Abstract
Yolk sac infection (YSI) and dead-in-shell mortality caused by Enterobacteriaceae in birds are not a rare phenomenon, however there are only a few reports indicating the association between these conditions and Klebsiella spp. among canary chicks (Serinus canaria). There have been reports of high mortality among 1-3 day old canary chicks in an indoor flock of canaries. In order to study the causative agent, yolk sac samples from dead-in-shell and day-old canary chicks were cultured. Klebsiella pneumonia was isolated and identified based on biochemical tests and using genus and species-specific multiplex PCR and later tested for their susceptibility to 13 antimicrobial agents. The isolates showed susceptibility to Gentamycin, Chloramphenicol, Florfenicol and Streptomycin.
Key Words: Antimicrobial susceptibility, Common canary (Serinus canaria), Klebsiella pneumoniae, Yolk sac infection
Introduction
Yolk retention and yolk sac infection (YSI) are considered as important causes of death in different domesticated birds such as chicken, guinea fowl, duck, turkey, quail and goose. Both YSI and dead-in-shell occur in chicks a few days after hatching, which result in decreased hatchability and increased mortality. Members of the Enterobacteriaceae family, such as Escherichia coli, Salmonella spp. and Klebsiella spp., along with other bacteria such as Staphylococcus, Pseudomonas and Clostridia species, and also Aspergillus fumigatus are common causes of YSI and dead-in-shell (Khan et al., 2004 ▶). Klebsiella pneumoniae is a bacillus, non-motile, oxidase negative, rod shape bacteria with a poly-saccharide based capsule, a late lactose fermenting organism and a member of the Enterobactriacea family that is a Gram-negative. This bacterium is a common saprophyte in many parts of the environment (Ryan and Ray, 2004 ▶) and it can be found everywhere in nature (Bagley, 1985 ▶). Klebsiella infections can cause a wide range of diseases in humans such as pneumonia, urinary tract infections, septicemia, meningitis, diarrhea, and soft-tissue infections, especially in those with weak immunity (Bagley, 1985 ▶; Podschun and Ullmann, 1998 ▶). They can also participate in the pathogenesis of the spondylo-arthropathies (Podschun and Ullmann, 1998 ▶). This bacterium, however, is an opportunistic pathogen that has been implicated in cases of mastitis (Braman et al., 1973 ▶), metritis (Brown et al., 1979 ▶), bacteraemia (Fecteau et al., 1997 ▶), pneumonia and urinary tract infections (Roberts et al., 2000 ▶), pneumonia and septicemia (Wilson and Madigan, 1989 ▶) and poly arthritis in cattle, mares, calves, dogs, foals and in humans, respectively. Additionally, Klebsiella can acquire resistance to multiple antibiotics and is an important cause of nosocomial wound and urinary tract infections of hospitalized humans and animals. Klebsiella species occasionally cause embryonic mortality and excess losses in young chickens and turkeys (Orajaka and Mohan, 1985 ▶).
Case description
An indoor aviculturist (250 canaries) reported dead-in-shell (10 of 24 eggs) and high mortality in his 1-3 day old chicks (11 of 26 chicks). They were depressed, unable to move and eat, showed abdominal distention and were found dead in the first three days after hatching. The breeding facility was located indoors, and the birds were kept in pairs in breeding cages (50 × 40 × 40 cm) with artificial lighting and used newspaper on the bottom of the cages as the substrate. The bird feeds mainly on seeds including: regular and traditional seed mixture (millets and linseed), soft food, cuttlefish bone, grit and conditioning seeds which are changed regularly.
Dead-in-shell and 1-3 day old canary chicks were brought to the Avian Medicine Division of the Veterinary Faculty of Ferdowsi University of Mashhad for further investigation. Necropsy revealed a distended yolk sac in chicks, that was hyperemic and yellow-greenish watery. There were signs of septicemia, including petechial hemorrhages on the serosal surface of visceral organs, enlargement of subcutaneous blood vessels and congestion and enlargement of the lungs, liver and kidneys.
Swabs were taken from yolk sac and liver of the two canaries for bacterial isolation. Samples were enriched in nutrient broth anaerobically/aerobically for 24-48 h at 37°C then cultured on Blood/MacConkey’s agar aerobically at 37°C for 24-48 h. Another 24 h of incubation at 37°C on MacConkey’s agar was done in order to purify the suspected colonies. Bacterial isolates were identified as Klebsiella spp. by non automated biochemical tests (Table 1).
Table 1.
List of primers used in this study
| Primer name | Sequence (5´-3´) | Target gene | Product size (bp) |
|---|---|---|---|
| KP (F) | CAA CGG TGT GGT TAC TGA CG | rpoB | 108 |
| (R) | ΤCΤ ΑCG ΑΑG ΤGG CCG ΤΤΤ ΤC | ||
| KO (F) | GAT ACG GAG TAT GCC TTT ACG GTG | pehX | 343 |
| (R) | TAG CCT TTA TCA AGC GGA TAC TGG | ||
| Kleb. (F) | CGC GTA CTA TAC GCC ATG AAC GTA | gyrA | 441 |
| (R) | ACC GTT GAT CAC TTC GGT CAG G |
Klebsiella isolates were recovered from dead-in-shell embryo and YSI and their susceptibility to a panel of 13 antimicrobial agents, determined by the agar disk-diffusion method, and also the interpretations of the results, were carried out according to the National Committee for Clinical Laboratory Standards Guidelines (CLSI)(1). The tested antimicrobial agents and their concentrations (μg) were as follows: ciprofloxacin (5), enrofloxacin (5), Chloramphenicol (30), cefazolin (30), cefixime (5), ampicillin (10), erythromycin (15), kanamycin (30), streptomycin (30), amikacin (30), gentamicin (10), florfenicol (30), and oxytetracycline (30). All anti-bacterial disks were purchased from Padtan Teb Co. (Tehran, Iran). The resistance of the isolates against antimicrobial agents was classified as susceptible, intermediate and resistant, based on the standard interpretation chart updated according to the current CLSI standard (CLSI, 2014).
The isolates were subjected to multiplex PCR to confirm biochemical test results by using Chander et al. (2011 ▶) method with some modification. The DNA was extracted from single colony using an extraction kit (Bioneer, South Korea) according to the manufacturer’s instructions. Briefly, 200 µL of the sample suspension was incubated with 200 µL of lysis buffer and 10 µL proteinase K at 65°C for 30 min. After incubation, 250 µL of binding buffer and 250 µL of ethanol, 80% were added to the lysate. The sample was then washed following the manufacturer’s recommendations. Nucleic acid was eluted with 100 µL of elution buffer provided in the kit (Chander et al., 2011 ▶).
Briefly, the PCR reaction mixture consisted of 5 μL template DNA, 12.5 μL Taq DNA Polymerase Master Mix RED kit (Ampliqon, Denmark) which contained Tris-HCl 150 mM pH = 8.5, (NH4)2SO4 40 mM, MgCl2 1.5 mM, 0.2% Tween 20, dNTPs 0.4 mM, Ampliqon Taq DNA Polymerase (0.05 Unit/ul), 1 μL (10 pmol/μL) of each forward and reverse of primers (Table 1) and distilled water to the final volume of 25 μL. Thermal cycling (Techne TC, 3000, England) was carried out at 95°C for 3 min followed by 35 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 1 min, and a final extension at 72°C for 10 min which terminated the PCR reaction (Chander et al., 2011 ▶). The PCR products were analyzed by 1% agarose gel and ethidium bromide staining followed by visualization with ultraviolet transillumi-nation 5 μL PCR products. All reactions contain positive control’s K. pneumoniae and Klebsiella oxytoca, kindly provided by Dr. M. Rad and Distilled water as negative control.
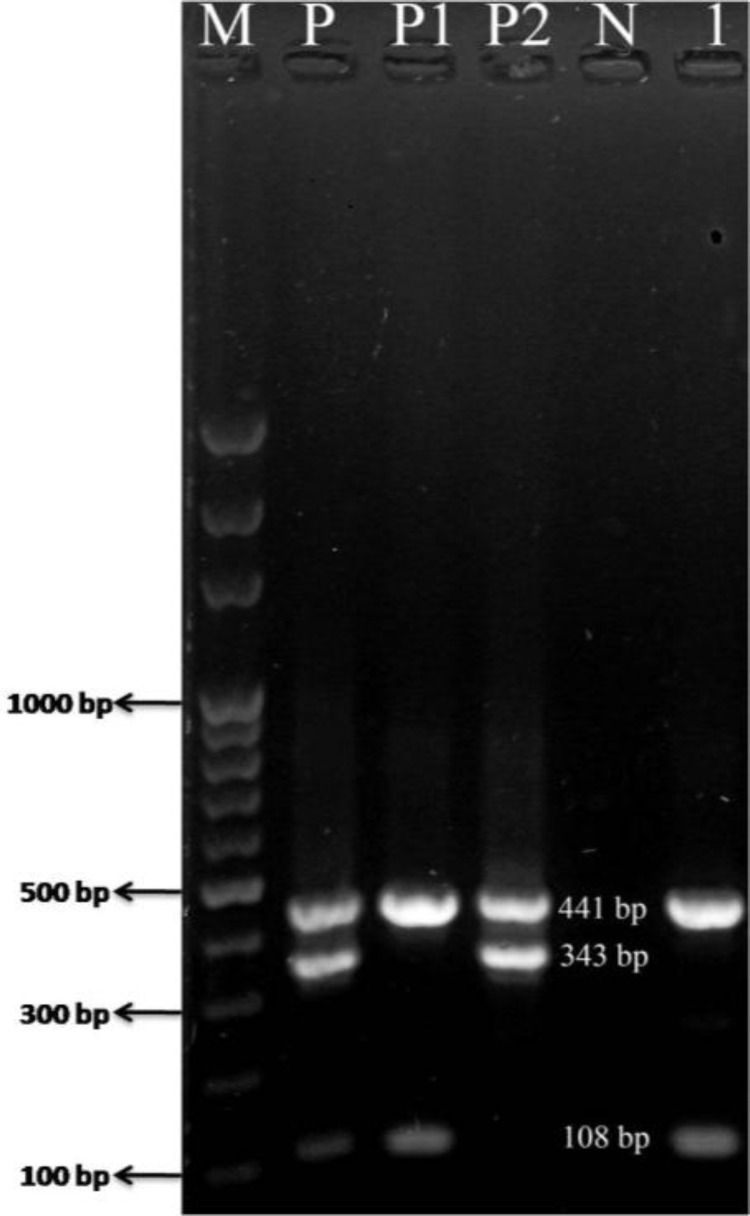
All isolates were identified as K. pneumonia by the biochemical tests and were also confirmed by using the PCR test (Fig. 1). Tested isolates were susceptible to Gentamycin, Chloramphenicol, Florfenicol and Strepto-mycin, intermediate susceptible to kanamycin, amikacin and oxytetracycline and resistant to ciprofloxacin, enrofloxacin, cefazolin, cefixime, ampicillin and erythro-mycin.
Fig. 1.
The presence of Klebsiella pneumonia was confirmed with mPCR assay. Lane M: 100 bp marker, Lane P: Positive control including K. pneumonia (108 bp) and Klebsiella oxcytoca (343 bp) and 441 bp PCR fragment of the gyrA gene (Klebsiella genus-specific gene), Lane P1: Positive control K. pneumonia, Lane P2: Positive control K. oxytoca, Lane N: negative control, and Lane 1: K. pneumonia isolated from the yolk sac sample
Discussion
Most reports present YSI and dead-in-shell or day-old mortality in poultry. However, few reports exist in the literature on companion birds. In this report, we present an outbreak of YSI caused by K. pneumoniae in a domestic flock of canaries.
Jahantigh (2010) ▶ reported Klebsiella spp. as 15% of bacterial flora of dead-in-shell embryos of ostrich along with Bacillus spp. (45%), Staphylococcus spp. (25%), E. coli (10%) and Proteus spp. (5%). Of 79 pooled samples containing 632 dead-in-shell chicken embryos, cultured from two hatcheries in Nigeria, 13 isolates were Klebsiella spp. (Orajaka and Mohan, 1985 ▶). Giacopello et al. (2014) ▶ reported the prevalence of Gram-negative bacteria among canaries with clinical disease, and the antimicrobial sensitivity patterns of the isolates. 6 of 88 isolates belonged to Klebsiella spp. (five K. oxytoca and one Klebsiella GR.47). The isolates in their study showed most susceptibility to Norfloxacin, Oxytetracy-cline, and Ciprofloxacin and most resistance to Amoxicillin, Cefadroxil, Erythromycin and Penicillin G (Giacopello et al., 2014 ▶); while in our study, the isolates were susceptible to Gentamycin, Chloramphenicol and Streptomycin, and resistant to ciprofloxacin, enrofloxa-cin, cefazolin, cefixime, ampicillin and erythromycin.
Husseina et al. (2008 ▶) studied on bacteriology, pathology and antimicrobial resistance of YSI in broiler chicks in Suleimani district and reported K. pneumonia as 12% of bacterial isolates from yolk sac samples and suggested Enrofloxacin, Florfenicol or Co-trimoxazole as being useful for the treatment of YSI. Ajayi and Egbebi (2011) ▶ recovered 90 strains of K. pneumonia from 150 faecal samples of local birds in Nigeria. The frequency of antibiotic resistance among the isolates from local birds ranged between 28% and 88% among K. pneumonia.
In conclusion, the results of the current study in a canary aviary focused on the importance of K. pneumonia as a primary pathogen to induce YSI and dead-in-shell in causing the high mortality of canary chicks during their first week of their post-hatching life and thus, posing a great threat to this small to large colonies for aviculturists in Iran. Identification of the causative agent of YSI and dead-in-shell is important in order to choose the effective treatment protocol. Blind antibiotic therapy could lead to the development of antibiotic-resistant strains, and this highlights the importance of considering antimicrobial sensitivity pattern results in the formulation of treatment protocols. Moreover, a further study has been carried out throughout the country on epidemiological investigation of YSI and dead-in-shell in canary aviaries and its economic impact. The study also tries to assess the origin tracking by molecular methods in K. pneumonia isolates obtained from human and bird’s sources, and finally, solutions to prevent and control the disease are encouraged.
Acknowledgements
The authors would like to thank Dr. M. Rad for kindly providing positive controls (Klebsiella oxcytoca and Klebsiella pneumoniae) and Mr. Ali Kargar for technical assistance.
References
- Ajayi AO, Egbebi AO. Antibiotic susceptibility of Salmonella typhi and Klebsiella pneumoniae from poultry and local birds in Ado-Ekiti, Ekiti-State, Nigeria. Ann. Biol. Res. 2011;2:431–437. [Google Scholar]
- Bagley ST. Habitat association of Klebsiella species Infect Control. Infect. Control Hosp. Epidemio. 1985;6:52–58. doi: 10.1017/s0195941700062603. [DOI] [PubMed] [Google Scholar]
- Braman SK, Eberhart RJ, Asbury MA, Hermann GJ. Capsular types of Klebsiella pneumoniae associated with bovine mastitis. J. Am. Vet. Med. Assoc. 1973;162:109–111. [PubMed] [Google Scholar]
- Brown JE, Corstvet RE, Stratton LG. A study of Klebsiella pneumoniae infection in the uterus of the mare. Am. J. Vet. Res. 1979;40:1523–1530. [PubMed] [Google Scholar]
- Chander Y, Ramakrishnan MA, Jindal N, Hansen K, Goyal SM. Differentiation of Klebsiella pneumoniae and Klebsiella oxytoca by multiplex poly-merase chain reaction. Int. J. Appl. Res. Vet. Med. 2011;9:138–142. [Google Scholar]
- Fecteau G, Van Metre DC, Pare J, Smith BP, Higgins R, Holmberg CA, Jang S, Guterbock W. Bacteriological culture of blood from critically ill neonatal calves. Can. Vet. J. 1997;38:95–100. [PMC free article] [PubMed] [Google Scholar]
- Giacopello C, Foti M, Fisichella V, Lo Piccolo F. Antibiotic-resistance patterns of gram-negative bacterial isolates from breeder canaries (Serinus canaria domestica) with clinical disease. J. Exotic Pet. Med. 2014;24:84–91. [Google Scholar]
- Husseina SA, Hassanb AH, Sulaimanc RR. Bacteriological and pathological study of yolk sac infection in broiler chicks in Sulaimani district. J. Dohuk Univ. 2008;11:48–56. [Google Scholar]
- Jahantigh M. Bacteriological study of dead-in-shell embryos of ostrich. Iranian J. Vet. Res. 2010;11:88–90. [Google Scholar]
- Khan KA, Khan SA, Aslam A, Rabbani M, Tipu MY. Factors contributing to yolk retention in poultry: a review. Pakistan Vet. J. 2004;24:46–51. [Google Scholar]
- Orajaka LJ, Mohan K. Aerobic bacterial flora from dead-in-shell chicken embryos from Nigeria. Avian Dis. 1985;29:583–589. [PubMed] [Google Scholar]
- Podschun R, Ullmann U. Klebsiella spp as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DE, McClain HM, Hansen DS, Currin P, Howerth EW. An outbreak of Klebsiella pneumoniae infection in dogs with severe enteritis and septicemia. J. Vet. Diagn. Invest. 2000;12:168–173. doi: 10.1177/104063870001200215. [DOI] [PubMed] [Google Scholar]
- Ryan KJ, Ray CJ. Sherris medical microbiology. 4th Edn. Mcgraw-Hill; 2004. ISBNO 8385-8399. [Google Scholar]
- Wilson WD, Madigan JE. Comparison of bacteriologic culture of blood and necropsy specimens for determining the cause of foal septicemia: 47 cases (1978-1987) J. Am. Vet. Med. Assoc. 1989;195:1759–1763. [PubMed] [Google Scholar]