Abstract
Postpartum breast cancers are a highly metastatic subset of young women’s breast cancers defined as breast cancers diagnosed in the postpartum period or within 5 years of last child birth. Women diagnosed with postpartum breast cancer are nearly twice as likely to develop metastasis and to die from breast cancer when compared with nulliparous women. Additionally, epidemiological studies utilizing multiple cohorts also suggest that nearly half of all breast cancers in women aged <45 qualify as postpartum cases. Understanding the biology that underlies this increased risk for metastasis and death may lead to identification of targeted interventions that will benefit the large number of young women with breast cancer who fall into this subset. Preclinical mouse models of postpartum breast cancer have revealed that breast tumor cells become more aggressive if they are present during the normal physiologic process of postpartum mammary gland involution in mice. As involution appears to be a period of lymphatic growth and remodeling, and human postpartum breast cancers have high peritumor lymphatic vessel density (LVD) and increased incidence of lymph node metastasis (1, 2), we propose that novel insight into is to be gained through the study of the biological mechanisms driving normal postpartum mammary lymphangiogenesis as well as in the microenvironment of postpartum tumors.
Keywords: breast cancer, lymphangiogenesis, lymphatic metastasis, macrophages, postpartum
Introduction to Postpartum Breast Cancer
Postpartum breast cancer is an under-recognized and highly metastatic subset of young women’s breast cancer, which we define as breast cancers diagnosed within 5 years of a women’s most recent child birth (3, 4). The distinction of postpartum cases from the various interactions of breast cancer and pregnancy, or pregnancy-associated breast cancer (PABC), arose from epidemiologic studies indicating that women diagnosed with breast cancer in the postpartum years are nearly three times as likely to develop metastasis and to die from breast cancer in comparison with nulliparous women (3–6). Importantly, this research highlighted the need to clearly separate breast cancer cases as nulliparous, pregnant, or postpartum, as opposed to defining PABC as cancers diagnosed both during and in the 1–2 years after parturition as one entity, to avoid diluting the risk signal (5). Epidemiological studies utilizing multiple cohorts also identify that ~45% of all breast cancers in Caucasian women aged ≤45 are diagnosed within 5–6 years of childbirth (5). More recently, it was identified that a breast cancer diagnosis of up to 10 years postpartum confers an ongoing measure of increased risk for metastasis, which would represent 60% or more of all young onset diagnosis in the US (7). Ideally, understanding the biology that underlies this epidemiologic risk for metastasis and death will lead to identification of targeted interventions that will benefit the large number of young women with breast cancer who fall into this subset (8).
To define the mechanisms of increased risk for metastasis, preclinical mouse models of postpartum breast cancer have revealed that tumors become more aggressive if they are exposed to the normal physiologic process of postpartum mammary gland involution. The process of postpartum breast or mammary gland involution is when the mammary epithelium regresses from the lactational state, undergoes a period of significant tissue remodeling, and resets to the pre-pregnant state. Multiple hallmarks of cancer are identified as also being important aspects of the involution process (9–37). Moreover, increased tumor growth, invasion, and metastasis are all identified when either human breast cancer or murine mammary tumors cells are implanted into postpartum hosts during involution compared with nulliparous controls (1, 36, 38–40). Mechanisms underlying this aggressive tumor promotional phenotype of involution include the induction of immunosuppression and lymphangiogenesis in the tumor microenvironment. Focusing on lymphangiogenesis, involution appears to be a period of significant lymphatic growth and remodeling, and human postpartum breast cancers have high peritumor lymphatic vessel density (LVD) and increased incidence of lymph node metastasis (2, 38, 41). Thus, we believe lymphangiogensis is an important pathway in the metastasis of postpartum breast cancer. Deeper understanding of the biological mechanisms driving normal postpartum mammary lymphangiogenesis offers potential novel insight into tumor-associated lymphangiogenesis.
Introduction to the Lymphatic System and Lymphangiogenesis
Lymphangiogenesis is the outgrowth of new lymphatic vessels, which is required for development of the immune system, fluid homeostasis, trafficking of lymphatic cells, normal wound healing, and tissue regeneration (42–47). Differential expression of lymphatic markers, which distinguish lymphatic vessels from blood vessels, has been described in detail over the past decade and has aided the field by allowing researchers to distinguish between newly formed neo- and mature lymphatics (43, 48–57). The adult lymphatic system consists of initial lymphatics, also known as lymphatic capillaries, which drain lymph fluid into pre-collecting lymphatics, followed by drainage into collecting lymphatics that then lead to the lymph node where foreign bodies can be trapped, immune reactions occur, and lymph fluid is concentrated (42, 44). While the lymphatic endothelial marker Lyve-1 is absent in the collecting lymphatics, it is highly expressed for the initial lymphatics or lymphatic capillaries (58). Furthermore, both the lymphatic capillaries and the collecting vessels exhibit high expression levels of Prox-1, VEGFR-3, and podoplanin (48–55, 59). These results suggest that Lyve-1 may be a marker that can be used to specifically measure new lymphatic formation or neo-lymphangiogenesis.
Neo-lymphangiogenesis occurs in adult tissues as an active normal response to infection, inflammation, and wound healing. Neo-lymphangiogenesis can be stimulated by the local production of the vascular endothelial growth factors VEGF-C, and -D within the damaged tissue and subsequent binding to VEGFR-2 and VEGFR-3 on nearby lymphatic endothelial cells (LECs), resulting in the expansion of lymphatics via sprouting from pre-existing lymphatic vasculature (50, 51, 59–69). Primary sources of VEGF-A, -C, and -D include fibroblasts, inflammatory cells, and macrophages (70–74). An alternative theory has emerged whereby bone marrow-derived cells, specifically macrophages, may also be recruited to contribute to lymphangiogenesis (75–77). In support of this theory, bone marrow transplanted from GFP+ mice into GFP− recipients revealed GFP+ cells localized and/or incorporated into new lymphatics during inflammation, and additional lineage tracing experiments support these findings (75, 76, 78). Furthermore, tissue-resident and bone marrow-derived macrophages express lymphatic markers, such as Lyve-1, Prox-1, and podoplanin (78–80), and the presence of macrophages at sites of neo-lymphangiogenesis during inflammation has been reported (78, 80). Thus, macrophages appear to be involved in neo-lymphangiogenesis. As macrophages are also an important part of the normal program of involution (19), we believe there is a role for macrophages in facilitating the neo-lymphangiogenesis seen during postpartum mammary involution.
Pro-Lymphatic Programs Observed During Postpartum Mammary Involution
Postpartum mammary involution has been extensively characterized using rodent mammary glands with more recent preliminary confirmation in human tissues (11, 12, 17, 19, 20, 26, 32, 35, 36, 40, 81–89). Postpartum mammary gland involution occurs in two distinct phases. During the first phase, which is reversible and lasts for 48 h (days 1–2 post weaning), apoptosis of the epithelium occurs and repopulation of the gland with adipocytes is observed. During the second phase (days 3–14), a remodeling program is initiated which results in additional cell death, increased expression of matrix remodeling proteases, degradation and remodeling of extracellular matrix components, and re-differentiation of adipocytes. Recently, we observed that neo- lymphangiogenesis occurs during postpartum mammary gland involution; however, the functional significance of these increased lymphatics has yet to be described (38, 41). Prior to our studies only a few reports had focused on mammary lymphatics, which are described below.
Expression of the VEGF family members has been characterized during the pregnancy/lactation/involution cycle, with a goal of understanding regulation of angiogenesis in the mammary gland. In these studies, VEGF-A expression was observed as increased during pregnancy and lactation where it likely drives angiogenesis and vascular permeability, which are important for milk production. In contrast, pro-lymphangiogenic VEGFC expression levels were overall lower over the course of pregnancy and lactation, remained extremely low during the first phase of involution (days 1 and 2), and then increased nearly twofold in the second phase (days 3 and 7); this provides evidence that pro-lymphangiogenic programs may be activated during postpartum mammary involution (90). Consistent with these findings, we have observed upregulation of pro-lymphangiogenic VEGF-C and VEGF-D mRNA expression, along with their receptors, VEGFR2/3, during postpartum involution in rat mammary tissues (38) (Figure 1A). Additional studies have utilized elegant high-resolution imaging of sectioned and/or whole mounted mouse mammary glands to better understand lymphatic development during the pregnancy/lactation/involution cycle of the mammary gland. These studies revealed that VEGF-C and -D are produced locally by the mammary epithelium and myo-epithelium and that Prox-1-positive lymphatic vessels were intimately associated with the mammary epithelium and the blood vasculature.
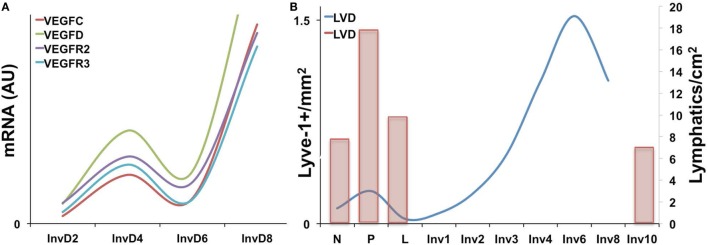
Figure 1.
(A) Pro-lymphangiogenic growth factor gene expression, as measured by qPCR is increased during early and late involution in whole rat mammary tissues [adapted from Lyons et al. (38)]. (B) Lyve-1+ lymphatic vessels per area (left axis) is increased during pregnancy and again during involution in mouse mammary tissues with peak levels observed at day 6. (Right axis) A previous study showing a similar increase in Prox-1+ lymphatic vessels during pregnancy as well as levels at involution day 10 [adapted from Betterman et al. (91)].
These studies, by Betterman et al., also examined Prox-1-positive lymph vessels per area to determine density. Interestingly, they observed peak LVD during pregnancy, which decreased during lactation and involution (91). However, their analysis included only a single timepoint during involution, involution day 10, which is near the end of the involution process in mice. In contrast, while our results also reveal that the number of Lyve-1 positive vessels per area in rodent mammary glands similarly drops from pregnancy to lactation, we observe that this drop is accompanied by a subsequent rise in LVD during the early phases involution, which peaks at involution day 6 in mouse and at day 10 in rat mammary tissue (38, 41) (Figure 1B). These results suggest that neo-lymphangiogenesis occurs during the active phase of mammary remodeling in rodents. Importantly, we also analyzed podoplanin positive vessels in normal breast tissue from women who were biopsied within 10 years postpartum to determine whether the increase in lymph vessels is also evident and whether the increase persisted over time, as has been suggested by a gene signature that contains pro-angiogenic molecules angiopoietin and VEGF-A (9). The results from our study showed that women who were within 1 year of giving birth, and no longer lactating, had the highest LVD compared with never been pregnant (NBP) women. In addition, women between 3 and 10 years of giving birth also had elevated LVD compared with nulliparous suggesting that neo-lymphangiogenesis occurs during postpartum breast involution in women, and the resulting lymphatics may persist beyond the period of remodeling (38).
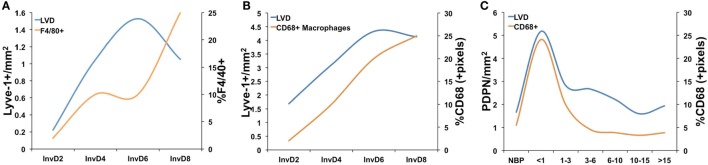
Consistent with a potential role for bone marrow-derived cells in neo-lymphangiogenesis, additional studies of postpartum mammary gland involution have revealed specific changes in immune cell populations, and regulation, during the involution process (12, 19, 20, 32, 36, 40, 81–83). Initial gene expression analyses during the pregnancy/lactation/involution cycle identified upregulation of genes important for acute inflammatory responses in the mammary epithelium during postpartum involution (12, 26). Specifically, Stat3 and NF-κB are primary mediators of involution in the mouse and are also known to be key mediators of acute-phase inflammatory response. Recently, and in support of these gene expression data, the postpartum mammary gland was shown to have a cascade of infiltrating immune cells, including T-cells, T regulatory cells, and dendritic cells during involution that mimic a wound-healing pattern (92). Furthermore, numerous studies have shown that macrophages are present during involution in mouse, rat, and human tissues and that macrophage ablation during the first phase of involution in mice blocks epithelial cell death and adipocyte repopulation. The details of these studies have been reviewed elsewhere (17, 20, 81). Importantly, we observe that macrophages and lymphatics may be similarly regulated during postpartum involution in mouse, rat, and human tissues (19, 38, 93) (Figure 2). We have also shown that macrophages present during postpartum involution in rodent and human tissues express markers of an M2-polarized phenotype, such as mannose receptor, arginase-1, and CD11b (19, 36, 81). CD11b+ macrophages produce pro-lymphangiogenic factors VEGF-C and -D (78, 80, 94). Moreover, subpopulations of CD11b+ positive macrophages express lymphatic endothelial markers Lyve-1 and VEGFR-3 (95, 96). Together, these findings indicate that the CD11b+ “involution macrophages” may either stimulate lymphangiogenesis through release of pro-lymphangiogenic cytokines or through expression of lymphatic markers and incorporation into existing lymphatics; evidence for both has been published in models of inflammation and cancer (68, 78, 80, 96, 97).
Figure 2.
A comparison of lymphatic vessel density (LVD) and macrophage infiltration in (A) mouse mammary tissue where macrophages were measured as %F4/80+ cells from whole mammary tissue by flow cytometry [data adapted from Lyons et al. (38) and Martinson et al. (36)], (B) rat mammary tissue where macrophages were measured by quantitative IHC as %CD68+ cells/pixel [data adapted from Lyons et al. (38) and O’Brien et al. (19)], and (C) in normal human breast tissues from women who had never been pregnant (NBP), were no longer lactating, and <1, 1–3, 3–6, 6–10, 10–15, and >15 years since last childbirth where lymphatics were measured as number of podopanin+ vessels per area and CD68 by quantitative IHC [data adapted from Lyons et al. (38) and Jindal et al. (93)].
Our analysis of lymphangiogenesis during postpartum involution also revealed that administration of a selective COX-2 inhibitor, celecoxib (CXB), during postpartum involution reduced Lyve-1-positive LVD at involution day 4 (38). These results are consistent with previous observations in tumor models (98–102). While, the mechanism by which CXB blocked lymphangiogenesis was not directly revealed by these studies, our in vitro data suggested that a product of COX-2 activity, PGE2, acts directly on the lymphatic endothelium via the EP2 receptor. However, PGE2 can also stimulate macrophages to an M2 phenotype (103, 104); thus, it is also possible that CXB inhibits lymphangiogenesis through macrophage-dependent mechanisms. Understanding the mechanisms underlying “involution macrophage” contribution to lymphangiogenesis during normal mammary gland development could lead to insight into macrophage-mediated lymphangiogenesis during breast cancer. We postulate that postpartum involution is a developmental window that allows for studies of mechanisms driving lymphangiogenesis.
Lymphatic Vasculature in Breast Cancer Metastasis
While expansion of the lymphatic vasculature has been linked to faster healing and greater ability to fight infection, lymphangiogenesis can also be pathologic. Pathologic lymphangiogenesis has been observed in graft-versus-host disease, in chronic inflammatory diseases (e.g., Rheumatoid arthritis and inflammatory bowel disease), and in the tumor microenvironment. Lymph node metastasis, lymphatic vessel presence at the tumor margin, and invasion of tumor cells into peritumor lymphatics are all poor prognostic factors for breast cancer patients (105). Further, increased LVD in the peritumor region correlates with increased metastasis in a number of human cancers, directly implicating new lymphatic vessel formation in tumor cell dissemination (106–108). A multitude of studies have examined mechanisms driving neo-lymphangiogenesis in the breast tumor environment, and VEGF-C, VEGF-D, macrophages, and COX-2/PGE2 have emerged as key players (51, 68, 72, 73, 80, 99–101, 109–118).
VEGF-C is secreted by macrophages and other lymphatic cells to stimulate lymphangiogenesis, but can also be secreted by tumor cells for the same purpose (51, 62, 72, 73, 94, 117, 119). Macrophages have also been shown to participate directly in lymphangiogenesis via inducing lymphatic vessel sprouting and incorporating into existing tumor-associated lymphatics (69, 80). COX-2 and its product PGE2 also promote lymphangiogenesis in the tumor microenvironment (38, 41, 98, 99, 101, 102, 116, 120, 121). Furthermore, VEGF-D promotes lymphatic vessel dilation through a COX-2-dependent mechanism. Dilation of pre-existing, peritumor, and intratumor lymphatics allows for the intravasation of tumor cells into the lymphatic vessels and subsequent transmigration to regional lymph nodes (109, 114, 119, 122). Together these results suggest there is a connection between COX-2-mediated lymphangiogenesis and lymphogenous tumor cell spread.
Lymphatic Vasculature in Postpartum Breast Cancer
Postpartum breast cancers are nearly three times as likely to metastasize when compared with breast cancers in nulliparous women (5, 123, 124). Furthermore, we have shown that postpartum breast cancers have increased peritumor LVD and increased lymph node involvement (1). It is anticipated that postpartum breast tumors will utilize mechanisms similar to those observed during postpartum involution to induce lymphangiogenesis. The first, and most obvious mechanism, is upregulation of COX-2 in the mammary epithelium (125), which results in increased PGE2 production to increase lymphangiogenesis. Indeed, in animals treated with celecoxib (CXB) during postpartum involution, the resultant postpartum tumors exhibit lower levels of LVD compared with untreated controls. In addition, COX-2 in the tumor cell appears to be required as well since tumors with stable siRNA knockdown of COX-2 exhibit decreased tumor-associated LVD. Finally, if postpartum tumors are re-implanted in nulliparous hosts they maintain their ability to drive tumor-associated lymphangiogenesis (38). Of interest, these results suggest that involution-induced pro-lymphangiogenic programs persist in tumor cells long after the process of involution is complete. These results are supported by gene-expression studies indicating that there is an involution signature observed in breast tissue of parous women that persists for 10 years postpartum (9).
In addition to a COX-2-dependent mechanism, if “involution macrophages” promote lymphangiogenesis during normal involution then postpartum tumor-associated macrophages (TAMs) may also acquire and utilize similar mechanisms to mediate lymphangiogenesis in the postpartum tumor microenvironment. CD11b+ is expressed by “involution macrophages” and by TAMs (96). TAMs also predict poor prognosis of breast cancer (126), and an association between TAMs and LVD has been reported for pancreatic cancer (127). Furthermore, TAMs express VEGF-C and may promote metastasis via lymphangiogenesis (71–73, 126, 128). Thus, the CD11b+ macrophages present during postpartum involution may promote lymphangiogenesis in a manner similar to TAMs, and we have preclinical data indicating that CD11b+ macrophages are also increased in the tumor microenvironment of involution/postpartum tumors compared with nulliparous controls (36).
Potential Clinical Implications: Anti-Lymphangiogenic Therapy
While it is not clear why the lymphatics are expanded during postpartum involution, it is clear that postpartum tumors hijack the lymphatic vessels in the postpartum gland to drive increased metastasis. In addition, the observed tumor-promoted neo-lymphangiogenesis offers a targetable mechanism to reduce cancer metastasis (123, 124, 129–131). Anti-lymphangiogenic therapies, such as anti-VEGFR2/3 antibodies and small molecule inhibitors that target VEGFR2/3, have been tested in clinical trials for multiple solid tumor types and have shown some successes and low toxicities (132–134). Since our studies suggest that COX-2 specific inhibitors may serve to reduce tumor-associated lymphangiogenesis, we suggest that identifying whether COX-2 inhibitors can be combined with current therapies, and/or with anti-lymphangiogenesis therapy, to reduce lymphogenous spread and metastatic recurrence should be explored for postpartum breast cancer patients.
Author Contributions
TL was responsible for overseeing writing and editing of the manuscript, assigning contributions from VB and AE, and for preparation of the figures. VB and AE contributed to writing and editing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was supported by NCI 1 R01 CA169175-01 and Robert F. and Patricia Young Connor Endowed Chair in Young Women’s Breast Cancer Research to VB. NCI R21CA185226-01, NIH 1KL2TR001080, and UL1 TR001082 to TL. TL was also supported by funding from the Cancer League of Colorado.
References
- 1.Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med (2011) 17:1109–15. 10.1038/nm.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neill T, Zoeller J. Matrix biology highlights. Matrix Biol (2014) 40:1–4. 10.1016/j.matbio.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 3.Borges VF, Schedin PJ. Pregnancy-associated breast cancer: an entity needing refinement of the definition. Cancer (2012) 118:3226–8. 10.1002/cncr.26643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia (2009) 14:87–98. 10.1007/s10911-009-9119-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat (2013) 138:549–59. 10.1007/s10549-013-2437-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden). Cancer Causes Control (2002) 13:299–305. 10.1023/A:1015287208222 [DOI] [PubMed] [Google Scholar]
- 7.Borges VF, Goddard E, Partridge AH, Schedin P. Abstract P6-08-08: postpartum breast cancer demonstrates increased liver and brain metastasis with a proposed role for postpartum involution. Cancer Res (2015). 10.1158/1538-7445.SABCS14-P6-08-08 [DOI] [Google Scholar]
- 8.Fornetti J, Martinson HA, Betts CB, Lyons TR, Jindal S, Guo Q, et al. Mammary gland involution as an immunotherapeutic target for postpartum breast cancer. J Mammary Gland Biol Neoplasia (2014) 19:213–28. 10.1007/s10911-014-9322-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asztalos S, Gann PH, Hayes MK, Nonn L, Beam CA, Dai Y, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila) (2010) 3:301–11. 10.1158/1940-6207.CAPR-09-0069 [DOI] [PubMed] [Google Scholar]
- 10.Atabai K, Sheppard D, Werb Z. Roles of the innate immune system in mammary gland remodeling during involution. J Mammary Gland Biol Neoplasia (2007) 12:37–45. 10.1007/s10911-007-9036-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarkson RW, Heeley JL, Chapman R, Aillet F, Hay RT, Wyllie A, et al. NF-kappaB inhibits apoptosis in murine mammary epithelia. J Biol Chem (2000) 275:12737–42. 10.1074/jbc.275.17.12737 [DOI] [PubMed] [Google Scholar]
- 12.Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res (2004) 6:R92–109. 10.1186/bcr754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol (2001) 152:693–703. 10.1083/jcb.152.4.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djonov V, Andres AC, Ziemiecki A. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc Res Tech (2001) 52:182–9. [DOI] [PubMed] [Google Scholar]
- 15.Green KA, Lund LR. ECM degrading proteases and tissue remodelling in the mammary gland. Bioessays (2005) 27:894–903. 10.1002/bies.20281 [DOI] [PubMed] [Google Scholar]
- 16.Hughes K, Wickenden JA, Allen JE, Watson CJ. Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. J Pathol (2012) 227:106–17. 10.1002/path.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monks J, Geske FJ, Lehman L, Fadok VA. Do inflammatory cells participate in mammary gland involution? J Mammary Gland Biol Neoplasia (2002) 7:163–76. 10.1023/A:1020351919634 [DOI] [PubMed] [Google Scholar]
- 18.Nickerson SC. Immunological aspects of mammary involution. J Dairy Sci (1989) 72:1665–78. 10.3168/jds.S0022-0302(89)79278-X [DOI] [PubMed] [Google Scholar]
- 19.O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol (2010) 176:1241–55. 10.2353/ajpath.2010.090735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien J, Schedin P. Macrophages in breast cancer: do involution macrophages account for the poor prognosis of pregnancy-associated breast cancer? J Mammary Gland Biol Neoplasia (2009) 14:145–57. 10.1007/s10911-009-9118-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pensa S, Watson CJ, Poli V. Stat3 and the inflammation/acute phase response in involution and breast cancer. J Mammary Gland Biol Neoplasia (2009) 14:121–9. 10.1007/s10911-009-9124-x [DOI] [PubMed] [Google Scholar]
- 22.Ramirez RA, Lee A, Schedin P, Russell JS, Masso-Welch PA. Alterations in mast cell frequency and relationship to angiogenesis in the rat mammary gland during windows of physiologic tissue remodeling. Dev Dyn (2012) 241:890–900. 10.1002/dvdy.23778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schedin P, Mitrenga T, McDaniel S, Kaeck M. Mammary ECM composition and function are altered by reproductive state. Mol Carcinog (2004) 41:207–20. 10.1002/mc.20058 [DOI] [PubMed] [Google Scholar]
- 24.Schedin P, Strange R, Mitrenga T, Wolfe P, Kaeck M. Fibronectin fragments induce MMP activity in mouse mammary epithelial cells: evidence for a role in mammary tissue remodeling. J Cell Sci (2000) 113(Pt 5):795–806. [DOI] [PubMed] [Google Scholar]
- 25.Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res (2004) 6:R75–91. 10.1186/bcr894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein T, Salomonis N, Gusterson BA. Mammary gland involution as a multi-step process. J Mammary Gland Biol Neoplasia (2007) 12:25–35. 10.1007/s10911-007-9035-7 [DOI] [PubMed] [Google Scholar]
- 27.Stein T, Salomonis N, Nuyten DS, van de Vijver MJ, Gusterson BA. A mouse mammary gland involution mRNA signature identifies biological pathways potentially associated with breast cancer metastasis. J Mammary Gland Biol Neoplasia (2009) 14:99–116. 10.1007/s10911-009-9120-1 [DOI] [PubMed] [Google Scholar]
- 28.Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development (1992) 115:49–58. [DOI] [PubMed] [Google Scholar]
- 29.Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol (1992) 118:1271–82. 10.1083/jcb.118.5.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson CJ. Post-lactational mammary gland regression: molecular basis and implications for breast cancer. Expert Rev Mol Med (2006) 8:1–15. 10.1017/S1462399406000196 [DOI] [PubMed] [Google Scholar]
- 31.Watson CJ. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res (2006) 8:203. 10.1186/bcr1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson CJ. Immune cell regulators in mouse mammary development and involution. J Anim Sci (2009) 87:35–42. 10.2527/jas.2008-1333 [DOI] [PubMed] [Google Scholar]
- 33.Werb Z, Ashkenas J, MacAuley A, Wiesen JF. Extracellular matrix remodeling as a regulator of stromal-epithelial interactions during mammary gland development, involution and carcinogenesis. Braz J Med Biol Res (1996) 29:1087–97. [PubMed] [Google Scholar]
- 34.Zhao L, Melenhorst JJ, Hennighausen L. Loss of interleukin 6 results in delayed mammary gland involution: a possible role for mitogen-activated protein kinase and not signal transducer and activator of transcription 3. Mol Endocrinol (2002) 16:2902–12. 10.1210/me.2001-0330 [DOI] [PubMed] [Google Scholar]
- 35.Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J, et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol (2011) 13:303–9. 10.1038/ncb2171 [DOI] [PubMed] [Google Scholar]
- 36.Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int J Cancer (2015) 136:1803–13. 10.1002/ijc.29181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaught DB, Cook RS. Clearance of dying cells accelerates malignancy. Oncotarget (2015) 6:24590–1. 10.18632/oncotarget.5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, et al. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J Clin Invest (2014) 124(9):3901–12. 10.1172/JCI73777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res (2007) 67:2062–71. 10.1158/0008-5472.CAN-06-3895 [DOI] [PubMed] [Google Scholar]
- 40.Stanford JC, Young C, Hicks D, Owens P, Williams A, Vaught DB, et al. Efferocytosis produces a prometastatic landscape during postpartum mammary gland involution. J Clin Invest (2014) 124:4737–52. 10.1172/JCI76375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swartz MA. Inflammatory lymphangiogenesis in postpartum breast tissue remodeling. J Clin Invest (2014) 124(9):1–4. 10.1172/JCI77765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Betterman K, Harvey N. The lymphatic vasculature: development and role in shaping immunity. Immunol Rev (2016) 27:276–92. 10.1111/imr.12413 [DOI] [PubMed] [Google Scholar]
- 43.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell (2010) 140:460–76. 10.1016/j.cell.2010.01.045 [DOI] [PubMed] [Google Scholar]
- 44.Butler MG, Isogai S, Weinstein BM. Lymphatic development. Birth Defects Res C Embryo Today (2009) 87:222–31. 10.1002/bdrc.20155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol (2011) 193:607–18. 10.1083/jcb.201012094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karpanen T, Makinen T. Regulation of lymphangiogenesis – from cell fate determination to vessel remodeling. Exp Cell Res (2006) 312:575–83. 10.1016/j.yexcr.2005.10.034 [DOI] [PubMed] [Google Scholar]
- 47.Karpanen T, Wirzenius M, Mäkinen T, Veikkola T, Haisma HJ, Achen MG, et al. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am J Pathol (2006) 169:708–18. 10.2353/ajpath.2006.051200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J (2002) 21:1505–13. 10.1093/emboj/21.7.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan Y, Wang WD, Yago T. Transcriptional regulation of podoplanin expression by Prox1 in lymphatic endothelial cells. Microvasc Res (2014) 94:96–102. 10.1016/j.mvr.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 50.Norrmén C, Tammela T, Petrova TV, Alitalo K. Biological basis of therapeutic lymphangiogenesis. Circulation (2011) 123:1335–51. 10.1161/CIRCULATIONAHA.107.704098 [DOI] [PubMed] [Google Scholar]
- 51.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol (2004) 5:74–80. 10.1038/ni1013 [DOI] [PubMed] [Google Scholar]
- 52.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol (1999) 154:385–94. 10.1016/S0002-9440(10)65285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norrmén C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol (2009) 185:439–57. 10.1083/jcb.200901104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrova TV, Karpanen T, Norrmén C, Mellor R, Tamakoshi T, Finegold D, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med (2004) 10:974–81. 10.1038/nm1094 [DOI] [PubMed] [Google Scholar]
- 55.Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J (2002) 21:4593–9. 10.1093/emboj/cdf470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev (2008) 22:3282–91. 10.1101/gad.1727208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin JW. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol Biol Cell (2005) 17:576–84. 10.1091/mbc.E05-04-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A (2002) 99:16069–74. 10.1073/pnas.242401399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dubé KN, et al. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature (2015) 522:62–7. 10.1038/nature14483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest (2014) 124:878–87. 10.1172/JCI71603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Breier G. Lymphangiogenesis in regenerating tissue: is VEGF-C sufficient? Circ Res (2005) 96:1132–4. 10.1161/01.RES.0000170976.63688.ca [DOI] [PubMed] [Google Scholar]
- 62.Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A (1998) 95:548–53. 10.1073/pnas.95.2.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, et al. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci U S A (1998) 95:14389–94. 10.1073/pnas.95.24.14389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao Y, Zhong W. Tumor-derived lymphangiogenic factors and lymphatic metastasis. Biomed Pharmacother (2007) 61:534–9. 10.1016/j.biopha.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 65.Cao Z, Shang B, Zhang G, Miele L, Sarkar FH, Wang Z, et al. Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. Biochim Biophys Acta (2013) 1836:273–86. 10.1016/j.bbcan.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 66.Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J, et al. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res (2004) 94:664–70. 10.1161/01.RES.0000118600.91698.BB [DOI] [PubMed] [Google Scholar]
- 67.Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer (2005) 5:735–43. 10.1038/nrc1693 [DOI] [PubMed] [Google Scholar]
- 68.Hall KL, Volk-Draper LD, Flister MJ, Ran S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One (2012) 7:e31794. 10.1371/journal.pone.0031794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ran S, Montgomery KE. Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) (2012) 4:618–57. 10.3390/cancers4030618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Björndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D, et al. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res (2005) 65:9261–8. 10.1158/0008-5472.CAN-04-2345 [DOI] [PubMed] [Google Scholar]
- 71.Gallego E, Vicioso L, Alvarez M, Hierro I, Pérez-Villa L, Blanes A, et al. Stromal expression of vascular endothelial growth factor C is relevant to predict sentinel lymph node status in melanomas. Virchows Arch (2011) 458:621–30. 10.1007/s00428-011-1044-7 [DOI] [PubMed] [Google Scholar]
- 72.Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, et al. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery (2006) 139:839–46. 10.1016/j.surg.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 73.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol (2002) 161:947–56. 10.1016/S0002-9440(10)64255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol (2001) 159:893–903. 10.1016/S0002-9440(10)61765-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Rüegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One (2009) 4:e7067. 10.1371/journal.pone.0007067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood (2005) 106:4184–90. 10.1182/blood-2005-01-0226 [DOI] [PubMed] [Google Scholar]
- 77.Jiang S, Bailey AS, Goldman DC, Swain JR, Wong MH, Streeter PR, et al. Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One (2008) 3:e3812. 10.1371/journal.pone.0003812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest (2005) 115:2363–72. 10.1172/JCI23874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci (2004) 45:2666–73. 10.1167/iovs.03-1380 [DOI] [PubMed] [Google Scholar]
- 80.Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest (2005) 115:2316–9. 10.1172/JCI26354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Brien J, Martinson H, Durand-Rougely C, Schedin P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development (2012) 139:269–75. 10.1242/dev.071696 [DOI] [PubMed] [Google Scholar]
- 82.Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res (2003) 5:R129–35. 10.1186/bcr622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watson CJ, Oliver CH, Khaled WT. Cytokine signalling in mammary gland development. J Reprod Immunol (2011) 88(2):124–9. 10.1016/j.jri.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 84.Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat (1989) 185:19–32. 10.1002/aja.1001850104 [DOI] [PubMed] [Google Scholar]
- 85.Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia (2000) 5:227–41. 10.1023/A:1026499523505 [DOI] [PubMed] [Google Scholar]
- 86.Schwertfeger KL, Richert MM, Anderson SM. Mammary gland involution is delayed by activated Akt in transgenic mice. Mol Endocrinol (2001) 15:867–81. 10.1210/mend.15.6.0663 [DOI] [PubMed] [Google Scholar]
- 87.Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, et al. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ (2005) 12:107–14. 10.1038/sj.cdd.4401517 [DOI] [PubMed] [Google Scholar]
- 88.Rudolph MC, McManaman JL, Hunter L, Phang T, Neville MC. Functional development of the mammary gland: use of expression profiling and trajectory clustering to reveal changes in gene expression during pregnancy, lactation, and involution. J Mammary Gland Biol Neoplasia (2003) 8:287–307. 10.1023/B:JOMG.0000010030.73983.57 [DOI] [PubMed] [Google Scholar]
- 89.Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod (2008) 78:586–94. 10.1095/biolreprod.107.065045 [DOI] [PubMed] [Google Scholar]
- 90.Pepper MS, Baetens D, Mandriota SJ, Di Sanza C, Oikemus S, Lane TF, et al. Regulation of VEGF and VEGF receptor expression in the rodent mammary gland during pregnancy, lactation, and involution. Dev Dyn (2000) 218:507–24. [DOI] [PubMed] [Google Scholar]
- 91.Betterman KL, Paquet-Fifield S, Asselin-Labat ML, Visvader JE, Butler LM, Stacker SA, et al. Remodeling of the lymphatic vasculature during mouse mammary gland morphogenesis is mediated via epithelial-derived lymphangiogenic stimuli. Am J Pathol (2012) 181:2225–38. 10.1016/j.ajpath.2012.08.035 [DOI] [PubMed] [Google Scholar]
- 92.Martinson H, Jindal S, Borges V, Schedin P. Immune cell influx during postpartum mammary gland involution reveals immunosuppression and tumor promotion. Cancer Res (2013):73. [Google Scholar]
- 93.Jindal S, Gao D, Bell P, Albrektsen G, Edgerton SM, Ambrosone CB, et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast Cancer Res (2014) 16:R31. 10.1186/bcr3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood (2009) 113:5650–9. 10.1182/blood-2008-09-176776 [DOI] [PubMed] [Google Scholar]
- 95.Hamrah P, Chen L, Cursiefen C, Zhang Q, Joyce NC, Dana MR. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) on monocytic bone marrow-derived cells in the conjunctiva. Exp Eye Res (2004) 79:553–61. 10.1016/j.exer.2004.06.028 [DOI] [PubMed] [Google Scholar]
- 96.Xu H, Chen M, Reid DM, Forrester JV. LYVE-1-positive macrophages are present in normal murine eyes. Invest Ophthalmol Vis Sci (2007) 48:2162–71. 10.1167/iovs.06-0783 [DOI] [PubMed] [Google Scholar]
- 97.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One (2009) 4:e6562. 10.1371/journal.pone.0006562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barnes NL, Warnberg F, Farnie G, White D, Jiang W, Anderson E, et al. Cyclooxygenase-2 inhibition: effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br J Cancer (2007) 96:575–82. 10.1038/sj.bjc.6603593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer (2006) 94:1154–63. 10.1038/sj.bjc.6603067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xin X, Majumder M, Girish GV, Mohindra V, Maruyama T, Lala PK. Targeting COX-2 and EP4 to control tumor growth, angiogenesis, lymphangiogenesis and metastasis to the lungs and lymph nodes in a breast cancer model. Lab Invest (2012) 92:1115–28. 10.1038/labinvest.2012.90 [DOI] [PubMed] [Google Scholar]
- 101.Liu H, Yang Y, Xiao J, Lv Y, Liu Y, Yang H, et al. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via VEGF-C. Anat Rec (Hoboken) (2009) 292:1577–83. 10.1002/ar.20940 [DOI] [PubMed] [Google Scholar]
- 102.Bhattacharjee RN, Timoshenko AV, Cai J, Lala PK. Relationship between cyclooxygenase-2 and human epidermal growth factor receptor 2 in vascular endothelial growth factor C up-regulation and lymphangiogenesis in human breast cancer. Cancer Sci (2010) 101:2026–32. 10.1111/j.1349-7006.2010.01647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.YlÖstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells (2012) 30:2283–96. 10.1002/stem.1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q, et al. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol (2012) 7(7):1091–100. 10.1097/JTO.0b013e3182542752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jain RK, Padera TP. Prevention and treatment of lymphatic metastasis by antilymphangiogenic therapy. J Natl Cancer Inst (2002) 94:785–7. 10.1093/jnci/94.11.785 [DOI] [PubMed] [Google Scholar]
- 106.Gudlaugsson E, Skaland I, Undersrud E, Janssen EA, Søiland H, Baak JP. D2-40/p63 defined lymph vessel invasion has additional prognostic value in highly proliferating operable node negative breast cancer patients. Mod Pathol (2011) 24:502–11. 10.1038/modpathol.2010.199 [DOI] [PubMed] [Google Scholar]
- 107.Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg (2004) 240:306–12. 10.1097/01.sla.0000133355.48672.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swartz MA, Skobe M. Lymphatic function, lymphangiogenesis, and cancer metastasis. Microsc Res Tech (2001) 55:92–9. 10.1002/jemt.1160 [DOI] [PubMed] [Google Scholar]
- 109.Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci (2008) 1131:225–34. 10.1196/annals.1413.020 [DOI] [PubMed] [Google Scholar]
- 110.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene (2012) 31:4499–508. 10.1038/onc.2011.602 [DOI] [PubMed] [Google Scholar]
- 111.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell (2002) 1:219–27. 10.1016/S1535-6108(02)00051-X [DOI] [PubMed] [Google Scholar]
- 112.Baldwin ME, Stacker SA, Achen MG. Molecular control of lymphangiogenesis. Bioessays (2002) 24:1030–40. 10.1002/bies.10173 [DOI] [PubMed] [Google Scholar]
- 113.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev (2002) 82:673–700. 10.1152/physrev.00005.2002 [DOI] [PubMed] [Google Scholar]
- 114.Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell (2012) 21:181–95. 10.1016/j.ccr.2011.12.026 [DOI] [PubMed] [Google Scholar]
- 115.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res (2001) 61:1786–90. [PubMed] [Google Scholar]
- 116.Liu H, Yang Y, Xiao J, Lv Y, Liu Y, Yang H, et al. COX-2-mediated regulation of VEGF-C in association with lymphangiogenesis and lymph node metastasis in lung cancer. Anat Rec (Hoboken) (2010) 293:1838–46. 10.1002/ar.21240 [DOI] [PubMed] [Google Scholar]
- 117.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med (2001) 7:192–8. 10.1038/84643 [DOI] [PubMed] [Google Scholar]
- 118.Wang CA, Jedlicka P, Patrick AN, Micalizzi DS, Lemmer KC, Deitsch E, et al. SIX1 induces lymphangiogenesis and metastasis via upregulation of VEGF-C in mouse models of breast cancer. J Clin Invest (2012) 122:1895–906. 10.1172/JCI59858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med (2001) 7:186–91. 10.1038/84635 [DOI] [PubMed] [Google Scholar]
- 120.Katoh H, Hosono K, Ito Y, Suzuki T, Ogawa Y, Kubo H, et al. COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. Am J Pathol (2010) 176:1469–83. 10.2353/ajpath.2010.090607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Su JL, Shih JY, Yen ML, Jeng YM, Chang CC, Hsieh CY, et al. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res (2004) 64:554–64. 10.1158/0008-5472.CAN-03-1301 [DOI] [PubMed] [Google Scholar]
- 122.Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J (2001) 20:4762–73. 10.1093/emboj/20.17.4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res (2006) 66:8065–75. 10.1158/0008-5472.CAN-06-1392 [DOI] [PubMed] [Google Scholar]
- 124.Quagliata L, Klusmeier S, Cremers N, Pytowski B, Harvey A, Pettis RJ, et al. Inhibition of VEGFR-3 activation in tumor-draining lymph nodes suppresses the outgrowth of lymph node metastases in the MT-450 syngeneic rat breast cancer model. Clin Exp Metastasis (2014) 31:351–65. 10.1007/s10585-013-9633-2 [DOI] [PubMed] [Google Scholar]
- 125.Fornetti J, Jindal S, Middleton KA, Borges VF, Schedin P. Physiological COX-2 expression in breast epithelium associates with COX-2 levels in ductal carcinoma in situ and invasive breast cancer in young women. Am J Pathol (2014) 184:1219–29. 10.1016/j.ajpath.2013.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lewis C, Pollard J. Distinct role of macrophages in different tumor microenvironments. Cancer Res (2006) 66(2):605–12. 10.1158/0008-5472.CAN-05-4005 [DOI] [PubMed] [Google Scholar]
- 127.Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, et al. M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas (2013) 42:155–9. 10.1097/MPA.0b013e318254f2d1 [DOI] [PubMed] [Google Scholar]
- 128.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet (2008) 371:771–83. 10.1016/S0140-6736(08)60241-X [DOI] [PubMed] [Google Scholar]
- 129.Burton JB, Priceman SJ, Sung JL, Brakenhielm E, An DS, Pytowski B, et al. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res (2008) 68:7828–37. 10.1158/0008-5472.CAN-08-1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst (2005) 97:14–21. 10.1093/jnci/dji003 [DOI] [PubMed] [Google Scholar]
- 131.Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res (2006) 66:2650–7. 10.1158/0008-5472.CAN-05-1843 [DOI] [PubMed] [Google Scholar]
- 132.Saif WM, Knost JA, Chiorean EG, Kambhampati SRP, Yu D, Pytowski B, et al. Phase I study of anti-VEGF receptor-3 (VEGFR-3) monoclonal antibody (Mab) LY3022856/IMC-3C5 (3C5). J Clin Oncol (2015) 33. [Google Scholar]
- 133.Mross K, Frost A, Scheulen ME, Krauss J, Strumberg D, Schultheiss B, et al. Phase I study of telatinib (BAY 57-9352): analysis of safety, pharmacokinetics, tumor efficacy, and biomarkers in patients with colorectal cancer. Vasc Cell (2011) 3:16. 10.1186/2045-824X-3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marx J. Cancer. Encouraging results for second-generation antiangiogenesis drugs. Science (2005) 308:1248–9. [DOI] [PubMed] [Google Scholar]