Abstract
This study was conducted to evaluate the efficacy of oral administration of fenbendazole (20 mg/kg body weight) prior to and after experimental infection of immunosuppressed rabbits with Encephalitozoon cuniculi. A total of thirty rabbits were divided into five groups: NN (non-immunosuppressed; non-infected), IN (immunosuppressed; non-infected), IPI (immunosuppressed; protected-infected), ITI (immunosuppressed; treated-infected), and II (immunosuppressed; infected) groups. Fenbendazole was administered as a prophylactic for seven successive days before infection with E. cuniculi and as a treatment for four weeks initiated on the 28th day post-challenge (PC). Experimental rabbits were infected with intraperitoneal injection of 2 × 105 E. cuniculi spores. Parameters evaluated were body weight, detection of spores in urine, serum antibody assay, hematological, biochemical and histopathological changes. The IPI and ITI groups showed a significant better final bwt than the II group. Spores were detected in urine of all infected rabbits from the 28th day PC until the end of the study. The IPI group showed the least values of antibodies (IgG) compared to the ITI and II groups. Concerning histopathological changes, the intensity of the lesions was marked particularly in the II rabbits and to a lesser extent in the ITI rabbits. Noticeable improvement was found in the IPI rabbits. It could be concluded that fenbendazole was effective to some extent in protection of rabbits against E. cuniculi infection, while when administered as a therapeutic no significant effects were observed.
Key Words: Encephalitozoon cuniculi, Fenbendazole, Immunocompromised, Rabbits
Introduction
Encephalitozoon cuniculi is an intracellular micros-poridian pathogen of mammals and birds with the rabbit as its main host (Wasson and Peper, 2000 ▶). Most of the knowledge offered now on microsporidia is based on this species (Kotkova et al., 2013 ▶). In rabbits, E. cuniculi causes mainly neurological signs, chronic renal failure or phacoclastic uveitis (Jordan et al., 2006 ▶).
There are currently no standardized treatment protocols, prophylactic method, or effective means of control for encephalitozoonosis in domestic rabbits (Künzel et al., 2008 ▶).
According to Beauvais et al. (1994) ▶, albendazole is considered as the most effective drug against microsporidiosis in humans. However, albendazole is known to be embryotoxic and teratogenic in rabbits (Kotler and Orenstein, 1999 ▶). Fenbendazole, another benzimidazole, has also been revealed to prevent and treat E. cunciuli infections in rabbits (Suter et al., 2001 ▶). Corticosteroids are often used for acute neurological signs associated with E. cuniculi. They appear to be effective and are specified to suppress the inflammatory response associated with cell rupture. However, they are generally not recommended for treatment as the immunosuppressive properties may deteriorate disease and generally have no effect on spore production or shedding (Jeklova et al., 2010 ▶).
The aim of the current study was to investigate the protective and therapeutic effects of fenbendazole in immunocompromised rabbits against E. cuniculi infection. Serological, hematological, biochemical and pathological parameters were used to assess such effects.
Materials and Methods
Fenbendazole
Fenbendazole was kindly provided by Pharmaswede Company. It was orally administered at a daily dose of 20 mg/kg body weight (bwt) (Suter et al., 2001 ▶) to the protection and treatment groups of rabbits.
Dexamethasone
Before infection, rabbits were immunosuppressed by intramuscular injection of dexamethasone (intervet), 2 mg/kg bwt every 72 h for 7 days and which was repeated once a week for the rest of the experimental period (Sheng et al., 1987 ▶).
Parasite
Spores of E. cuniculi (strain ATCC 50503) were given to the experimental rabbits through intraperitoneal (ip) injection at dose of 2 × 105 E. cuniculi spores (Salát et al., 2001 ▶).
Animals and experimental design
Thirty rabbits, 8 weeks of age, were housed in mental cages. Pelleted commercial feed (Ibex Co., Cairo, Egypt) and water were supplied ad libitum. Rabbits received humane care in compliance with the animal care guidelines of the National Institute of Health, and the local ethical committee approved this study. After one week of acclimatization, rabbits were divided into five equal groups: The NN group as a negative control (non-immunosuppressed; non-infected); the IN group as control (immunosuppressed; non-infected); the IPI group was the immunosuppressed protected-infected group; the ITI was the immunosuppressed treated-infected group, and the II group as a positive control (immuno-suppressed; infected). Fenbendazole was administered as a prophylactic agent to the IPI group for seven successive days just before challenge with E. cuniculi. Challenge was performed on the 8th day after administration of fenbendazole. Fenbendazole was administered as a therapeutic agent to the ITI group daily for four weeks initiated on day 28 post-challenge (PC), when infection was confirmed by serology and detection of E. cuniculi spores in urine of infected rabbits. The experimental design is presented in Table 1. Sera of all rabbits were tested before infection for presence of specific anti E. cuniculi antibodies using an enzyme-linked immunosorbent assay (ELISA). The experiment was terminated and all rabbits were slaughtered 8 weeks PC.
Table 1.
Experimental design
| Group | Immunosuppression (Dexamethasone) |
Infection (2 × 105 E. cuniculi spores) |
Fenbendazole (20 mg/kg bwt) |
|
|---|---|---|---|---|
| Protection | Treatment | |||
| NN (Non-immunosuppressed, non-infected), negative control | - | - | - | - |
| IN (Immunosuppressed, non-infected) | √ | - | - | - |
| IPI (Immunosuppressed, protected, infected) | √ | √ | √ | - |
| ITI (Immunosuppressed, treated, infected) | √ | √ | - | √ |
| II (Immunosuppressed, infected), positive control | √ | √ | - | - |
Parameters evaluated
Body weight
At the beginning of the experiment, all rabbits were weighed and there was no appreciable difference in body weight of rabbits in different groups. They were again weighed at the end of the experiment.
Detection of E. cuniculi spores in urine
Urine samples were collected weekly from each group for parasitological examination beginning on the 21st day PC until the end of the experiment. Smears were prepared from sediments of urine samples and were left to dry, then fixed with methanol and stained with Weber’s green Modified Trichrome Stain (MTS). Stained smears were examined microscopically with oil immersion lens (Kokoskin et al., 1994 ▶).
Serum antibody assay
Three rabbits from each group were bled from the ear vein at weekly intervals from the 3rd to 8th week PC. Sera were separated from the clotted blood samples by centrifugation at 2500 g for 10 min and tested for IgG antibodies to E. cuniculi using ELISA to measure the relative levels of specific serum antibodies among different groups.
Preparation of E. cuniculi antigen (Akerstedt, 2002 ▶)
Following three cycles of freezing/thawing, spores of E. cuniculi were sonicated (30 min, 60 W) in BRANSON sonicator. The protein content of the supernatant was estimated according to Lowry et al. (1951) ▶. The soluble antigen solution was stored at -20°C until use.
Reference sera
A negative reference serum was kindly supplied by (Division of Microbiology, Tulane National Primate Research Center, Covington, LA, USA). A positive reference serum was obtained by experimental infection of rabbits with E. cuniculi spores. Sera were collected after 3 weeks post infection (PI), stored at -20°C, and used as the positive control.
ELISA procedures
Rabbit sera were examined by indirect ELISA as described by Akerstedt (2002) ▶. Briefly, polystyrene microliters plates (Immunoplate Maxisorb, Nunc, Roskilde, Denmark) were coated with 50 µL/well soluble antigen at a concentration of 2 μg/ml. The plates were incubated overnight at 4°C, washed with 200 μL PBS/Tween 20, and treated with the blocking solution (3% bovine serum albumin (BSA) with 0.05% Tween 20). A total of 50 μL each of diluted tested rabbit serum, negative control sera, and positive control sera (1:10) were added to each well and incubated at 37°C for 1 h. After incubation, the plates were washed 3 times with 200 μL PBS/0.05% Tween 20. After washing, horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG (KOMABIOTECH) was diluted at 1:5000 in PBS-T and added to the plates (100 μL/well). Finally, the plates were washed 3 times with PBS/0.05% Tween 20, and the enzyme activity of bound peroxidase was revealed by adding 100 μL of ortho-phenylenediamine substrate (OPD) (Laboratories Inc., San Diego, CA, USA) to each well. After incubation in darkness (45 min), the enzymatic color reaction was stopped by adding 100 μL of 1 M phosphoric acid to each well, and the optical density was read at 490 nm using a microplate reader (Corona Electrical, Japan). Cutoff value was calculated according to Crowther (2009) ▶:
Cutoff= Mean of negatives + (3 × SD of negatives)
Median values represent the readings of ELISA reader; each value is the average of 3 readings of 3 individuals for each group.
Hematological and Biochemical assays
At the end of the experiment (8 weeks PC), blood samples were collected during slaughtering from all groups. Some blood samples were collected in tubes containing dipotassium salt of EDTA as anticoagulant and used for evaluation of hematological parameters. Another part of samples was placed in plain centrifuge tubes and centrifuged at 3000 rpm for 15 min for separation of serum. The clear serum was separated and kept at -20°C for biochemical analysis. The hematological parameters including: red blood cells (RBCs) count, packed cell volume (PCV%), hemoglobin (Hb) concentration, the erythrocytic indices (mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC)), total leukocytic count (TLC) and the differential leukocytic counts were estimated automatically using a veterinary hematology analyzer (boule medical for multispecies veterinary applications, Stockholm, Sweden). The serum activities of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were estimated colorimetrically according to Reitman and Frankel (1957) ▶. The concentration of blood urea was colorimetrically assayed according to the method described by Tabacco et al. (1979) ▶ and creatinine was determined by colorimetric kinetic method according to Fabiny and Ertingshausen (1971) ▶.
Postmortem and histopathological examinations
At the end of the study, gross pathological examination was performed and tissue specimens were collected from the brain, kidneys, liver, lungs, intestines, heart and spleen. These tissue specimens were fixed in 10% formalin for 24 h and processed by paraffin embedding technique. Five µm-thick sections were stained by hematoxylin and eosin (H&E) (Bancroft and Stevens, 1996 ▶).
Statistical analysis
Data were analyzed using the GLM procedure of the Statistical Analysis System software (SAS, 2002 ▶). The analysis model included the effect of group. Means were compared using the least squares means (LSM) of the same program, and the level of significance was P<0.05.
Results
Clinical diagnosis
No clinical signs and no mortalities were recorded in all experimental animals throughout the study. Encephalitozoon cuniculi infection significantly reduced the final bwt of all infected groups compared to the NN group. The reduction in growth was more pronounced in the II and IN groups. However, the IPI group showed significantly better final bwt than the ITI group (Table 2).
Table 2.
Effect of administration of fenbedazole on body weight, hematological and serum biochemical parameters in different groups of rabbits
| Parameter | Groups |
||||
|---|---|---|---|---|---|
| NN | IN | IPI | ITI | II | |
| Initial body weight (g) | 1783 ± 40.1a | 1788 ± 42.7a | 1792 ± 45.5a | 1783 ± 33.3a | 1783 ± 38.0a |
| Final body weight (g) | 2912 ± 91.6a | 2480 ± 120.0bc | 2675 ± 47.9ab | 2535 ± 70.3bc | 2361 ± 95.2c |
| WBCs (103/ml) | 8.50 ± 0.35ab | 5.17 ± 0.03b | 5.67 ± 0.61b | 6.20 ± 1.15b | 10.07 ± 1.76a |
| RBCs (106/ml) | 6.20 ± 0.29a | 5.73 ± 0.03a | 5.60 ± 0.35a | 6.10 ± 0.17a | 6.10 ± 0.06a |
| Hemoglobin (g/dl) | 12.80 ± 0.52a | 11.40 ± 0.12b | 11.90 ± 0.46ab | 12.37 ± 0.09ab | 12.67 ± 0.15a |
| PCV (%) | 41.80 ± 0.46a | 39.77 ± 0.32a | 38.67 ± 1.88a | 40.40 ± 0.64a | 41.20 ± 0.17a |
| MCV (fL) | 67.64 ± 2.41a | 69.25 ± 0.34a | 69.22 ± 1.12a | 66.31 ± 0.56a | 67.50 ± 0.45a |
| MCH (pg) | 20.66 ± 0.12ab | 19.86 ± 0.14b | 21.34 ± 0.55a | 20.28 ± 0.35b | 20.72 ± 0.01ab |
| MCHC (g/dl) | 30.60 ± 0.92a | 28.70 ± 0.06b | 30.83 ± 0.32a | 30.57 ± 0.26a | 30.70 ± 0.23a |
| Lymphocytes (103/ml) | 5.11 ± 0.36a | 2.15 ± 0.03c | 2.25 ± 0.26c | 1.87 ± 0.07c | 3.50 ± 0.11b |
| Monocytes (103/ml) | 0.60 ± 0.04a | 0.40 ± 0.00a | 0.39 ± 0.12a | 0.45 ± 0.09a | 0.63 ± 0.07a |
| Granulocytes (103/ml) | 2.81 ± 0.24b | 2.60 ± 0.00b | 3.01 ± 0.23ab | 3.88 ± 1.07ab | 5.92 ± 1.66a |
| Lymphocytes (%) | 60.00 ± 1.73a | 41.77 ± 0.32b | 39.70 ± 0.35b | 32.47 ± 6.03b | 37.00 ± 6.12b |
| Monocytes (%) | 7.10 ± 0.17a | 7.77 ± 0.03a | 6.67 ± 1.36a | 7.20 ± 0.06a | 6.40 ± 0.40a |
| Granulocytes (%) | 32.90 ± 1.44b | 50.50 ± 0.29a | 53.67 ± 1.70a | 60.37 ± 5.98a | 56.60 ± 6.52a |
| AST (U/L) | 30.70 ± 2.14b | 31.60 ± 1.10b | 30.57 ± 1.65b | 33.37 ± 1.13b | 47.40 ± 2.66a |
| ALT (U/L) | 28.40 ± 1.67b | 30.30 ± 0.69b | 39.00 ± 1.44a | 41.87 ± 0.09a | 39.87 ± 0.43a |
| Urea (mg/dl) | 36.80 ± 1.67a | 37.60 ± 1.27a | 37.50 ± 1.21a | 39.47 ± 2.22a | 39.70 ± 1.10a |
| Creatinine (mg/dl) | 1.30 ± 0.06b | 1.40 ± 0.06ab | 1.30 ± 0.06b | 1.40 ± 0.00ab | 1.50 ± 0.06a |
Urine examination
Microscopic examination of MTS-stained smears showed spores of E. cuniculi as oval bodies with pink outline, central vacuole and polar eminent. All infected groups of rabbits showed spores in their urine smears. The first spore output in urine was observed on the 28th PC and spores were shed until the end of the study in all experimentally infected groups.
Serology
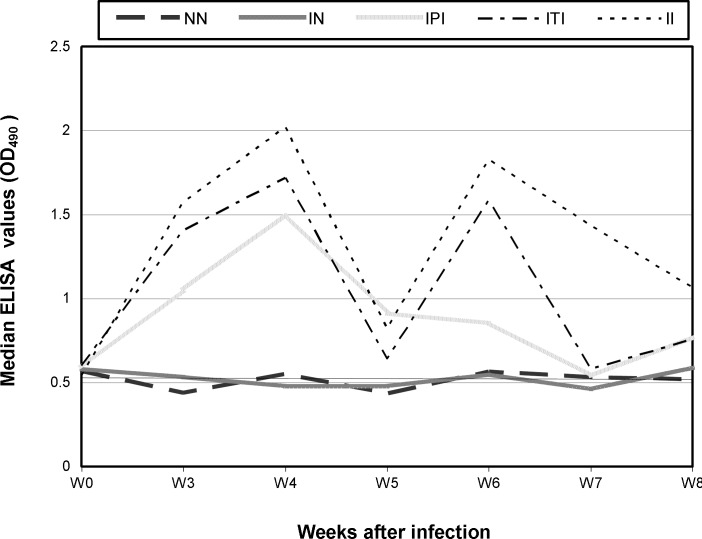
Median ELISA values are given in Fig. 1. High levels of specific antibodies to E. cuniculi were demonstrated in sera of infected rabbits from the 21st day PC till the end of the experiment. The circulating antibody response was episodic response in all infected groups; where there was a rise of ELISA values from the 3rd week PC, reached the highest around the 4th week and then decreased to the minimum at the 5th week. Thereafter, they retain a second peak at the 6th week and then decreased at the 7th week and until the end of the experiment. The levels of antibodies were the highest in the II rabbits and lower to some extent in the ITI rabbits. The IPI group showed the lowest ELISA values compared to the ITI and II groups and reached the normal level around the 7th week. The antibody values of NN and IN groups remained at the preinoculation level throughout the trial.
Fig. 1.
Humoral antibody response in different groups of experimental rabbits after infection with E. cuniculi. NN: Non-immunosuppressed non-infected group, IN: Immunosuppressed non-infected group, IPI: Immunosuppressed protected-infected group, ITI: Immunosuppressed treated-infected group and II: Immunosuppressed infected group. W: Week and OD: Optical density
Hematological and biochemical assays
Results of hematological and serum biochemical profiles for all groups of experimental animals at the end of the experiment are shown in Table 2. The levels of blood lymphocytes in dexamethasone-immunosuppress-ed groups (IN, IPI, ITI, and II) of rabbits significantly decreased compared to NN group. No significant difference in PCV%, Hb content and RBCs counts was observed in E. cuniculi infected protected or treated rabbits. Monocyte values were comparable in all groups while granulocytes percentage significantly increased in all treated groups compared to NN group. Encepha-litozoon cuniculi infection significantly enhanced serum ALT enzyme activities compared to the NN and IN groups. While values of serum AST enzyme were improved in the IPI and ITI groups compared to the II group. The serum urea levels in all treated groups exhibited non-significant changes when compared to NN group. The serum creatinine level was significantly elevated only in the II group.
Postmortem findings
At necropsy, lungs of the ITI and II rabbits were heavy, meaty in appearance and failed to collapse. Kidneys of II and ITI rabbits were pale with adherence of the capsule to the parenchyma and presence of numerous subcapsular pits on their surface or few whitish foci, however, kidneys of IPI rabbits showed a few subcapsular pits on their surface.
Histopathological findings
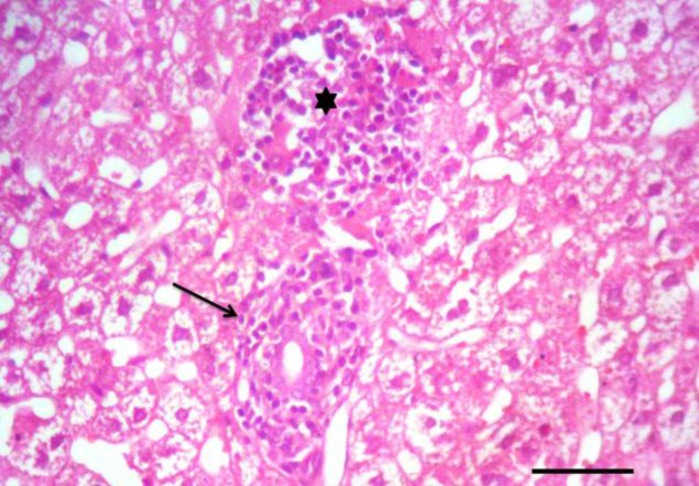
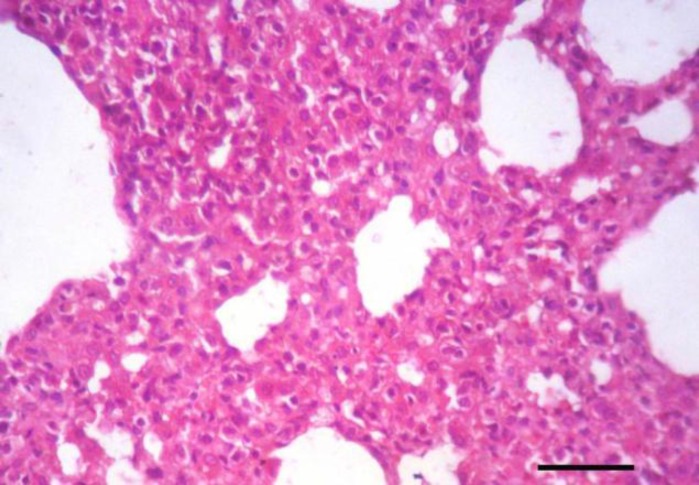
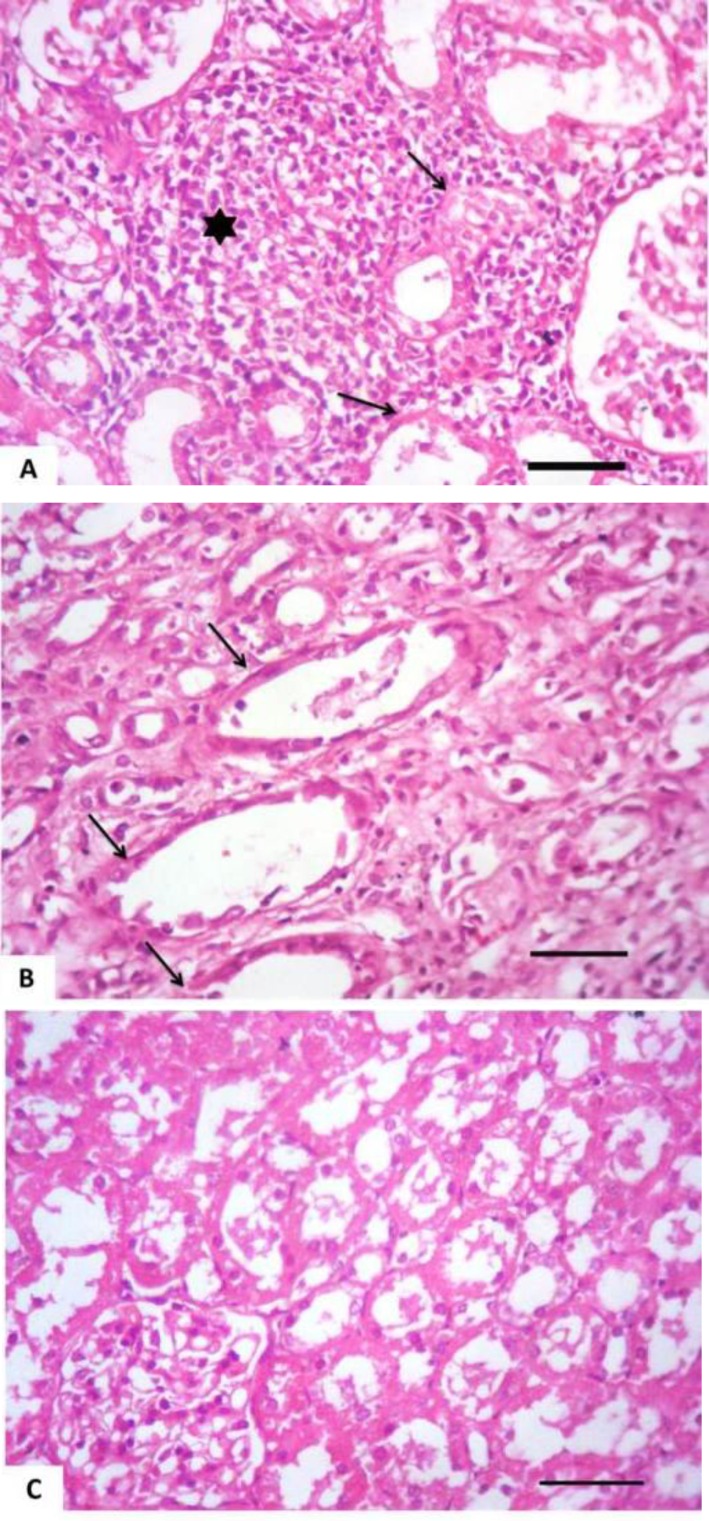
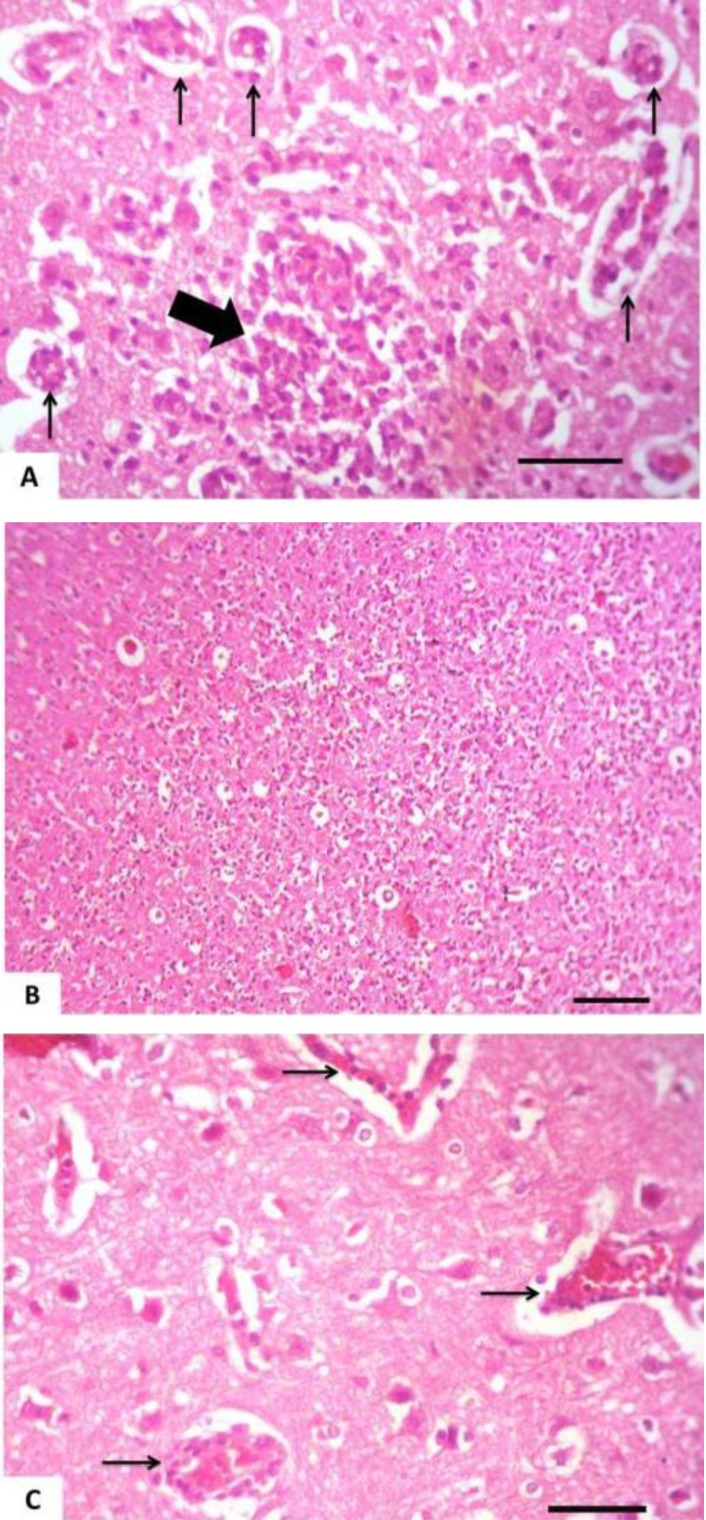
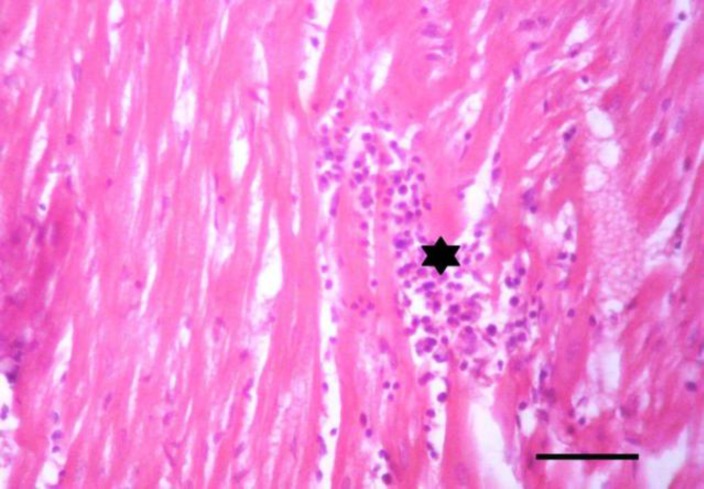
Tissue specimens of organs collected from the NN and IN rabbits had nearly normal histologic structure. Incidence and severity of histopathological lesions of the experimental groups are summarized in Table 3. The intensity of the lesions was high particularly in the II rabbits and to a lesser extent in the ITI rabbits. A Values are means ± SE. WBCs: White blood cells count, RBCs: Red blood corpuscles count, PCV: Packed cell volume, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, AST: Aspartate aminotransferase, and ALT: Alanine aminotransferase. NN: Non-immunosuppressed non-infected group, IN: Immunosuppressed non-infected group, IPI: Immunosuppressed protected-infected group, ITI: Immunosuppressed treated-infected group, and II: Immunosuppressed infected group. Means in the same row without a common letter differ significantly (P<0.05) noticeable improvement was found in the IPI rabbits. Liver of the II rabbits showed moderate portal hepatitis and few focal areas of hepatocytic coagulative necrosis associated with mononuclear cell infiltrates (Fig. 2). Liver of the IPI and ITI rabbits showed moderate to marked dilatation of hepatic sinusoids and veins and mild portal mononuclear cell infiltrates without occurrence of hepatic necrosis. Pulmonary lesions were similar in severity in both II and ITI groups (Fig. 3) and less severe in IPI rabbits. These lesions consisted of thickening of the interalveolar septa with mononuclear cell infiltrates and some alveoli contained necrotic epithelial cells accompanied by alveolar macrophages. Hyperplasia of bronchial associated lymphoid tissue (BALT) with or without bronchial epithelial necrosis and desquamation were noticed. Kidneys of both II and ITI rabbits showed multifocal tubular necrosis with epithelial cell desquamation accompanied by lymphocytic interstitial infiltrates (Fig. 4A) and fibrosis (Fig. 4B). Renal lesions were mild in the IPI group and consisted of few focal areas of tubular necrosis with mild interstitial lymphocytic cell infiltrations. Most of the renal parenchyma in IPI rabbits was not affected by the parasite (Fig. 4C). Brain lesions of the II rabbits were minute multifocal cerebral granulomas (Fig. 5A) with prominent diffuse gliosis (Fig. 5B) and perivascular cuffs were clear in the cerebrum and meninges. Fenbendazole treatment ameliorated cerebral lesions to some extent (Fig. 5C) and no significant cerebral lesions were observed in the prophylactic group of rabbits. Moreover, there were multifocal aggregates of lymphocytes within the myocardium of group II rabbits (Fig. 6) and to a lesser extent ITI rabbits showed focal lymphocytic aggregates that disappeared in IPI rabbits. Spleen of the experimental animals showed normal histologic limits.
Table 3.
Incidence and severity of histopathological lesions in the liver, lungs, kidneys, brain and heart of the experimental groups
| Organ/lesion | Incidence * and severity † of histopathological lesions |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPI |
ITI |
II |
||||||||||
| Absent (-) |
Mild (+) |
Moderate (++) |
Severe (+++) |
Absent (-) |
Mild (+) |
Moderate (++) |
Severe (+++) |
Absent (-) |
Mild (+) |
Moderate (++) |
Severe (+++) |
|
| Lymphocytic aggregates | 6 | 0 | 0 | 0 | 4 | 2 | 0 | 0 | 4 | 0 | 2 | 0 |
| I. Liver | ||||||||||||
| Congestion | 1 | 1 | 4 | 0 | 0 | 0 | 2 | 4 | 0 | 0 | 6 | 0 |
| Portal mononuclear cell infiltrates | 3 | 3 | 0 | 0 | 2 | 4 | 0 | 0 | 1 | 1 | 4 | 0 |
| Hepatocytic coagulative necrosis | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 3 | 0 | 3 | 0 |
| II. Lungs | ||||||||||||
| Thickened interalveolar septa | 1 | 1 | 4 | 0 | 0 | 1 | 1 | 4 | 0 | 0 | 1 | 5 |
| Hyperplasia of BALT | 0 | 3 | 3 | 0 | 0 | 1 | 2 | 3 | 0 | 0 | 3 | 3 |
| Bronchial epithelial necrosis | 3 | 2 | 1 | 0 | 2 | 1 | 3 | 0 | 0 | 0 | 3 | 3 |
| III. Kidneys | ||||||||||||
| Renal tubular necrosis | 0 | 4 | 2 | 0 | 0 | 1 | 4 | 1 | 0 | 0 | 1 | 5 |
| Interstitial lymphocytic cell infiltrates | 0 | 4 | 2 | 0 | 0 | 1 | 2 | 3 | 0 | 0 | 0 | 6 |
| Interstitial fibrosis | 3 | 3 | 0 | 0 | 0 | 1 | 3 | 2 | 0 | 0 | 2 | 4 |
| IV. Brain | ||||||||||||
| Cerebral granulomas | 6 | 0 | 0 | 0 | 4 | 2 | 0 | 0 | 2 | 0 | 4 | 0 |
| Diffuse gliosis | 6 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 1 | 0 | 2 | 3 |
| Perivascular cuffs | 6 | 0 | 0 | 0 | 0 | 1 | 3 | 2 | 0 | 0 | 0 | 6 |
| V. Heart | ||||||||||||
Number of rabbits with lesions per total examined (6 rabbits per group). IPI: Immunosuppressed protected-infected group, ITI: Immunosuppressed treated-infected group and II: Immunosuppressed infected group. † Severity of lesions: (−) absence of the lesion= 0%, (+) mild= 1–10%, (++) moderate= 11–59%, and (+++) severe= 60% of the examined tissue sections
Fig. 2.
Liver of an immunosuppressed rabbit infected with E. cuniculi. Portal mononuclear cell infiltration (arrow) and focal periportal hepatic necrosis with mononuclear cell infiltrates (asterisk), (H&E, Bar= 50 µm
Fig. 3.
Lung of an immunosuppressed rabbit infected with E. cuniculi after 28 days treatment with fenbendazole. Marked thickening of the interalveolar septa with mononuclear cell infiltrates, (H&E, Bar=50 µm
Fig. 4.
Kidney of an immunosuppressed rabbit infected with E. cuniculi. (A) Cortical tubular necrosis (arrows) with epithelial cell desquamation that accompanied by lymphocytic interstitial infiltrates (asterisk), (B) 28 days fenbendazole treatment. Cortical tubular necrosis (arrows) and interstitial fibrosis, (C) Seven successive days prophylaxis with fenbendazole; the majority of the renal parenchyma nearly normal except lumina of some renal tubules contained eosinophilic material, (H&E, Bar= 50 µm
Fig. 5.
Brain of an immunosuppressed rabbit infected with E. cuniculi. (A) Cerebral granuloma (thick arrow) with lymphocytic perivascular cuffing (thin arrows), (B) Prominent diffuse gliosis, (C) 28 days fenbendazole treatment. Lymphocytic perivascular cuffing (arrows), (H&E, Bar (A), (C) = 50 µm, (B) = 100 µm
Fig. 6.
Heart of an immunosuppressed rabbit infected with E. cuniculi. Lymphocytic aggregates within the myocardium (asterisk), (H&E, Bar= 50 µm
Discussion
Fenbendazole as one of benzimidazoles block certain metabolic pathways of parasites, such as the transport and uptake of glucose, without affecting the host (Praag, 2011).
In the present study, E. cuniculi infection significantly reduced the final bwt of all infected groups compared to the NN group. Weight loss may be due to subclinical neurological or renal disease caused by chronic granulomatous lesions (Harcourt-Brown, 2005 ▶) or chronic azotemia that lead to weight loss and emaciation despite a normal dietary intake (Csokai et al., 2009 ▶). However, the protected group of rabbits (IPI) showed significantly better final bwt than ITI and II groups. These results agreed with those of Suter et al. (2001) ▶. ELISA was used to confirm infection with E. cuniculi as serological examination has been established as a reliable diagnostic test for E. cuniculi infection (Boot et al., 2000 ▶). The median values of antibodies were high in infected rabbits suggesting intense spore multiplication. The change in these values can provide a rough estimation of the progression of microsporidiosis (Garcia, 2002 ▶). In our study, the circulating antibody response was episodic. This observation agreed with those recorded by Kunstyr et al. (1986) ▶ who observed three types of responses; short, long and episodic. They explained this by the probable genetic heterogeneity of random-bred rabbits and by individual differences in responsiveness. Moreover, inconsistent IgG pattern and differing responses may be influenced by E. cuniculi exposure load and individual variation in immune response (Latney et al., 2014 ▶). Nevertheless, the protected group of rabbits (IPI) showed lower values of IgG antibodies followed by the ITI and finally the II groups. These results agreed with those of Suter et al. (2001) ▶.
Regarding the hematological examination, the lymphocytic counts were significantly decreased in all treated groups in comparison with the NN group, indicating a depression of cell mediated immunity evoked by administration of dexamethasone and/or E. cuniculi infection. Heterophil percentages were increased in infected rabbits since they are the initial responders to numerous pathogens and irritants (Redmond et al., 2009 ▶) and to adapt stress condition (Aengwanich, 2007 ▶). Additionally, E. cuniculi infection significantly enhanced serum ALT and AST enzymes activities parallel with the subsequent detrimental alterations in the hepatic tissues and biliary system. The significant elevation of the serum creatinine level in II group could be attributed to the marked renal lesions induced by the parasite.
Regarding the histopathological findings, the intensity of the lesions was high particularly in the II rabbits and to a lesser extent in the ITI rabbits. Encephalitozoon cuniculi infection in rabbits usually follows a chronic course, taking weeks to months to develop a significant parasite burden which may or may not lead to clinical symptoms. Firstly, tissue alterations were present in the kidney, liver and lung while the brain is not affected at the beginning, but after about 3 months, the most significant alterations are seen in the kidneys and the brain. At that time, lesions disappear in lung and liver, and parasites are usually absent from these organs. The heart can also be involved, although usually to a minor extent (Cox et al., 1979 ▶). In our study, the hepatic lesions were similar to those recorded by Fuentealba et al. (1992) ▶ and pulmonary lesions were agreed with Anete Lallo et al. (2013) ▶. The classic histopathological findings in rabbits were noticed and consisted of chronic interstitial nephritis and granulomatous meningoence-phalitis. These findings were established by Csokai et al. (2009) ▶. A noticeable improvement was found in the IPI rabbits.
In conclusion, the oral administration of fenbenda-zole prior to experimental infection was effective to some extent in protection of rabbits against E. cuniculi infection, while when administered as a therapeutic no significant effects were observed.
Acknowledgements
The study was funded by the Alexandria University Research Fund (AURF), Grants, Innovation and Technology Transfer Center (GITTC), Research Enhancement Program (ALEX REP), 2011-2012 (project code, AGRV-11). The authors gratefully thank Dr. Esther van de Ven, QM Diagnostics (231QM), Nijmegen, Netherlands for supplying E. cunniculi spores and Lisa Bowers (Division of Microbiology, Tulane National Primate Research Center, Covington, LA, USA) for providing a negative reference serum.
References
- Aengwanich W. Comparative ability to tolerate heat between Thai indigenous chicken, Thai indigenous chicken crossbred and broilers by using heterophil/lymphocyte ratio. Pak. J. Biol. Sci. 2007;10:1840–1844. doi: 10.3923/pjbs.2007.1840.1844. [DOI] [PubMed] [Google Scholar]
- Akerstedt J. An indirect ELISA for detection of Encephalitozoon cuniculi infection in farmed blue foxes (Alopex lagopus) Acta Vet. Scand. 2002;43:211–220. doi: 10.1186/1751-0147-43-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anete Lallo M, Vidoto da Costa LF, Manoel de Castro J. Effect of three drugs against Encepha-litozoon cuniculi infection in immunosuppressed mice. Antimicrob. Agents Chemother. 2013;57:3067–3071. doi: 10.1128/AAC.00157-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft JD, Stevens A. Theory and practice of histological technique. 4th Edn. New York: Churchill Livingstone; 1996. pp. 391–410. [Google Scholar]
- Beauvais B, Sarfati C, Challier S, Derouin F. In vitro model to assess effect of antimicrobial agents on Encephalitozoon cuniculi. Antimicrob. Agents Chemother. 1994;38:2440–2448. doi: 10.1128/aac.38.10.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot R, Hansen CK, Nozari N, Thuis HC. Comparison of assays for antibodies to Encephalitozoon cuniculi in rabbits. Lab. Anim. 2000;34:281–289. doi: 10.1258/002367700780384726. [DOI] [PubMed] [Google Scholar]
- Cox JC, Hamilton RC, Attwood HD. An investigation of the route and progression of Encepha-litozoon cuniculi infection in adult rabbits. J. Protozool. 1979;26:260–265. doi: 10.1111/j.1550-7408.1979.tb02776.x. [DOI] [PubMed] [Google Scholar]
- Crowther JR. The ELISA guidebook. Series: methods in molecular biology. 2nd Edn. Totowa, New Jersey: Humana Press; 2009. pp. 516–566. [Google Scholar]
- Csokai J, Gruber A, Kunzel F, Tichy A, Joachim A. Encephalitozoonosis in pet rabbits (Oryctolagus cuniculus): pathohistological findings in animals with latent infection versus clinical manifestation. Parasitol. Res. 2009;104:629–635. doi: 10.1007/s00436-008-1239-2. [DOI] [PubMed] [Google Scholar]
- Fabiny DL, Ertingshausen G. Automated reaction-rate method for determination of serum creatinine with certificated chemicals. Clin. Chem. 1971;17:696–700. [PubMed] [Google Scholar]
- Fuentealba IC, Mahoney NT, Shadduck JA, Harvill J, Wicher V, Wicher K. Hepatic lesions in rabbits infected with Encephalitozoon cuniculi adminis-tered per rectum. Vet. Pathol. 1992;29:536–540. doi: 10.1177/030098589202900608. [DOI] [PubMed] [Google Scholar]
- Garcia LS. Laboratory identification of the micros-poridia. J. Clin. Microbiol. 2002;40:1892–1901. doi: 10.1128/JCM.40.6.1892-1901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt-Brown, F. Encephalitozoon cuniculi in pet rabbits; 56th International Congress; SCIVAC, Rimini, Italy. 2005. [DOI] [PubMed] [Google Scholar]
- Jeklova E, Jekl V, Kovarcik K, Hauptman K, Koudela B, Neumayearova H, Knotek Z, Faldyna M. Usefulness of detection of specific IgM and IgG antibodies for diagnosis of clinical encephalitozoonosis in pet rabbits. Vet. Parasitol. 2010;170:143–148. doi: 10.1016/j.vetpar.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Jordan C, Zajac AM, Lindsay DS. Encepha-litozoon cuniculi infection in rabbits. Comp. Cont. Educ. Pract. 2006;28:108–116. [Google Scholar]
- Kokoskin E, Gyorkos TW, Camus A, Cedilotte L, Purtill T, Ward B. Modified technique for efficient detection of Microsporidia. J. Clin. Microbiol. 1994;32:1074–1075. doi: 10.1128/jcm.32.4.1074-1075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotkova M, Sak B, Kvetonova D, Kvac M. Latent microsporidiosis caused by Encephalitozoon cuniculi in immunocompetent hosts: a murine model demonstrating the ineffectiveness of the immune system and treatment with albendazole. Plos One. 2013;8:1–7. doi: 10.1371/journal.pone.0060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler DP, Orenstein JM. Clinical syndromes associated with microsporidiosis. In: Wittner M, Weiss LM, editors. The microsporidia and microsporidiosis. Washington D.C.: American Society of Microbiology Press; 1999. pp. 258–292. [Google Scholar]
- Kunstyr I, Lev L, Naumann S. Humoral antibody response of rabbits to experimental infection with Encephalitozoon cuniculi. Vet. Parasitol. 1986;21:223–232. doi: 10.1016/0304-4017(86)90048-8. [DOI] [PubMed] [Google Scholar]
- Künzel F, Gruber A, Tichy A, Edelhofer R, Nell B, Hassan J, Leschnik M, Thalhammer JG, Joachim A. Clinical symptoms and diagnosis of encepha-litozoonosis in pet rabbits. Vet. Parasitol. 2008;151:115–124. doi: 10.1016/j.vetpar.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Latney LV, Bradley CW, Wyre NR. Encephalitozoon cuniculi in pet rabbits: diagnosis and optimal management. Vet. Med. Res. Rep. 2014;5:169–180. doi: 10.2147/VMRR.S49842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Praag EV. Various treatment options for Encepha-litozoon cuniculi, a protozoal parasite of the nervous system in rabbits? 2011. Available from http://www.medirabbit.com/EN/Neurology/cuniculi/pyrimethamine.htm.
- Redmond SB, Chuammitri P, Andreasen CB, Palić D, Lamont SJ. Chicken heterophils from commercially selected and non-selected genetic lines express cytokines differently after in vitro exposure to Salmonella enteritidis. Vet. Immunol. Immunopathol. 2009;132:129–134. doi: 10.1016/j.vetimm.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Reitman S, Frankel S. A coloremetric method for determination of oxaloacetic transaminase and serum glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Salát J, Braunfuchsová P, Kopecky J. Experimental infection of immunocompetent and immuno-deficient mice with Encephalitozoon cuniculi. Folia Parasitol. 2001;48:249–254. [PubMed] [Google Scholar]
- SAS. Statistical Analysis System. Version 9. Cary NC, USA: SAS Institute Inc.; 2002. [Google Scholar]
- Sheng FC, Freischlag JA, Backstrom B, Kelly D, Busuttil RW. The effect of dexamethasone in vivo on blood and peritoneal neutrophils (PMN) in rabbits with peritonitis. J. Surg. Res. 1987;43:296–301. doi: 10.1016/0022-4804(87)90084-9. [DOI] [PubMed] [Google Scholar]
- Suter C, Müller-Doblies UU, Deplazes P, and Hatt JM. Prevention and treatment of Encephalitozoon cuniculi infection in rabbits with fenbendazole. Vet. Rec. 2001;148:478–480. doi: 10.1136/vr.148.15.478. [DOI] [PubMed] [Google Scholar]
- Tabacco A, Meiattini F, Moda E, and Tarli E. Simplified enzymatic/colorimetric serum urea nitrogen determination. Clin. Chem. 1979;25:336–337. [PubMed] [Google Scholar]
- Wasson K, Peper RL. Mammalian micros-poridiosis. Vet. Pathol. 2000;37:113–128. doi: 10.1354/vp.37-2-113. [DOI] [PubMed] [Google Scholar]