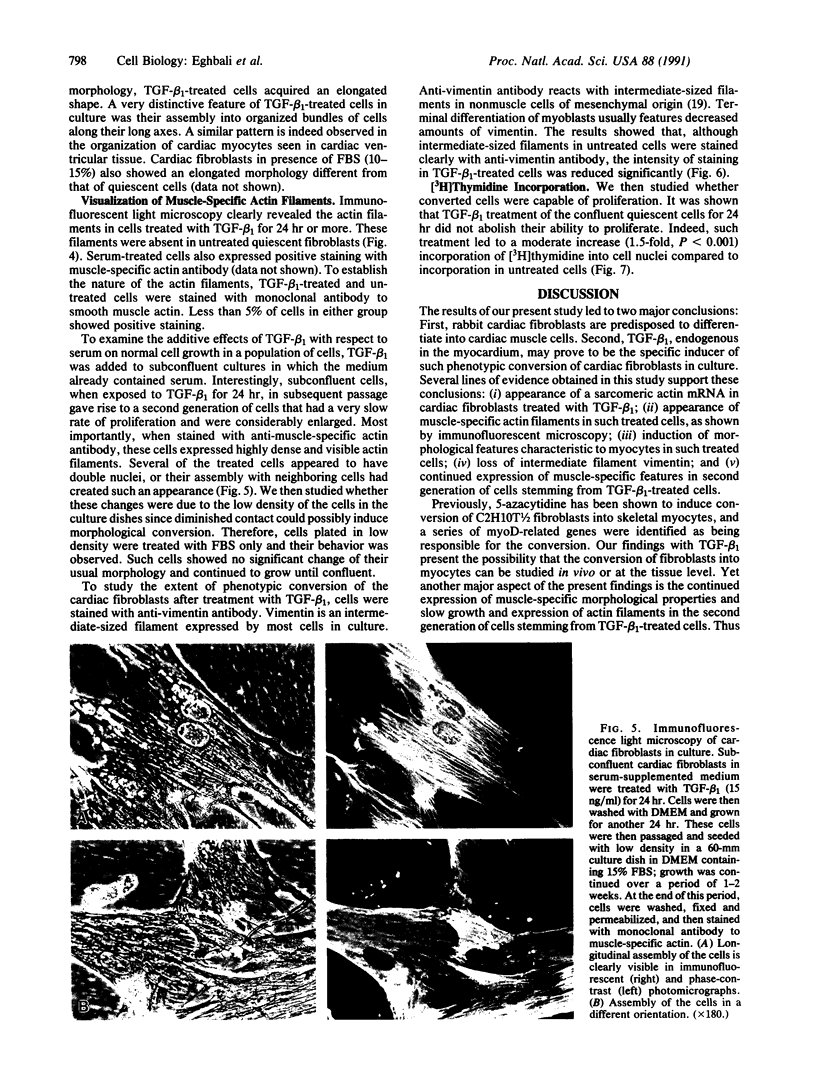
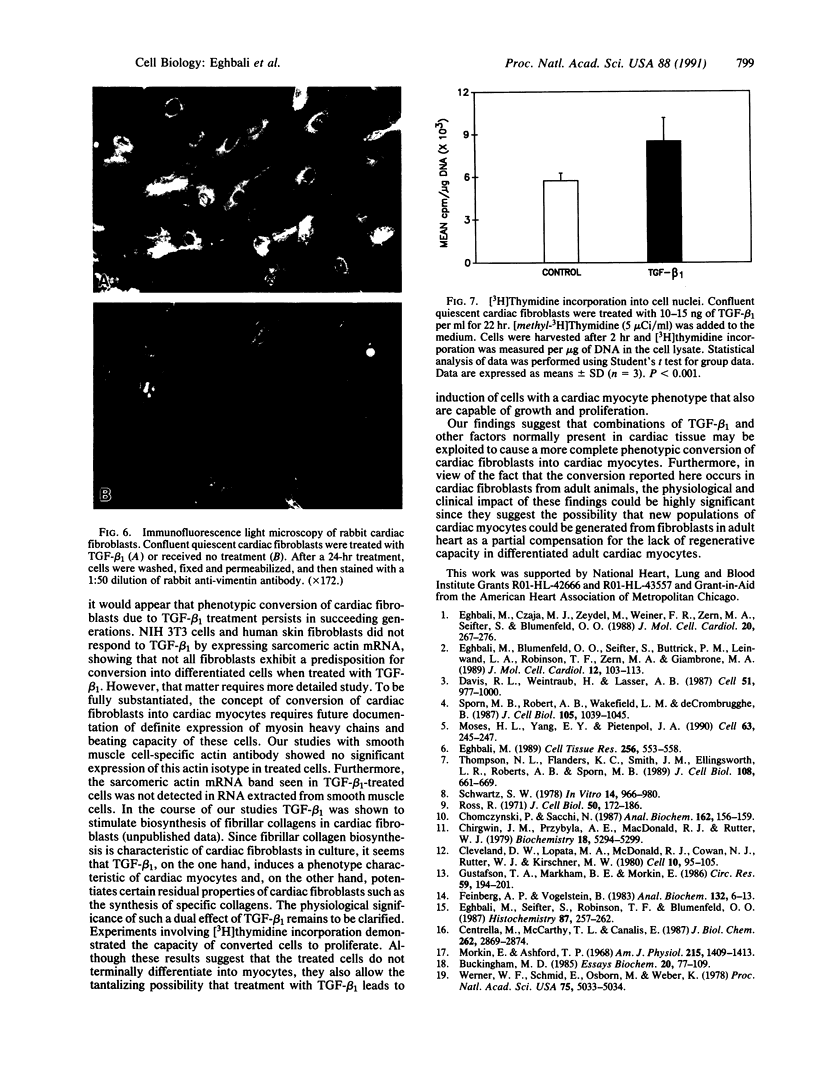
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckingham M. E. Actin and myosin multigene families: their expression during the formation of skeletal muscle. Essays Biochem. 1985;20:77–109. [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987 Feb 25;262(6):2869–2874. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Blumenfeld O. O., Seifter S., Buttrick P. M., Leinwand L. A., Robinson T. F., Zern M. A., Giambrone M. A. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol. 1989 Jan;21(1):103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- Eghbali M. Cellular origin and distribution of transforming growth factor-beta in the normal rat myocardium. Cell Tissue Res. 1989 Jun;256(3):553–558. doi: 10.1007/BF00225603. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Czaja M. J., Zeydel M., Weiner F. R., Zern M. A., Seifter S., Blumenfeld O. O. Collagen chain mRNAs in isolated heart cells from young and adult rats. J Mol Cell Cardiol. 1988 Mar;20(3):267–276. doi: 10.1016/s0022-2828(88)80059-2. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Seifter S., Robinson T. F., Blumenfeld O. O. Enzyme-antibody histochemistry. A method for detection of collagens collectively. Histochemistry. 1987;87(3):257–262. doi: 10.1007/BF00492419. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T. A., Markham B. E., Morkin E. Effects of thyroid hormone on alpha-actin and myosin heavy chain gene expression in cardiac and skeletal muscles of the rat: measurement of mRNA content using synthetic oligonucleotide probes. Circ Res. 1986 Aug;59(2):194–201. doi: 10.1161/01.res.59.2.194. [DOI] [PubMed] [Google Scholar]
- Morkin E., Ashford T. P. Myocardial DNA synthesis in experimental cardiac hypertrophy. Am J Physiol. 1968 Dec;215(6):1409–1413. doi: 10.1152/ajplegacy.1968.215.6.1409. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Flanders K. C., Smith J. M., Ellingsworth L. R., Roberts A. B., Sporn M. B. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989 Feb;108(2):661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]