Abstract
The FLOQSwab™ is a specimen collection device worldwide recognised for its superior performance in the clinical diagnostics. The aim of this work was to evaluate FLOQSwab™ for the recovery of microbiological samples from surfaces compared to the traditional swab (rayon tipped swab) as per ISO 18593:2004 standard. The FLOQSwab™, thanks to its innovative manufacturing technology, allows improving the efficiency of recovery and release of analyte. The study has been divided into two experiments. In the first experiment the two swabs were evaluated for their capacity to recover and release the analyte (three different bacterial loads of Escherichia coli). In the second experiment, the two swabs were evaluated for their capacity to recover three different bacterial loads of E. coli from two different surface materials (stainless steel and polypropylene). In all experiments the flocked swab demonstrated a higher recovery rate compared to the traditional rayon tipped swab. The data obtained from this preliminary study demonstrated that the FLOQSwab™ could be a good food surfaces collection device, which improves the recovery of the analyte and thus produces accurate results. Based on the outcomes of the study, a larger field study is in progress using the FLOQSwab™ for samples collection to improve both environmental monitoring and the efficacy of the hygiene controls for food safety.
Key words: Swab, Environmental contamination, Sampling
Introduction
The Copan flocked swab (FLOQSwab™; Copan Italia S.p.A., Brescia, Italy) is an innovative pre-analytical specimen collection device that is compatible with all diagnostic tests, from culture to molecular amplifications assays. The flocked swab contains short nylon fibre strands attached to molded plastic, with a hydrophilic layer of nylon pile that results in efficient collection and release of particulate matter.
Flocked swabs are produced in a variety of sizes and shapes and are used to collect specimens from premature babies to adults and have become the pre-analytical device of choice for the investigation of infectious diseases.
In recent publications in the Journal of Clinical Microbiology, endocervical flocked swabs were reported to enhance the analytical sensitivity of antigen detection, culture and nucleic acid amplification assays (Chernesky et al., 2006). In another research study, the nasopharyngeal flocked swab design yielded significantly more total respiratory epithelial cells and more infected respiratory epithelial cells. This two- to three-fold increase in cell yield with the flocked swabs has a greater effect on diagnostic sensitivity compared to traditional fibre swabs (Daley et al., 2006).
Flocked swabs are currently used worldwide in Italy, Europe, Canada, US, South America, Australia, Japan, and China and have been validated with all the state of the art diagnostics and forensic investigations.
In food safety, microbiological work surface controls play an important role in the monitoring of contamination from the environment (Kusumaningrum et al., 2003; Verran et al., 2010). There is great interest and continued commitment of all the entities involved, food business operators, laboratories and companies producing laboratory principals, in order to guarantee greater security concerning the aspect of hygiene of work surfaces and its verification (Moore and Griffith, 2002; Ismail et al., 2013). In this context, the possibility of adopting a new type of collection device for the recovery of bacteria from food surfaces for environmental monitoring has been taken into account (Probst et al., 2010; Lahou and Uyttendaele, 2014). Clinical data and previous studies have demonstrated flocked swab superiority over traditional collection devices (Dalmaso et al., 2008) in terms of recovery and release. Based on these studies, it was decided to verify the applicability of flocked swabs in the food industry for the recovery of bacteria from surfaces for environmental monitoring and detection of specific pathogens.
Materials and Methods
In the Microbiology Laboratory of the Institute for Experimental Veterinary Medicine of Lombardy and Emilia Romagna a study to compare two different types of swabs – a FLOQSwab™ (Copan Italia S.pA) and a traditional rayon tipped swab (Copan Italia S.p.A.) – was conducted. The study was divided into two different experiments.
In the first experiment the two type of swabs were evaluated for their capacity to recover and release an analyte (microorganism) in vitro. Suspensions with three different loads equal to 105 colony forming unit (CFU)/mL 104 and 103 CFU/mL were prepared from overnight culture of Escherichia coli (reference strain ATCC 25922). For each load different tubes containing 100 µL of the suspension were prepared. The two different swabs were put inside the tubes containing the different bacterial load suspensions in order to absorb the inocula within 10 sec. Five replicates for both swabs were analysed for each load according to ISO 4833-2:2013 Cor 1:2014 (ISO, 2014) for total mesophilic count at 30°C. The gold standard for the three different loads was also verified placing directly the suspensions onto nutrient agar plates and incubating them at the appropriate conditions. After incubation for 72 h, plates were counted and results recorded.
In the second experiment (Figure 1), the two swabs were evaluated for their capacity to recover a bacterial load from contaminated surfaces. Two different surface materials – stainless steel (SS) and polypropylene (PP) – were cleaned either by autoclaving (SS) or disinfectant (quaternary ammonium compounds), and let air dry under a laminar flow for 2 h. Suspensions with three different bacterial loads equal to 106, 105, and 104 CFU/mL were prepared from overnight culture of E. coli (reference strain ATCC 25922). The testing surfaces were contaminated by dispensing 1 mL of different E. coli suspensions distributed on an approximate 10×10 cm area using a sterile spreader in order to obtain approximately 104, 103, 102 CFU/cm2. The contaminated surfaces were let air dry for 2 h and then sampled in parallel area with the two swabs, according to ISO 18593:2004 (ISO, 2004; Figure 1). Four replicates side by side of both swabs were analysed for each load both for the SS and the PP surface, according to ISO 4833-2:2013 Cor 1:2014 (ISO, 2014) for total mesophilic count at 30°C. After the incubation time plates were counted and the results were recorded. The difference between means value were detected by the t-test and evaluations were based on a confidence interval of 95%.
Figure 1.
Surface sampling according to ISO 18593:2004 (ISO, 2004).
Results
Experiment 1
E. coli counts recorded using the FLOQSwab™ were expressed as mean of the replicates, and the values resulted as 4.60×103 CFU/mL for the lower, 4.12×104 CFU/mL for the middle, and 3.14×105 CFU/mL for the higher load. Analysis for FLOQSwab™ results, in comparison with the gold standard values, showed a recovery rate of 128% for the lower, 76% for the middle, and 112% for the higher load of the original concentration of microorganism used in the trial. E. coli counts recorded using a traditional rayon tipped swab were 1.94×103 CFU/mL for the lower, 2.84×104 CFU/mL for the middle, and 1.19×105 CFU/mL for the higher load. Analysis for the traditional rayon tipped swab results, in comparison with the gold standard values, showed a recovery rate of 54% for the lower, 53% for the middle, and 42% for the higher load of the original concentration of microorganism used in the trial (Table 1). Differences observed in means of values for the three different loads were statistically significant (P<0.05).
Table 1.
Escherichia coli counts observed in experiment 1.
| Concentration (CFU/mL) | Gold standard | Traditional swab | FLOQSwabTM |
|---|---|---|---|
| 1000 | 3.60×103 | 1.94×103 (54%) | 4.60×103 (128%) |
| 10,000 | 5.40×104 | 2.84×104 (53%) | 4.12×104 (76%) |
| 100,000 | 2.80×105 | 1.19×105 (42%) | 3.14×105 (112%) |
CFU, colony forming unit. Values are expressed as means of five replicates for each concentration considered in the trial.
Experiment 2
E. coli counts recorded using the FLOQSwab™ to recover the bacterial load from SS surfaces (expressed as mean of the replicates for each bacterial load) were 9.00 CFU/cm2 for the lower, 8.95×101 CFU/cm2 for the middle, and 3.18×103 CFU/cm2 for the higher load. Using the FLOQSwab™ and considering the original inoculum concentration used to contaminate the surface, the recovery rates were 9% for the lower, 9% for the middle, and 32% for the higher load (Figure 2). On the surface using the traditional rayon tipped swab, E. coli counts – expressed as mean of the replicates for each bacterial load – were 1.00, 4.09×101, and 4.45×102 CFU/cm2. With the traditional rayon tipped swab and considering the original inoculum concentration, the recovery rates were 1% for the lower, 1% for the middle, and 4% for the higher load. Differences observed in means of value for the three different loads were statistically significant (P<0.05). For the PP surfaces, E. coli counts recorded using the FLOQSwab™ and expressed as means of the replicates for each bacterial load, were 1.30×101 CFU/cm2 for the lower, 2.13×102 CFU/cm2 for the middle, and 2.08×103 CFU/cm2 for the higher load. The FLOQSwab™ and the original inoculum concentration considered, the recovery rates were 13% for the lower, 21% for the middle, and 21% for the higher load (Figure 3).
Figure 2.
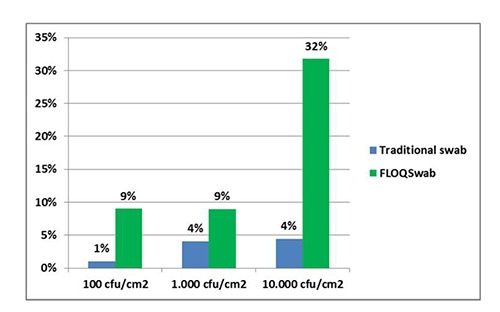
Experiment 2: Escherichia coli counts observed on stainless steel surface. Values are expressed as percentage of means of replicates, compared to expected concentration.
Figure 3.
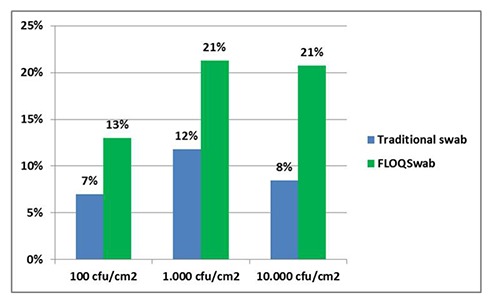
Experiment 2: Escherichia coli counts observed on polypropylene surface. Values are expressed as percentage of means of replicates, compared to expected concentration.
On the same surface using the traditional rayon tipped swab, E. coli counts (expressed as means of the replicates for each bacterial load) were 7.00, 1.18×102, and 8.45×102 CFU/cm2. In this case, the recovery rates were 7% for the lower, 12% for the middle, and 8% for the higher load. Differences observed in means of value were statistically significant (P<0.05) for lower and higher loads, while for the middle load there was no statistical difference (P>0.05).
Discussion
In the first experiment, FLOQSwab™ adsorbed the analyte and then released it, applying a routine analytical protocol, in an amount comparable to what recovered in the gold standard analyses for all the three loads considered. Regarding the lower bacterial load result, the fact that the FLOQSwab™ recovered more than the original concentration has to be addressed to variables and errors that can be produced, and to the uncertainty inherent in microbiological counts. Instead, the traditional rayon tipped swab showed a lower recovery rate. Indeed, only 50% of microorganisms were recovered comparing to gold standards values.
In the second experiment, the FLOQSwab™ showed a better performance also when used as a collection tool as per ISO 18593:2004 (ISO, 2004) from the two different surface materials tested. In particular, on the SS surface the recovery was more consistent especially at high bacterial load, compared to the traditional rayon tipped swab. In general, 1 log recovery improvement was recorded. On the PP surface the results were always better for the FLOQSwab™ than the traditional rayon tipped swab, even if the differences in the percentage of recovery were lower.
Conclusions
In food safety, microbiological work surface controls play a fundamental role in the prevention of contamination from the environment. The sampling techniques usually applied underrate the level of contamination of different surfaces that are in contact with food during food process. This could give food business operators false information about the level of cleanliness of processing environments.
Results obtained in this study, where the use of an innovative collection tool – the FLOQSwab™ – was proposed as an improvement in collecting environmental hygiene controls, seem to be encouraging. Indeed, this kind of swab, both in vitro and in experimental surface sampling tests, demonstrated to be more efficient compared to a traditional rayon tipped swab, which has been the state of the art so far and has been also suggested by the ISO 18593:2004 (ISO, 2004) to be widely used in the food industries.
The use of swabs for the environmental sampling was discouraged in the past due to the poor recovery and then for the risk of an erroneous or underestimated result. The introduction of the FLOQSwab™ allows the operator to reintroduce the use of swab as a sampling tool for all the difficult-to-reach areas to be monitored for hygiene assessment.
Based on these preliminary results, it was decided to verify the applicability of the flocked swab, even with other laboratory confirmatory tests, directly in the food industry. Companies should be mainly implicated in dairy and meat-derived production, and the recovery of bacteria should be done from a wider variety of surfaces (wood, SS, PP or other plastic and ceramic surfaces) for the sake of environmental monitoring and research of specific pathogens commonly involved in food poisoning event, like Salmonella spp. and Listeria monocytogenes.
Aknowledgements
The swab devices used in this study were provided by Copan Italia S.p.A., Brescia, Italy.
References
- Chernesky M, Castriciano S, Jang D, Smieja M, 2006. Use of flocked swabs and a universal transport medium to enhance molecular detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 44:1084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmaso G, Bini M, Paroni R, Ferrari M, 2008. Qualification of high-recovery, flocked swabs as compared to traditional rayon swabs for microbiological environmental monitoring of surfaces. PDA J Pharm Sci Tech 62:191-9. [PubMed] [Google Scholar]
- Daley P, Castriciano S, Chernesky M, Smieja M, 2006. Comparison of flocked swabs and rayon swabs for collection of respiratory epithelial cells from volunteers and symptomatic patients. J Clin Microbiol 44:2265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail R, Aviat F, Michel V, Le Bayon I, Gay-Perret P, Kutnik M, Federighi M, 2013. Methods for recovering microorganisms from solid surfaces used in the food industry: a review of literature. Int J Environ Res Public Health 10:6169-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO, 2004. Microbiology of food and animal feeding stuffs: horizontal method for sampling techniques from surfaces using contact plates and swabs. ISO Norm 18593:2004. International Standardization Organization ed., Geneva, Switzerland. [Google Scholar]
- ISO, 2014. Microbiology of the food chain: horizontal method for the enumeration of microorganisms. Part 2: colony count at 30°C by surface plating technique Corrigendum 1. ISO Norm 4833-2:2013 Cor 1:2014. International Standardization Organization ed., Geneva, Switzerland. [Google Scholar]
- Kusumaningrum HD, Riboldi G, Hazeleger WC, Beumer RR, 2003. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int J Food Microbiol 85:227-36. [DOI] [PubMed] [Google Scholar]
- Lahou E, Uyttendaele M, 2014. Evaluation of three swabbing devices for detection of Listeria monocytogenes on different types of contact surfaces. Int J Environ Res Public Health 11:804-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G, Griffith CA, 2002. Comparison of surface sampling methods for detecting coliforms on food contact surfaces. Food Microbiol 19:65-73. [Google Scholar]
- Probst A, Facius R, Wirth R, Moissl-Eichinger C, 2010. Validation of a nylon flocked swab protocol for efficient recovery of bacterial spores from smooth and rough surfaces. Appl Environ Microb 76:5148-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verran J, Redfern J, Smith LA, Whitehead KA, 2010. A critical evaluation of sampling methods used for assessing microorganism on surfaces. Food Bioprod Process 88:335-40. [Google Scholar]


