Abstract
In this work, two complexes of ruthenium(III) ([Ru(phen)2Cl2]Cl·2H2O and [Ru(phen)2(G)Cl]2Cl·H2O) were synthesized from 1,10-phenanthroline alone as well as from both 1,10-phenanthroline and guanide. The synthesis was checked using halide test, conductance measurement, and spectroscopic (ICP-OES, FTIR, and UV/Vis) analysis. Their in vitro antibacterial activities were also investigated on two Gram-positive (Staphylococcus aureus (S. aureus) and methicillin resistant Staphylococcus aureus (MRSA)) and two Gram-negative (Escherichia coli (E. coli) and Klebsiella pneumoniae (K. pneumoniae)) bacteria. These complexes showed wide-range better activities than the commercially available controls (Chloramphenicol and Ciprofloxacin) against even the most drug resistant K. pneumoniae. [Ru(phen)2(G)Cl]2Cl·H2O inhibited S. aureus, MRSA, E. coli, and K. pneumoniae by 17.5%, 27.4%, 16%, and 52%, respectively, better than Chloramphenicol. It also inhibited these pathogens by 5.9%, 5.1%, 2.3%, and 17.2%, respectively, better than Ciprofloxacin. Similarly, [Ru(Phen)2(Cl)2]Cl·2H2O inhibited these pathogens by 11%, 8.7%, 0.1%, and 31.2%, respectively, better than Chloramphenicol. Therefore, after in vivo cytotoxicity investigations, these compounds can be considered as potential antibiotic drugs.
1. Introduction
Coordination chemistry is about tuning properties of metal ions using different ligands [1–3]. This includes stabilization of different oxidation states and modulation of the solvophilicity and electrophilic and nucleophilic properties of the metal ion [4–6]. While coordinating, the properties of the ligands themselves are also modified. For instance, the pharmacological activities and their crucial role in DNA/RNA base pairing through several hydrogen-bonding patterns of free oxypurines such as guanine can significantly change after complex formation [7, 8]. Based on this, synthesis of different coordination compounds with desired properties by ligand tailoring has become a fascinating research field. Designing new coordination compounds with therapeutic abilities has been part of this activity [9–17]. From this perspective, there has been a growing interest in the chemistry community to examine the biological activities of ruthenium complexes [18, 19]. Ruthenium (5s 24d 6) frequently accesses +2 and +3 oxidation states at physiological conditions and can interact with nucleic acids, proteins, sulfur, or oxygen containing compounds and water in the cells [20–24]. Its interaction kinetics with the latter can be controlled using advantages of the unique properties of each kind of ligand. This enables the ligand exchange rates of ruthenium complexes to be close to those of cellular processes which make them suitable for therapeutic applications.
In this respect, numerous investigations in the properties and applications of ruthenium(II) complexes with 1,10-phenanthroline (phen) as a ligand or mixed with other ligands are reported [25–27]. Nevertheless, there is no report on the chemistry of ruthenium(III) complex containing 1,10-phenanthroline alone or 1,10-phenanthroline and guanide mixed.
The ideally placed nitrogen atoms along with their rigid planar structure and hydrophobic, electron-poor heteroaromatic, and π-acidic properties cooperatively made 1,10-phenanthroline a classic chelating bidentate ligand. These properties enable it to have stacking interaction ability with DNA base pairs [28–31]. Guanine is a chemically inert oxypurine heteroaromatic molecule. Its inertness is changed by complex formation. The latter is favored in its guanide (G−) form which is derived by deprotonation of guanine.
The purpose of this study is to examine the effects of 1,10-phenanthroline alone and mixed with guanide on the biological activity of Ru(III). The complex would orchestrate the binding ability of ruthenium with a range of biomolecules, the unique stacking interaction ability of 1,10-phenanthroline on cell genetic material, and the interaction of guanide through hydrogen bonding with cytosine residue of the genetic material.
2. Experimental
2.1. Chemicals
All chemicals used in the present work are as follows: 1,10-phenanthroline monohydrate (BDH Chemical Ltd., Poole, England), guanine (99%, ACROS), RuCl3, silver nitrate, sodium hydroxide, acetone, chloroform, sulfuric acid (Sigma Aldrich), methanol (Hi Media Laboratories Ltd., India), KBr, dichloromethane, Mueller Hinton agar, and barium chloride (BLULUX Laboratories Ltd., India), and nitric acid (T.V. Industrial Estate, India).
2.2. Instruments and Methods
The electronic conductance was measured using 10−3 M solution of each complex in deionized water with JENWAY 4200 conductivity meter at room temperature. The electronic spectra were recorded in the 200–800 nm region on Sanyo SP65 UV/Vis spectrophotometer. IR spectra were recorded using KBr discs in the 4000–400 cm−1 region on AVATAR 330 FTIR, Thermo Nicolet spectrophotometer. Ruthenium content was determined by PerkinElmer, Optima 7300 V HF Version ICP-OES spectrometer, digesting 10 mg of each complex in concentrated nitric acid and diluting them using distilled water. Melting points were determined using STONE, STAFFORDSHIRE, ST15 OSA, UK, digital melting point apparatus. Chloride ions were determined thermogravimetrically using the AgCl precipitate obtained from the mixture of 10 mL solution of 1 mg of each complex in distilled water with excess AgNO3 solution.
2.3. Synthesis
2.3.1. Synthesis of [Ru(Phen)2(Cl)2]Cl·2H2O
Ethanolic solution of 1,10-phenanthroline (1 g, 5 mmol) was added from a dropping funnel to an ethanolic solution of RuCl3 (0.5 g, 2.5 mmol) being stirred magnetically in an ice bath. The mixture was allowed to stir for 3 h at room temperature. Reddish-brown homogeneous solution was obtained. The solvent was removed in vacuum. Reddish-brown powder was collected and washed three times with acetone to remove any unreacted 1,10-phenanthroline. It was recrystallized from ethanol to remove any unreacted RuCl3 (yield: 1.2 g, 80%).
2.3.2. Synthesis of [Ru(Phen)2(G)(Cl)]Cl·H2O
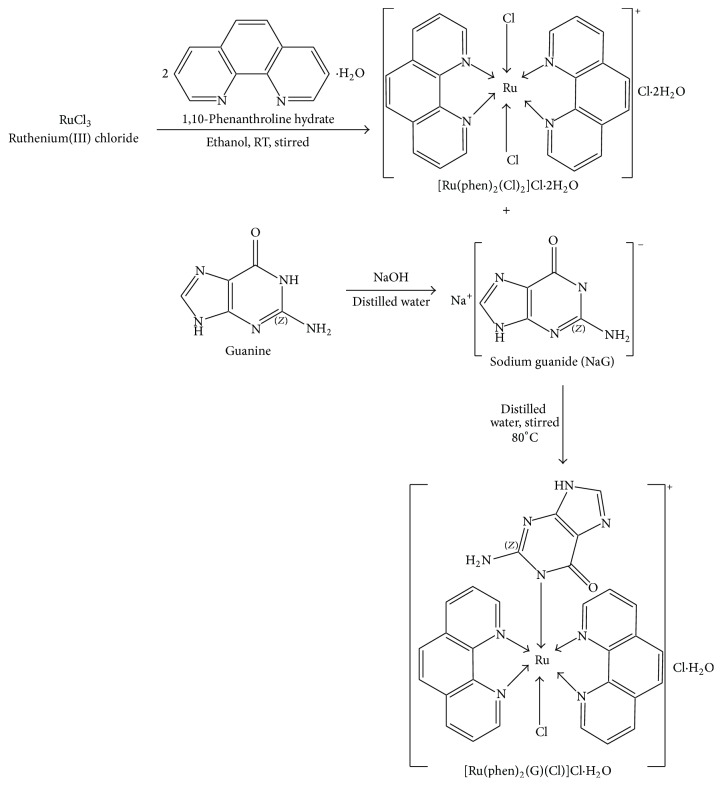
An aqueous solution of sodium guanide obtained from a reaction between guanine (0.125 g, 8.0 mmol) and sodium hydroxide (0.032 g, 8.0 mmol) was added from a dropping funnel to an aqueous solution of [Ru(Phen)2(Cl)2]Cl·2H2O (0.5 g, 8.0 mmol) while stirring in an oil bath at 80°C. The mixture was allowed to stir for 3 h. The resulting orange-red homogeneous solution was mixed with 100 mL dichloromethane and stirred for 1 h and allowed to stand overnight. The organic (dichloromethane) phase was separated using separatory funnel. The dichloromethane was removed in vacuum and dark brown powder was collected and recrystallized from ethanol (yield: 0.546 g, 87%). The reaction paths of the two synthetic complexes are shown in Scheme 1.
Scheme 1.
Synthesis of [Ru(phen)2(Cl)2]Cl·2H2O and [Ru(Phen)2(G)(Cl)]Cl·2H2O.
2.4. Antibacterial Activity Testing
The ligands and their metal complexes were evaluated for in vitro antibacterial activities against strains of two Gram-positive (S. aureus (ATCC 25923) and methicillin resistant S. aureus (clinical isolate)) and two Gram-negative (E. coli (ATCC255922) and K. pneumoniae (ATCC986605)) bacteria. The bacterial strains were maintained in the appropriate blood agar base at 4°C. Antibiotic discs (Ciprofloxacin 5 μg and Chloramphenicol 30 μg) were used as reference. The minimum inhibitory concentration (MIC) against each bacterium was determined by preparing aqueous solutions of different concentrations of the complexes by serial dilution (200 μg/mL, 300 μg/mL, 400 μg/mL, 500 μg/mL, 600 μg/mL, 800 μg/mL, and 1000 μg/mL). The experiments were repeated three times to obtain consistent results. The antibacterial tests were carried out at the Amhara Regional Health Research Microbiology Laboratory Center, Bahir Dar, Ethiopia.
3. Results and Discussion
The analytical data of the complexes are indicated in Table 1.
Table 1.
Analytical data of the complexes.
| Complex (color) | Melting point/°C | Yield (%) | Elemental estimation Calculated (found) (%) |
Molar conductivity ΛM (S cm2 mol−1) |
|
|---|---|---|---|---|---|
| Ru | Cl | ||||
| [Ru(phen)2(Cl)2]Cl·2H2O (reddish brown) | >300 | 80 | 16.74 (16.62) | 5.88 (5.66) | 121.50 |
| [Ru(phen)2(G)(Cl)]Cl·H2O (orange red) | >300 | 87 | 14.47 (14.23) | 5.07 (4.89) | 87.26 |
3.1. Molar Conductance of the Metal Complexes
The conductance measurements, recorded for 10−3 M solutions of the metal complexes in deionized water, are listed in Table 1. The data shows that both complexes are 1 : 1 electrolytes [32]. The lower conductance of [Ru(phen)2(G)(Cl)]Cl·H2O compared to [Ru(phen)2Cl2]Cl·2H2O is a consequence of increase in molar mass and the surface area. Hence the speed of mobility of the cation decreases as a result of the decrease in the kinetic energy imparted by the electric field from measurement instrument [33].
3.2. Electronic Spectra
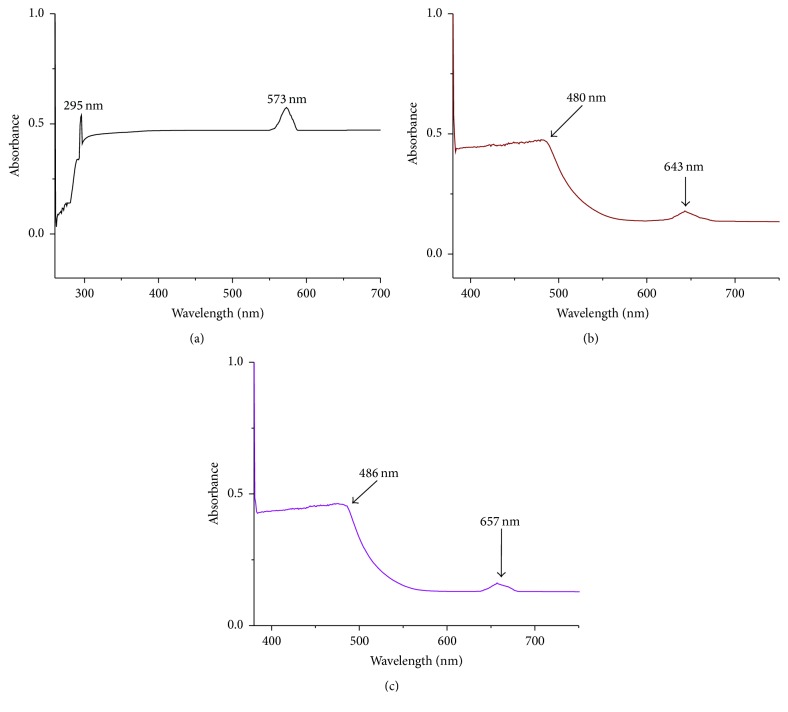
The electronic spectra of the complexes are displayed in Figure 1 and Table 2.
Figure 1.
Electronic spectra of (a) RuCl3, (b) [Ru(phen)2Cl2]Cl·2H2O, and (c) [Ru(phen)2(G)Cl]Cl·H2O.
Table 2.
Electronic spectral data of the salt and complexes.
| Complex | Band position (nm) | Assignment |
|---|---|---|
| RuCl3 | 295, 573 | ∗LMCT, d-d(2T2g → 2T1g) |
| [Ru(phen)2(Cl)2]Cl·2H2O | 643 | d-d(2T2g → 2T1g) |
| [Ru(phen)2(G)(Cl)]Cl·H2O | 657 | d-d(2T2g → 2T1g) |
∗LMCT: ligand to metal charge transfer.
The complexes exhibited simple characteristic d-d transitions. The difference in the band position for d-d transition absorption of RuCl3 and the complexes may be explained by assuming different environments around the metal ion following the coordination [34, 35]. The coordination of 1,10-phenanthroline to Ru(III) results in a distorted octahedral geometry. Consequently, the e g orbitals split to dz2 and dx2-y2 resulting in two transitions (t2g → e g(dz2) and t2g → e g(dx2-y2)). [Ru(Phen)2(G)(Cl)]Cl·H2O demonstrated absorption at longer wavelength (Figure 1(c)) than [Ru(Phen)2(Cl)2]Cl·2H2O (Figure 1(b)). This is probably because guanide (G−) formed a shorter and stronger bond that narrowed the transition (t2g → e g) gap (Figure 1 and Table 2).
3.3. IR Spectroscopy
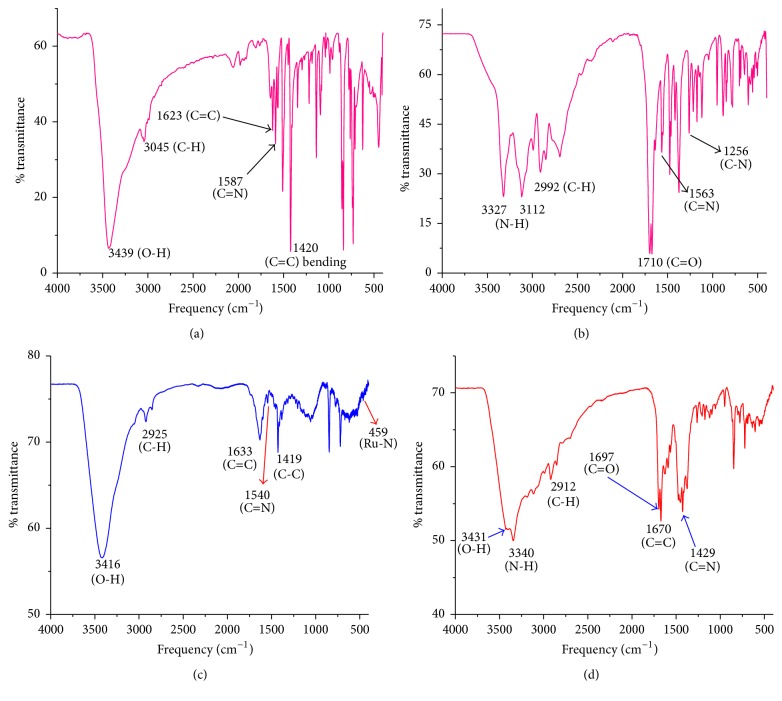
The infrared spectra of the ligands and the complexes are indicated in Figure 2 and selected characteristic frequencies are indicated in Table 3.
Figure 2.
FTIR spectra of (a) 1,10-phenanthroline monohydrate, (b) guanine, (c) [Ru(phen)2(Cl)2]Cl·2H2O, and (d) [Ru(phen)2(G)(Cl)]Cl·H2O.
Table 3.
Important characteristic IR bands of the ligands and complexes, cm−1.
| Compound | Absorption frequencies, cm−1 | ||||||
|---|---|---|---|---|---|---|---|
| ν(O-H) | ν(N-H) | ν(C-H) | ν(C-N) | ν(C=C) | ν(C=N) | ν(C=O) | |
| 1,10-Phenanthroline monohydrate | 3439 (s, b) | — | 3045 (w) | 1290 (w) | 1623 (s) | 1587 (s) | — |
| Guanine | — | 3335, 3112 (d) | 2992 | 1256 (w) | 1692 | 1563 (w) | 1710 (s) |
| [Ru(Phen)2(Cl)2]Cl·2H2O | 3416 (s, b) | — | 2925 (w) | 1208 (w) | 1633 (w) | 1540 (w) | — |
| [Ru(Phen)2(G)(Cl)]Cl·H2O | 3431 | 3340 (s) | 2912 (w) | 1270 | 1670 | 1429 | 1697 |
s: strong, b: broad, w: weak, and d: doublet.
The bands at 1623 cm−1 (s) and 1587 cm−1 (s), characteristic for ν C=C and ν C=N stretching in the free 1,10-phenanthroline monohydrate (Figure 2(a)), appear at 1670 cm−1 (w) and 1429 cm−1 (w), respectively, in [Ru(phen)2(G)(Cl)]Cl·H2O (Figure 2(d)). They also appeared at 1633 cm−1 (w) and 1540 cm−1 (w), respectively, in [Ru(phen)2(Cl)2]Cl·2H2O (Figure 2(c)). Similarly, the characteristic bands of guanide at 3335 cm−1 (s), 3112 cm−1 (s) ν N-H(NH2), and 1710 cm−1 (s) (ν C=O) (Figure 2(b)) are displaced towards 3340 cm−1 (w) and 1697 cm−1 (w) (Figure 2(d)), respectively. The changes in absorption frequencies and strength suggest that 1,10-phenanthroline and guanide are coordinated. The strong and broad bands at 3439 cm−1 and 3416 cm−1 characteristic for ν O-H(H2O) in the free 1,10-phenanthroline monohydrate and [Ru(phen)2(Cl)2]Cl·2H2O, respectively (Figures 2(a) and 2(b)), appear at 3431 cm−1 obscured in the band characteristic for ν N-H(NH2) in [Ru(phen)2(G)(Cl)]Cl·H2O (Figure 2(d)). This change in the absorption frequency of water explains the change in the nature of its interaction. Moreover, the change in the intensity may explain the change in the relative amount of water molecules in 1,10-phenanthroline monohydrate and the complexes. The latter argument supports the proposed formula of the complexes.
3.4. Antibacterial Activity Testing
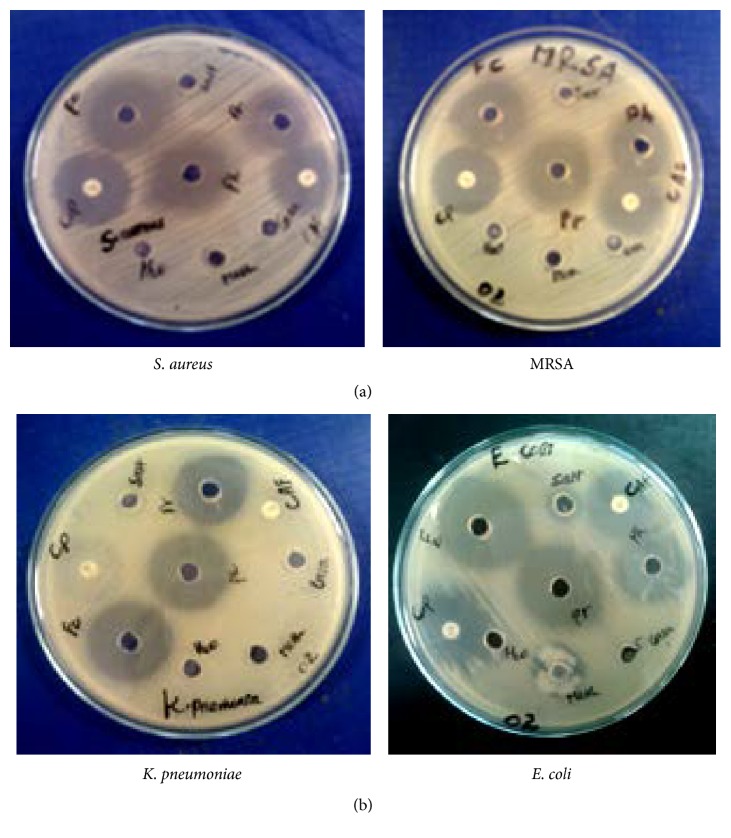
This observation shows that the complexes demonstrated biological activities against all the tested strains (Figure 3 and Table 4). The observed increase in antibacterial activity can be explained on the basis of Overtone's concept [36] and Tweedy's chelation theory [37]. The lipid membrane that surrounds the cell favors the passage of only lipid soluble materials which is an important condition for antimicrobial activity. On coordination, the polarity of the metal ion will be reduced to a greater extent due to the overlap of the ligand orbitals and partial sharing of the positive charge of the metal ion with the donor groups. Further, it increases the delocalization of π electrons over the whole chelate ring and hence enhances the liposolubility of the complexes. This increased liposolubility enhances the penetration of the complexes into the lipid membrane and interferes in the normal activities of the bacteria [38].
Figure 3.
Inhibition zone of ligands, complexes, salt, and commercial antibiotics against (a) Gram-positive (S. aureus and MARSA) and (b) Gram-negative (K. pneumoniae and E. coli) bacteria.
Table 4.
Antibacterial activities of the complexes, free ligands, metal salt, and commercially available antibiotics.
| Compound | Inhibition zone (mm) | |||
|---|---|---|---|---|
| S. aureus (ATCC 25923) | MRSA (clinical isolate) |
E. coli
(ATC 255922) |
K. pneumonia (ATCC 986605) | |
| 1,10-Phenanthroline | 27.40 ± 0.12 | 26.4 ± 0.11 | 28.20 ± 0.12 | 26.00 ± 0.14 |
| Guanine | — | — | — | — |
| RuCl3 | — | — | 12.20 ± 0.21 | — |
| [Ru(Phen)2(Cl)2]Cl·2H2O | 25.54 ± 0.15 | 24.56 ± 0.2 | 26.53 ± 0.13 | 26.50 ± 0.13 |
| [Ru(Phen)2(G)(Cl)]Cl·H2O | 29.65 ± 0.13 | 28.8 ± 0.21 | 30.70 ± 0.11 | 30.70 ± 0.11 |
| Ciprofloxacin | 28.00 ± 0.14 | 27.4 ± 0.13 | 30.00 ± 0.10 | 26.20 ± 0.32 |
| Chloramphenicol | 25.24 ± 0.14 | 22.6 ± 0.15 | 26.50 ± 0.23 | 20.20 ± 0.35 |
The percent activity indexes of the complexes against the reference antibiotics demonstrated significant comparative inhibitions (Table 5). [Ru(phen)2(G)(Cl)]Cl·H2O showed better activity than the two commercial antibiotics (Ciprofloxacin and Chloramphenicol) against all the strains studied including the most drug resistant Gram-negative K. pneumoniae. [Ru(phen)2(Cl)2]Cl·2H2O also showed nearly equal activities as Chloramphenicol against S. aureus and E. coli and better activities against MARSA and K. pneumoniae (Table 5). The better activities demonstrated by [Ru(phen)2(G)(Cl)]Cl·H2O compared to [Ru(phen)2(Cl)2]Cl·2H2O are due to its additional interaction with the genetic material of the cell by guanide.
Table 5.
The % activity index data of the complexes against the tested bacteria compared to (a) Chloramphenicol and (b) Ciprofloxacin.
(a).
| Microorganism | ||||
|---|---|---|---|---|
| Compound | S. aureus | MRSA | E. coli | K. pneumoniae |
| [Ru(Phen)2(Cl)2]Cl·H2O | 11.00% | 8.70% | 0.10% | 31.20% |
| [Ru(Phen)2(G)(Cl)]Cl·H2O | 17.50% | 27.40% | 15.85% | 52.00% |
(b).
| Microorganism | ||||
|---|---|---|---|---|
| Compound | S. aureus | MRSA | E. coli | K. pneumoniae |
| [Ru(Phen)2(Cl)2]Cl·2H2O | −8.80% | −10.00% | −12.00% | 1.01% |
| [Ru(Phen)2(G)(Cl)]Cl·H2O | 5.90% | 5.10% | 2.30% | 17.17% |
MRSA: methicillin resistant S. aureus.
3.5. Minimum Inhibitory Concentration (MIC) Determination
MIC is the lowest concentration that completely inhibited the growth of microorganisms for 24 hours.
Around 300 μg/mL [Ru(Phen)2(G)(Cl)]Cl·H2O is sufficient to inhibit the growth of E. coli while around 400 μg/mL is necessary to start inhibiting S. aureus and K. pneumonia (Table 6).
Table 6.
MIC assay of [Ru(Phen)2(G)(Cl)]Cl2·H2O against four bacterial pathogens.
| Microorganism | Minimum concentration of microorganism growth | ||||||
|---|---|---|---|---|---|---|---|
| 200 µg/mL | 300 µg/mL | 400 µg/mL | 500 µg/mL | 600 µg/mL | 800 µg/mL | 1000 µg/mL | |
| S. aureus | + | + | − | − | − | − | − |
| MRSA | + | + | + | + | − | − | − |
| K. pneumoniae | + | + | − | − | − | − | − |
| E. coli | + | − | − | − | − | − | − |
Note: +: bacteria growth and −: no bacteria growth.
4. Conclusions
In this synthesis, Ru(III) and the ligands are brought together with rigid configuration. This resulted in delocalization of π electrons over the whole cationic unit and hence the reduction of the polarity of the complexes which increased the liposolubility. This has enhanced the penetration of the complexes into the lipid membrane and inhibited the growth of the tested Gram-positive and Gram-negative bacteria. The latter phenomenon demonstrates the wide-range activities of the complexes.
Acknowledgments
The authors express sincere thanks to Bahir Dar University for financial support.
Competing Interests
There is no conflict of interests among the authors and the funding institution.
References
- 1.Lawrence G. A. Introduction to Coordination Chemistry. New South Wales, Australia: John Wiley & Sons, University of Newcastle; 2010. [Google Scholar]
- 2.Sears R. B., Joyce L. E., Turro C. Electronic tuning of ruthenium complexes by 8-quinolate ligands. Photochemistry and Photobiology. 2010;86(6):1230–1236. doi: 10.1111/j.1751-1097.2010.00814.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilkins R. G. The Study of Kinetics and Mechanism of Reactions of Transition Metal Complexes. Boston, Mass, USA: Allyn and Bacon; 1974. [Google Scholar]
- 4.Boyer J. L., Rochford J., Tsai M.-K., Muckerman J. T., Fujita E. Ruthenium complexes with non-innocent ligands: electron distribution and implications for catalysis. Coordination Chemistry Reviews. 2010;254(3-4):309–330. doi: 10.1016/j.ccr.2009.09.006. [DOI] [Google Scholar]
- 5.Goo Y. R., Maity A. C., Cho K.-B., et al. Tuning the reactivity of Chromium(III)-superoxo species by coordinating axial ligands. Inorganic Chemistry. 2015;54(21):10513–10520. doi: 10.1021/acs.inorgchem.5b02068. [DOI] [PubMed] [Google Scholar]
- 6.Tolman C. A. Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis. Chemical Reviews. 1977;77(3):313–348. doi: 10.1021/cr60307a002. [DOI] [Google Scholar]
- 7.Mohapatra B., Verma S. Crystal engineering with modified 2-aminopurine and group 12 metal ions. Crystal Growth & Design. 2013;13(7):2716–2721. doi: 10.1021/cg4006168. [DOI] [Google Scholar]
- 8.Patel D. K., Domínguez-Martín A., del Pilar Brandi-Blanco M., Choquesillo-Lazarte M. D., Nurchi V. M., Niclós-Gutiérrez J. Metal ion binding modes of hypoxanthine and xanthine versus the versatile behaviour of adenine. Coordination Chemistry Reviews. 2012;256(1-2):193–211. doi: 10.1016/j.ccr.2011.05.014. [DOI] [Google Scholar]
- 9.Motswainyana W. M., Ajibade P. A. Anticancer activities of mononuclear ruthenium(II) coordination complexes. Advances in Chemistry. 2015;2015:21. doi: 10.1155/2015/859730.859730 [DOI] [Google Scholar]
- 10.Rafique S., Idrees M., Nasim A., Akbar H., Athar A. Transition metal complexes as potential therapeutic agent. Biotechnology and Molecular Biology Reviews. 2010;5(2):38–45. [Google Scholar]
- 11.Bruijnincx P. C. A., Sadler P. J. Controlling platinum, ruthenium, and osmium reactivity for anticancer drug design. Advances in Inorganic Chemistry. 2009;61:1–62. doi: 10.1016/S0898-8838(09)00201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruijnincx P. C., Sadler P. J. New trends for metal complexes with anticancer activity. Current Opinion in Chemical Biology. 2008;12(2):197–206. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suntharalingam K., Johnstone T. C., Bruno P. M., Lin W., Hemann M. T., Lippard S. J. Bidentate ligands on osmium(VI) nitrido complexes control intracellular targeting and cell death pathways. Journal of the American Chemical Society. 2013;135(38):14060–14063. doi: 10.1021/ja4075375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carreira M., Calvo-Sanjuán R., Sanaú M., Marzo I., Contel M. Organometallic palladium complexes with a water-soluble iminophosphorane ligand as potential anticancer agents. Organometallics. 2012;31(16):5772–5781. doi: 10.1021/om3006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra A., Jung H., Park J. W., et al. Anticancer activity of self-assembled molecular rectangles via arene-ruthenium acceptors and a new unsymmetrical amide ligand. Organometallics. 2012;31(9):3519–3526. doi: 10.1021/om2012826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glans L., Ehnbom A., de Kock C., et al. Ruthenium (II) arene complexes with chelating chloroquine analogue ligands: synthesis, characterization and in vitro antimalarial activity. Dalton Transactions. 2012;41(9):2764–2773. doi: 10.1039/c2dt12083f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vela L., Contel M., Palomera L., Azaceta G., Marzo I. Iminophosphorane-organogold(III) complexes induce cell death through mitochondrial ROS production. Journal of Inorganic Biochemistry. 2011;105(10):1306–1313. doi: 10.1016/j.jinorgbio.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allardyce C. S., Dyson P. J. Metal-based drugs that break the rules. Dalton Transactions. 2016;45(8):3201–3209. doi: 10.1039/c5dt03919c. [DOI] [PubMed] [Google Scholar]
- 19.Kratz F., Messori L. Spectral characterization of ruthenium(III) transferrin. Journal of Inorganic Biochemistry. 1993;49(2):79–82. doi: 10.1016/0162-0134(93)85017-3. [DOI] [PubMed] [Google Scholar]
- 20.van Rijt S. H., Sadler P. J. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discovery Today. 2009;14(23-24):1089–1097. doi: 10.1016/j.drudis.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabrowiak J. C. R. Metals in Medicine. New York, NY, USA: John Wiley & Sons; 2009. Titanium and gallium for cancer; pp. 149–189. [Google Scholar]
- 22.Alessio E. Bioinorganic Medicinal Chemistry. New York, NY, USA: John Wiley & Sons; 2011. [Google Scholar]
- 23.Page S., Wheeler R. Ruthenium compounds as anticancer agents. Education in Chemistry, 2012, http://www.rsc.org/eic.
- 24.Reedijk J. Metal-ligand exchange kinetics in platinum and ruthenium complexes: significance for effectiveness as anticancer drugs. Platinum Metals Review. 2008;52(1):2–11. doi: 10.1595/147106708x255987. [DOI] [Google Scholar]
- 25.Adeloye A. O., Ajibade P. A. Synthesis and characterization of a Ru(II) complex with functionalized phenanthroline ligands having single-double linked anthracenyl and 1-methoxy-1-buten-3-yne moieties. Molecules. 2010;15(11):7570–7581. doi: 10.3390/molecules15117570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turel I., Golobič A., Kljun J., Samastur P., Batista U., Sepčić K. New synthetic routes for the preparation of ruthenium-1,10-phenanthroline complexes. Tests of cytotoxic and antibacterial activity of selected ruthenium complexes. Acta chimica Slovenica. 2015;62(2):337–345. doi: 10.17344/acsi.2014.1130. [DOI] [PubMed] [Google Scholar]
- 27.Bonneson P., Walsh J. L., Pennington W. T., Cordes A. W., Durham B. Six-coordinate complexes with 1,10-phenanthroline ligands in the trans configuration. Preparation of trans-bis(1,10-phenanthroline)ruthenium(II) complexes and crystal structure of trans-bis(1,10-phenanthroline)bis(pyridine)ruthenium(II) hexafluorophosphate. Inorganic Chemistry. 1983;22(12):1761–1765. doi: 10.1021/ic00154a013. [DOI] [Google Scholar]
- 28.Schaeffer F., Rimsky S., Spassky A. DNA-stacking interactions determine the sequence specificity of the deoxyribonuclease activity of 1,10-phenanthroline-copper ion. Journal of Molecular Biology. 1996;260(4):523–539. doi: 10.1006/jmbi.1996.0419. [DOI] [PubMed] [Google Scholar]
- 29.Lincoln P., Nordén B. DNA binding geometries of ruthenium(II) complexes with 1,10-phenanthroline and 2,2′-bipyridine ligands studied with linear dichroism spectroscopy. Borderline cases of intercalation. The Journal of Physical Chemistry B. 1998;102(47):9583–9594. doi: 10.1021/jp9824914. [DOI] [Google Scholar]
- 30.Coury J. E., Anderson J. R., McFail-Isom L., Williams L. D., Bottomley L. A. Scanning force microscopy of small ligand-nucleic acid complexes: tris(o-phenanthroline)ruthenium(II) as a test for a new assay. Journal of the American Chemical Society. 1997;119(16):3792–3796. doi: 10.1021/ja9623774. [DOI] [Google Scholar]
- 31.Pyle A. M., Rehmann J. P., Meshoyrer R., Kumar C. V., Turro N. J., Barton J. K. Mixed-ligand complexes of ruthenium(II): factors governing binding to DNA. Journal of the American Chemical Society. 1989;111(8):3051–3058. doi: 10.1021/ja00190a046. [DOI] [Google Scholar]
- 32.Bard A. J., Faulkner L. R., Leddy J., Zoski C. G. Electrochemical Methods: Fundamentals and Applications. New York, NY, USA: Wiley; 1980. [Google Scholar]
- 33.Atkins P. W. Physical Chemistry. 5th. Oxford, UK: Oxfored University Press; 1994. [Google Scholar]
- 34.Housecroft C. E., Sharpe A. G. Inorganic Chemistry. 2nd. England, UK: Pearson Education; 2005. [Google Scholar]
- 35.Missler G. L., Tarr D. A. Inorganic Chemistry. 3rd. Northfield, Minn, USA: Pearson Education, St. Olaf College; 2004. [Google Scholar]
- 36.Dharmaraj N., Viswanathamurthi P., Natarajan K. Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Transition Metal Chemistry. 2001;26(1-2):105–109. doi: 10.1023/A:1007132408648. [DOI] [Google Scholar]
- 37.Tweedy B. G. Plant extracts with metal ions as potential antimicrobial agents. Phytopathology. 1964;55:910–914. [Google Scholar]
- 38.Anantha Lakshmi P. V., Reddy P. S., Raju V. J. Synthesis, characterization and antimicrobial activity of 3d transition metal complexes of a biambidentate ligand containing quinoxaline moiety. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2009;74(1):52–57. doi: 10.1016/j.saa.2009.05.007. [DOI] [PubMed] [Google Scholar]