Abstract
BACKGROUND
The outcome of kidney transplantation in human immunodeficiency virus (HIV)–positive patients who receive organs from HIV-negative donors has been reported to be similar to the outcome in HIV-negative recipients. We report the outcomes at 3 to 5 years in HIV-positive patients who received kidneys from HIV-positive deceased donors.
METHODS
We conducted a prospective, nonrandomized study of kidney transplantation in HIV-infected patients who had a CD4 T-cell count of 200 per cubic millimeter or higher and an undetectable plasma HIV RNA level. All the patients were receiving antiretroviral therapy (ART). The patients received kidneys from deceased donors who tested positive for HIV with the use of fourth-generation enzyme-linked immunosorbent assay at the time of referral. All the donors either had received no ART previously or had received only first-line ART.
RESULTS
From September 2008 through February 2014, a total of 27 HIV-positive patients underwent kidney transplantation. Survivors were followed for a median of 2.4 years. The rate of survival among the patients was 84% at 1 year, 84% at 3 years, and 74% at 5 years. The corresponding rates of graft survival were 93%, 84%, and 84%. (If a patient died with a functioning graft, the calculation was performed as if the graft had survived.) Rejection rates were 8% at 1 year and 22% at 3 years. HIV infection remained well controlled, with undetectable virus in blood after the transplantation.
CONCLUSIONS
Kidney transplantation from an HIV-positive donor appears to be an additional treatment option for HIV-infected patients requiring renal-replacement therapy.
South africa has one of the highest incidences of human immunodeficiency virus (HIV) infection in Africa. The rollout of antiretroviral therapy (ART) in South Africa has been tremendously successful in extending the lives of HIV-infected persons. Consequently, more patients who would have died before the availability of ART are now receiving a diagnosis of HIV-associated nephropathy.1
The rates of disease progression and death in the population of HIV-positive patients with chronic kidney disease can be modified by ART, which reduces the risk of advanced chronic kidney disease among patients with HIV-associated nephropathy by approximately 60%.2,3 It has been estimated that the prevalence of chronic kidney disease among HIV-infected patients receiving treatment is between 8% and 22%4–7; among untreated patients, it is estimated to be between 20% and 27%.8,9 Confronted with a high burden of HIV disease and limited resources, South Africa faces considerable challenges in providing renal-replacement therapy for the large numbers of HIV-infected persons in whom chronic kidney disease will develop during their lifetime.
State-funded renal-replacement programs in South Africa have limited resources. Previous reports showing that outcomes in HIV-infected recipients who have received a transplant are equivalent to those in other high-risk candidates argued strongly for HIV-infected patients to be considered as candidates for renal-replacement therapy.10,11 In 2008, in response to the clinical need of our patients and to the fact that HIV infection was considered to be a contraindication to acceptance for dialysis treatment or receipt of a kidney from an HIV-seronegative donor, we performed four renal transplantations in HIV-positive recipients using kidneys from HIV-infected donors. The preliminary results from that study showed 100% graft survival and patient survival at 1 year.12 We now present the 5-year follow-up for these patients and for additional recipients in the HIV-positive–to–HIV-positive renal transplantation program at Groote Schuur Hospital, Cape Town.
METHODS
STUDY DESIGN
Groote Schuur Hospital has an active transplantation program in which 50 to 70 kidneys per year are transplanted from living donors (30% of the donors) or deceased donors (70%). After the first four transplantations from HIV-positive donors to HIV-positive recipients in 2008, the human research ethics committee of the Faculty of Health Sciences at the University of Cape Town undertook a comprehensive review of the inclusion criteria, protocols, and procedures for HIV-positive renal-replacement therapy at Groote Schuur Hospital. In 2009, the exclusion of HIV-infected persons from the general renal-transplantation program was lifted, enabling HIV-infected patients to receive kidneys from HIV-negative donors or from HIV-positive deceased donors as part of the present study.
All HIV-positive patients with stage 5 chronic kidney disease who underwent transplantation with kidneys from HIV-positive donors at our hospital from September 2008 through February 2014 were included in this study; the protocol is available with the full text of this article at NEJM.org. The University of Cape Town human research ethics committee granted permission for this prospective, nonrandomized study, and all the participants provided written informed consent. The data were gathered and analyzed by the first author, who also designed the study. The first author wrote the initial draft of the manuscript, with contributions from all the authors. The first author vouches for the integrity of the data and the analyses reported and for the fidelity of the study to the protocol.
DONOR SELECTION
All HIV-infected deceased donors who were referred to the transplantation unit at Groote Schuur Hospital from September 2008 through February 2014 were included in the study. No living donors who were HIV positive were included. HIV infection was confirmed by means of a fourth-generation enzyme-linked immunosorbent assay (Abbott). Exclusion criteria were severe sepsis, active tuberculosis, World Health Organization stage 4 HIV disease (i.e., the acquired immunodeficiency syndrome [AIDS]), and abnormal renal function as assessed by the serum creatinine level, the presence of proteinuria on the urine dipstick, or a microalbumin-to-creatinine ratio of more than 300 μg of microalbumin per milligram of creatinine (3.4 mg of microalbumin per millimole of creatinine). Eligible donors either had not received ART or had been receiving first-line treatment and had an undetectable plasma HIV RNA viral load (<50 copies per milliliter). All donor kidneys were biopsied at the time of implantation.
RECIPIENTS
Recipients were eligible for inclusion in the study if they had been receiving ART for at least 3 months, had a CD4 T-cell count of 200 per cubic millimeter or higher, and had an undetectable plasma HIV RNA viral load. Patients with a history of any opportunistic infection that suggested a diagnosis of AIDS were excluded. Patients with a history of drug-sensitive tuberculosis were included only after completing a minimum of 6 months of treatment.
IMMUNOSUPPRESSION
The immunosuppression protocol included antibody induction therapy with rabbit antithymocyte globulin (either Thymoglobuline [Sanofi], at a dose of 1.5 mg per kilogram of body weight per day for 5 to 7 days, or ATG [Fresenius], at a dose of 2 mg per kilogram per day for 5 to 7 days). Maintenance immunosuppression therapy that was started on day 0 included prednisone at a dose of 30 mg per day, tapered to 5 mg per day over the first 3 months after transplantation, mycophenolate mofetil at a dose of 1 g every 12 hours, and tacrolimus, which was started at 0.2 mg per kilogram, with the dose adjusted to maintain trough levels between 6 and 8 ng per milliliter. All drugs were purchased at full cost.
ANTIRETROVIRAL THERAPY
Initially, patients who were receiving nonnucleoside reverse-transcriptase inhibitor (NNRTI)–based first-line ART were switched to a boosted protease inhibitor–based regimen at the time of transplantation to increase the likelihood that donor-virus replication would be suppressed by the recipient’s ART regimen and to lower the costs of immunosuppressive therapy by taking advantage of the inhibitory effect of ritonavir on calcineurin-inhibitor metabolism. After concerns were raised regarding calcineurin-inhibitor toxicity as a cause of graft dysfunction, recipients continued to receive their pretransplantation ART regimen.
PROPHYLAXIS FOR OPPORTUNISTIC INFECTIONS
Prophylaxis for opportunistic infections included lifelong therapy with trimethoprim–sulfamethoxazole, at a daily dose of 80 mg of trimethoprim and 400 mg of sulfamethoxazole, to prevent pneumocystis pneumonia, and isoniazid, at a dose of 300 mg once daily, to prevent Mycobacterium tuberculosis infection. Valganciclovir, at a dose of 900 mg daily (adjusted according to renal function), was administered for the first 3 months to prevent cytomegalovirus reactivation.
CLINICAL PROTOCOL
Demographic data and medical and ART history were recorded, and baseline plasma HIV RNA level (according to the Abbott RealTime assay) and CD4 T-cell count were measured. All the patients received induction therapy, and no adverse events occurred while the drug was being infused. After transplantation, recipients were followed weekly for the first month and monthly thereafter. Urea and creatinine levels were monitored monthly, and the CD4 T-cell count and plasma HIV RNA viral load were monitored every 6 months. Allograft biopsies were performed if there was clinical suspicion of acute rejection (an increased creatinine level, low urine output, or pain over the graft site); in addition, allograft biopsies were performed yearly as part of routine follow-up. Acute rejection was defined according to the Banff classification,13 and all rejection episodes required confirmation by means of biopsy. One histopathologist reviewed all the biopsies.
The final follow-up date for each patient was March 15, 2014, or the date of death, for the analysis of patient survival, or the date of resumption of dialysis treatment at any time after the first week after the transplantation, for the analysis of graft survival. Patient survival and graft survival were the primary outcomes.
STATISTICAL ANALYSIS
Estimates of patient survival, graft survival, and allograft rejection were calculated with the use of the Kaplan–Meier method. Associated 95% confidence intervals were calculated with the use of Greenwood’s formula. The analysis of graft survival followed individual kidney transplants until graft failure occurred. If a patient died with a functioning graft, the calculation was done as if the graft had survived.
RESULTS
DONORS
During the study period, a total of 23 HIV-infected potential donors (22 persons who were brain-dead and 1 after circulatory death) were referred to the transplantation unit at the Groote Schuur Hospital. A total of 6 potential donors were excluded because of opportunistic infection, presence of proteinuria, or tuberculosis. Two potential donors were excluded because they presented during the period in 2009 when program was stopped for review by the University of Cape Town research ethics committee.
The median age of the donors was 30 years (interquartile range, 23 to 36). A total of 13 donors had died as a result of trauma, 1 from an overdose, and 1 from a subarachnoid hemorrhage. Only 1 donor had been receiving ART (NNRTI-based first-line therapy); all the other donors in the study had not received ART previously.
RECIPIENTS
The baseline characteristics of the 27 recipients are shown in Table 1. Survivors were followed for a median of 2.4 years. The median age of the recipients was 41 years (interquartile range, 34 to 46), and nearly all were ethnically African. HIV-associated nephropathy was the predominant cause of chronic kidney disease. The median CD4 T-cell count at the time of transplantation was 288 cells per cubic millimeter (interquartile range, 236 to 511). The percentage of recipients with hepatitis B virus coinfection mirrored the seroprevalence of hepatitis B virus in the general population. A total of 16 patients were receiving NNRTI-based regimens, and 11 were receiving protease inhibitor–based ART.
Table 1.
Characteristics of HIV-Positive Kidney-Transplant Recipients at Baseline.*
| Characteristic | Patients (N = 27) |
|---|---|
| Age — yr | |
| Median | 41 |
| Interquartile range | 39–43 |
| Male sex — no. (%) | 15 (56) |
| Race — no (%)† | |
| Black | 26 (96) |
| Mixed race | 1 (4) |
| Cause of chronic kidney disease — no. (%) | |
| Hypertension | 1 (4) |
| Membranous glomerulonephritis | 1 (4) |
| Biopsy-confirmed HIV-associated nephropathy | 5 (19) |
| Presumed HIV-associated nephropathy | 20 (74) |
| CD4 T-cell count — cells/mm3 | |
| Median | 288 |
| Interquartile range | 236–511 |
| Positive for hepatitis B surface antigen — no. (%) | 3 (11) |
| ART regimen — no. (%) | |
| Protease inhibitor–based therapy | 11 (41) |
| NNRTI-based therapy | 16 (59) |
| Antibody induction therapy‡ | |
| ATG | |
| No. of patients (%) | 10 (37) |
| Cumulative dose — mg | |
| Median | 475 |
| Interquartile range | 400–750 |
| Thymoglobuline | |
| No. of patients (%) | 17 (63) |
| Cumulative dose — mg | |
| Median | 600 |
| Interquartile range | 450–1000 |
ART denotes antiretroviral therapy, HIV human immunodeficiency virus, and NNRTI nonnucleoside reverse-transcriptase inhibitor.
Race was self-reported.
Antibody induction therapy was performed with the use of rabbit antithymocyte globulin (ATG [Fresenius] or Thymoglobuline [Sanofi]).
PATIENT AND GRAFT SURVIVAL
Two of the 27 patients had delayed graft function and required dialysis during the first week after the transplantation: 1 patient had graft failure owing to venous thrombosis on day 1, and the second patient had acute severe antibody-mediated rejection, refractory to plasmapheresis, which necessitated removal of the graft after 2 weeks. The remaining 25 patients had well-functioning grafts at the end of the first year. The median serum creatinine level at 1 year was 111.5 μmol per liter (1.3 mg per deciliter; interquartile range, 102.5 to 117.0 μmol per liter [1.2 to 1.3 mg per deciliter]).
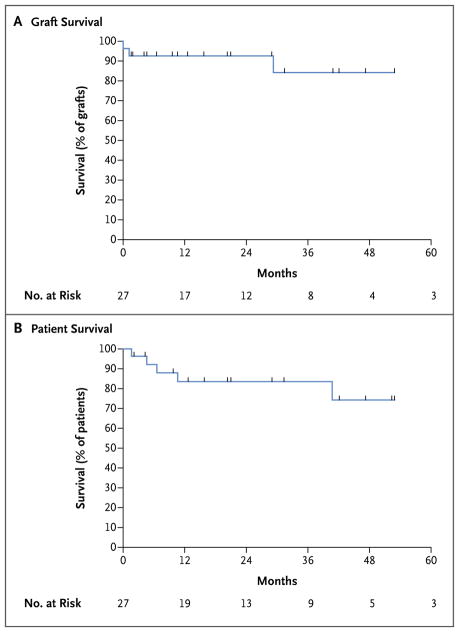
The cumulative rates of survival were 84% (95% confidence interval [CI], 62 to 94) at 1 year and 3 years and 74% (95% CI, 45 to 89) at 5 years. The rate of graft survival at 1 year was 93% (95% CI, 74 to 98), decreasing to 84% at 3 years and 5 years (95% CI, 55 to 95). Data regarding graft survival were censored at the time of a patient’s death (Fig. 1). The rate of survival among the HIV-negative patients in our unit was 91% (95% CI, 63 to 92) at 1 year and 85% (95% CI, 56 to 94) at 5 years. The rate of graft survival among HIV-negative patients was 88% (95% CI, 47 to 91) at 1 year and 75% (95% CI, 43 to 88) at 5 years.
Figure 1. Graft and Patient Survival among 27 Human Immunodeficiency Virus (HIV)–Positive Patients Who Received Kidney Transplants from HIV-Positive Donors.
Data on graft survival were censored (tick marks) at the time of the patient’s death. The analysis of graft survival followed individual kidney transplants until graft failure occurred. If a patient died with a functioning graft, the calculation was done as if the graft had survived.
Five patients died after transplantation, despite a functioning graft. One patient died of sepsis and acute pancreatitis within 1 month after transplantation. Escherichia coli and pseudomonas species were identified from the blood culture. A duodenal perforation was found at the postmortem examination. A second patient died of a myocardial infarction 6 months after transplantation. Two patients died of infection during the first year after transplantation. One patient had recurrent gram-negative septicemia due to recurrent urinary tract infections and died in the hospital after he was infected by a nosocomial pathogen (a carbapenem-resistant Klebsiella pneumoniae was identified on blood culture). The second patient had rapidly progressive, invasive pulmonary aspergillosis. Surgical resection was attempted, but the patient’s condition did not improve, and the patient died during the same admission. The fifth patient died of pulmonary squamous-cell carcinoma 5 years after transplantation.
ALLOGRAFT REJECTION
Eight episodes of biopsy-confirmed acute rejection occurred in five patients (Table 2). Rejection rates were 8% at 1 year and 22% at 3 years. Six of the eight episodes of acute rejection were successfully reversed either by treatment with glucocorticoids or rabbit antithymocyte globulin (ATG or Thymoglobuline) or by means of plasmapheresis. Two recipients had graft failure — the first at 1 week after transplantation owing to acute severe antibody-mediated rejection, and the second after 2 years, with the graft showing features of chronic scarring and fibrosis.
Table 2.
Rejection Episodes after Transplantation.
| Time from Transplantation to Rejection Episode | Treatment | Outcome | |
|---|---|---|---|
| Medication or Other Treatment | Total Dose | ||
| Patient 5 | |||
|
| |||
| 2 yr | Methylprednisolone | 2000 mg | Functioning graft |
|
| |||
| 3 yr 3 mo | ATG | 700 mg | Functioning graft |
|
| |||
| Patient 7 | |||
|
| |||
| 1 yr 7 mo | Methylprednisolone | 2000 mg | Functioning graft |
|
| |||
| 2 yr 3 mo | Methylprednisolone | 2000 mg | Graft failure |
|
| |||
| Patient 9 | |||
|
| |||
| 4 mo | Methylprednisolone | 2000 mg | Functioning graft |
|
| |||
| 2 yr 9 mo | Methylprednisolone | 2000 mg | Functioning graft |
|
| |||
| Patient 17 | |||
| 1 wk | Thymoglobuline and plasmapheresis | 800 mg | Graft failure |
| Patient 27 | |||
| 1 wk | Thymoglobuline and plasmapheresis | 500 mg | Functioning graft |
PHARMACOLOGIC INTERACTIONS
The type of ART regimen had a considerable effect on the tacrolimus dosing. The median tacrolimus dose required to maintain a trough level of 6.0 to 8.0 mg per milliliter was 0.5 mg every 7 to 10 days in patients receiving a protease inhibitor–based regimen, as compared with 8.5 mg (interquartile range, 7.0 to 11.0) every 12 hours in patients receiving an NNRTI-based regimen. The side effects of tacrolimus were more pronounced in patients receiving a protease inhibitor–based regimen, five of whom had evidence on renal biopsy of calcineurin-inhibitor toxicity.
PROGRESSION OF HIV DISEASE
CD4 T-Cell Counts
The median CD4 T-cell count decreased to 179 per cubic millimeter (interquartile range, 141 to 310) in the first year after transplantation, as would be expected with induction therapy. There was a slow increase in the CD4 T-cell count, with a median of 386 cells per cubic millimeter (inter-quartile range, 307 to 484) at 3 years in the nine patients who reached this stage.
Viral Load
All the recipients in the study were receiving suppressive ART before surgery, with a plasma viral load of less than 50 copies per milliliter. The HIV viral load remained suppressed during the follow-up period in all the recipients.
Histologic Findings
In three patients, routine renal biopsies revealed changes typical of early HIV-associated nephropathy that had not been present in the baseline biopsy specimens. Prominent epithelial cells and protein reabsorption granules within the podocytes, as well as central mesangial sclerosis and increased cells in the mesangium, were noted. None of these patients had clinically significant renal impairment or clinically significant proteinuria, and none required dialysis.
Infection and Hospitalization
Episodes of infection that responded to treatment but required hospitalization included six episodes of urinary tract infection in three patients (Patients 3, 12, and 16), osteomyelitis (Patient 1), respiratory infection (Patient 3), gastroenteritis (Patients 3, 16, 23, and 25), meningitis (Patient 3), and extrapulmonary tuberculosis (Patient 10). Three patients had infectious complications that led to death — pancreatitis (E. coli and pseudomonas species identified on blood culture in Patient 22), urinary tract infection complicated by carbapenem-resistant K. pneumoniae sepsis (Patient 24), and rapidly progressive, invasive pulmonary aspergillosis (Patient 19).
DISCUSSION
This study showed the medium-term follow-up of renal transplantation from an HIV-positive donor to an HIV-positive recipient. The cumulative rates of patient survival, 84% at 1 year and 74% at 5 years, and the cumulative rates of graft survival, 93% at 1 year and 84% at 5 years, compare favorably with reported outcomes in HIV-positive patients who received kidneys from HIV-negative donors.14
The proposal to perform transplantations from an HIV-positive donor to an HIV-positive recipient raised several ethical issues, the first being the validity of the informed consent. When the program commenced in 2008, these patients did not have an alternative option, because they had been denied access to dialysis. Therefore, the choice was between an experimental, unproven procedure and death. Even though HIV-positive patients became eligible for dialysis after the first year of the study, dialysis is still a very limited resource, and many patients with stage 5 chronic kidney disease, regardless of HIV status, do not qualify.
Another major concern was the risk of transmission of a new and possibly resistant strain of HIV from donor to recipient. Previous reports on the outcomes in HIV-positive patients with superinfection transmitted through sexual intercourse have yielded conflicting results, owing in part to methodologic difficulties.15,16 The patients in our study did not have any increase in plasma viral load, and viral levels remained undetectable after transplantation. Detailed viral sequencing in this group of patients is being undertaken currently. The appearance in the postoperative biopsy specimens of early changes related to HIV-associated nephropathy that were not present in the baseline biopsy specimens was of some concern. Findings from a recent study showed the presence of HIV type 1 in podocytes and tubular cells after transplantation of a kidney from an HIV-negative donor into an HIV-positive recipient.17 Infection in podocytes was associated with a faster decline in graft function than infection in tubular cells, possibly related to poorer glomerular filtration rates.
In South Africa, transmitted resistance to first-line antiretroviral drugs is low (approximately 5% of HIV-infected persons).18–22 Most patients who do not have a response to second-line ART do not have a response because of nonadherence to therapy, and 67% have wild-type virus on genotyping.23,24 All the donors in this study had not received ART previously or had received first-line treatment with no known virologic evidence that would suggest resistance, although genotyping was not performed.
The interaction between antiretroviral drugs and immunosuppressants introduced several challenges. Ritonavir, an inhibitor of the cytochrome P-450 enzyme system, decreases the metabolism of tacrolimus by competing with it for the cytochrome P-450 enzyme–binding site, resulting in an increase by a factor of 5 in the area under the curve and a decrease of more than 80% in the clearance of tacrolimus.25,26 The patients receiving this drug combination required minimal doses of tacrolimus every 7 to 10 days to maintain adequate levels. In contrast, the NNRTIs, efavirenz and nevirapine, induce the cytochrome P-450 enzyme system, which increases the metabolism of tacrolimus, necessitating much higher doses (median, 8.5 mg twice daily) to maintain adequate levels.
Both types of therapies have cost implications because there is a substantial cost saving with the former. The patients who received the inhibitors of the cytochrome P-450 enzyme had a higher incidence of calcineurin-inhibitor nephrotoxic effects, as observed on biopsy, than did the patients who received NNRTIs. Therefore, a decision was made after the first 2 years of the study not to switch patients routinely to protease inhibitors after transplantation if the patients were to be switched only in the interest of cost.
Rejection rates among HIV-positive recipients have been reported to be approximately 3 times as high as those among HIV-negative recipients.14 The reason for this is still unknown, but two hypotheses have been proposed. The first is immune dysregulation, and the second is the challenge of managing the drug interactions between the antiretroviral agents and immunosuppressants.27 Owing to the very high risk of rejection, all the patients in this study received induction therapy with anti–T-cell antibody and maintenance immunosuppression with tacrolimus, mycophenolate mofetil, and glucocorticoids. Despite this potent immunosuppressive regimen, five patients (19%) had an acute rejection episode.
All the patients had CD4 T-cell counts of more than 200 per cubic millimeter at the time of transplantation. The marked decrease in CD4 T-cell levels during the first year after transplantation was probably related to induction therapy with anti–T-cell antibody; this hypothesis is supported by the fact that all the patients had an increase in the CD4 T-cell count after the first year. Opportunistic infection remains a major cause of complications and death in patients who have received a transplant.28 Surprisingly few opportunistic infections were documented during the first year of follow-up, when CD4 T-cell counts were at their lowest. We do not report here on the outcome of two different HIV strains in the recipient, because viral sequencing was not available from the start of the study.
In conclusion, our study showed that kidneys from HIV-positive deceased donors can be transplanted into carefully selected HIV-positive recipients, with the expectation that the outcome would be similar to that observed in kidney-transplantation programs involving high-risk patients without HIV infection.
Acknowledgments
Supported by a research grant from Sanofi South Africa and by the Roche Organ Transplantation Research Foundation.
We thank the members of the Groote Schuur Hospital transplantation team, including the coordinators and the medical and nursing colleagues who were involved with patient care.
Footnotes
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Laurinavicius A, Hurwitz S, Rennke HG. Collapsing glomerulopathy in HIV and non-HIV patients: a clinicopathological and follow-up study. Kidney Int. 1999;56:2203–13. doi: 10.1046/j.1523-1755.1999.00769.x. [DOI] [PubMed] [Google Scholar]
- 2.Okpechi I, Swanepoel C, Duffield M, et al. Patterns of renal disease in Cape Town South Africa: a 10-year review of a single-centre renal biopsy database. Nephrol Dial Transplant. 2011;26:1853–61. doi: 10.1093/ndt/gfq655. [DOI] [PubMed] [Google Scholar]
- 3.Wearne N, Swanepoel CR, Boulle A, Duffield MS, Rayner BL. The spectrum of renal histologies seen in HIV with outcomes, prognostic indicators and clinical correlations. Nephrol Dial Transplant. 2012;27:4109–18. doi: 10.1093/ndt/gfr702. [DOI] [PubMed] [Google Scholar]
- 4.Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis. 2008;197:1548–57. doi: 10.1086/587994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winston J, Deray G, Hawkins T, Szczech L, Wyatt C, Young B. Kidney disease in patients with HIV infection and AIDS. Clin Infect Dis. 2008;47:1449–57. doi: 10.1086/593099. [DOI] [PubMed] [Google Scholar]
- 6.Wyatt CM, Winston J. Renal disease in patients with HIV. Curr Infect Dis Rep. 2006;8:76–81. doi: 10.1007/s11908-006-0038-0. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt CM, Winston JA, Malvestutto CD, et al. Chronic kidney disease in HIV infection: an urban epidemic. AIDS. 2007;21:2101–3. doi: 10.1097/QAD.0b013e3282ef1bb4. [DOI] [PubMed] [Google Scholar]
- 8.Peters PJ, Moore DM, Mermin J, et al. Antiretroviral therapy improves renal function among HIV-infected Ugandans. Kidney Int. 2008;74:925–9. doi: 10.1038/ki.2008.305. [DOI] [PubMed] [Google Scholar]
- 9.Mulenga LB, Kruse G, Lakhi S, et al. Baseline renal insufficiency and risk of death among HIV-infected adults on anti-retroviral therapy in Lusaka, Zambia. AIDS. 2008;22:1821–7. doi: 10.1097/QAD.0b013e328307a051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roland ME, Stock PG. Review of solid-organ transplantation in HIV-infected patients. Transplantation. 2003;75:425–9. doi: 10.1097/01.TP.0000046943.35335.18. [DOI] [PubMed] [Google Scholar]
- 11.Stock PG, Roland ME. Evolving clinical strategies for transplantation in the HIV-positive recipient. Transplantation. 2007;84:563–71. doi: 10.1097/01.tp.0000279190.96029.77. [DOI] [PubMed] [Google Scholar]
- 12.Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362:2336–7. doi: 10.1056/NEJMc0900837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mengel M, Sis B, Haas M, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–70. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363:2004–14. doi: 10.1056/NEJMoa1001197. Erratum, N Engl J Med 2011;364:1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waters L, Smit E. HIV-1 superinfection. Curr Opin Infect Dis. 2012;25:42–50. doi: 10.1097/QCO.0b013e32834ef5af. [DOI] [PubMed] [Google Scholar]
- 16.Streeck H, Li B, Poon AF, et al. Immune-driven recombination and loss of control after HIV superinfection. J Exp Med. 2008;205:1789–96. doi: 10.1084/jem.20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canaud G, Dejucq-Rainsford N, Avettand-Fenoël V, et al. The kidney as a reservoir for HIV-1 after renal transplantation. J Am Soc Nephrol. 2014;25:407–19. doi: 10.1681/ASN.2013050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs GB, Laten A, van Rensburg EJ, et al. Phylogenetic diversity and low level antiretroviral resistance mutations in HIV type 1 treatment-naive patients from Cape Town, South Africa. AIDS Res Hum Retro-viruses. 2008;24:1009–12. doi: 10.1089/aid.2008.0028. [DOI] [PubMed] [Google Scholar]
- 19.Parboosing R, Naidoo A, Gordon M, Taylor M, Vella V. Resistance to antiretroviral drugs in newly diagnosed, young treatment-naive HIV-positive pregnant women in the province of KwaZulu-Natal, South Africa. J Med Virol. 2011;83:1508–13. doi: 10.1002/jmv.22143. [DOI] [PubMed] [Google Scholar]
- 20.Nwobegahay JM, Bessong PO, Masebe TM, Mavhandu LG, Iweriebor BC, Selabe G. Prevalence of antiretroviral drug resistance mutations and HIV-I subtypes among newly-diagnosed drug-naïve persons visiting a voluntary testing and counselling centre in northeastern South Africa. J Health Popul Nutr. 2011;29:303–9. doi: 10.3329/jhpn.v29i4.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nwobegahay J, Selabe G, Ndjeka NO, Manhaeve C, Bessong PO. Low prevalence of transmitted genetic drug resistance in a cohort of HIV infected naïve patients entering antiretroviral treatment programs at two sites in northern South Africa. J Med Virol. 2012;84:1839–43. doi: 10.1002/jmv.23348. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann CJ, Charalambous S, Sim J, et al. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis. 2009;49:1928–35. doi: 10.1086/648444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levison JH, Orrell C, Gallien S, et al. Virologic failure of protease inhibitor-based second-line antiretroviral therapy without resistance in a large HIV treatment program in South Africa. PLoS One. 2012;7(3):e32144. doi: 10.1371/journal.pone.0032144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levison JH, Wood R, Scott CA, et al. The clinical and economic impact of genotype testing at first-line antiretroviral therapy failure for HIV-infected patients in South Africa. Clin Infect Dis. 2013;56:587–97. doi: 10.1093/cid/cis887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S, Yung Chan S, Cher Goh B, et al. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet. 2005;44:279–304. doi: 10.2165/00003088-200544030-00005. [DOI] [PubMed] [Google Scholar]
- 26.Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008;9:310–22. doi: 10.2174/138920008784220664. [DOI] [PubMed] [Google Scholar]
- 27.Iordanskiy S, Santos S, Bukrinsky M. Nature, nurture and HIV: the effect of producer cell on viral physiology. Virology. 2013;443:208–13. doi: 10.1016/j.virol.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenner L, Reid SE, Fox MP, et al. Tuberculosis and the risk of opportunistic infections and cancers in HIV-infected patients starting ART in Southern Africa. Trop Med Int Health. 2013;18:194–8. doi: 10.1111/tmi.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]