Abstract
Plant derived immunostimulants are a promising alternative to chemotherapeutics and also perhaps vaccines. In the present study, we examined the immunostimulating properties of aqueous leaf extract of Phyllanthus niruri, an Indian traditional medicinal herb, on neutrophil activation and antibody response of Oreochromis mossambicus (Peters). Serial ten-fold diluted doses of P. niruri ranging from 0.002 mg to 20 mg were administered to two groups of O. mossambicus (n=8). One group of fishes was administered with sheep red blood cells and the primary and secondary antibody responses were estimated using direct haemagglutination assay. The other group of fishes was administered heat-aggregated BSA to assess the ability of plant extract to elicit neutrophil activation. Our results indicate a significant enhancement of both neutrophil activation and antibody response. Among the various doses tested, fishes administered 20 mg/kg body weight caused the maximal enhancement of both primary and secondary antibody response and 0.002 mg/kg showed higher neutrophil activation compared to that of the control group. This short study indicates that aqueous leaf extract of P. niruri has the potential to be used as an immunostimulant and after confirming its immunostimulatory properties by a battery of tests on other nonspecific and specific parameters and disease-protective property by challenging the fish with virulent fish pathogens, it can be used either as a routine feed supplement to activate the immune system of farmed fishes or as an adjuvant to enhance the efficacy of vaccines.
Key Words: Antibody response, Aqueous extract, Immunostimulant, Neutrophil activation
Introduction
Aquaculture has been gaining importance over capture fisheries since 1990 and growing at a rate of 6% annually (Reverter et al., 2014 ▶). Poor culture practices, overcrowding the fish, poor water quality, etc. causes diseases and disease-outbreaks in aquaculture on a large scale (Reverter et al., 2014 ▶) resulting in substantial loss in production (Gabriel et al., 2015 ▶).
Plant derived immunostimulants are better alter-natives to conventional chemotherapeutics and antibiotics (Vaseeharan and Thaya, 2014 ▶). Immuno-stimulation by plant extracts are better because they confer protection to fish against a wide range of infectious agents compared to vaccines that are specific to single pathogenic organism (Anderson, 1992 ▶).
Phyllanthus niruri is an important ethno-botanical species of India and widely used in Ayurveda formulations (Narendra et al., 2012 ▶). Phyllanthus niruri was shown to possess anti-hepatitis (Venkateswaran et al., 1987 ▶) activity and diuretic properties (Boim et al., 2010 ▶). The phytochemicals present in P. niruri and their pharmacological effects were reviewed elsewhere (Bagalkotkar et al., 2006 ▶). In the present study, we demonstrate the efficacy of aqueous extracts of P. niruri leaves in positively modulating specific and nonspecific immune responses of O. mossambicus.
Materials and Methods
Oreochromis mossambicus of both sexes weighing 25-30 g were collected from a local fish farmer. All the experiments were carried out in circular plastic tanks of 70 L capacity at ambient temperature with daily renewal of water; fishes were fed ad libitum with a balanced diet prepared in this laboratory (Table 1).
Table 1.
Preparation of balanced fish diet. The ingredients are dried and powdered separately, sieved through fine strainers and autoclaved. Finally, 10 g vitamin mix was added to mixture and a dough was prepared using 300 ml double distilled water. The dough is then pressed through fine pored plate, dried, and stored in air-tight container at 15°C
| Ingredients | Quantity in gram |
|---|---|
| Dried fish | 420 |
| Groundnut oil cake | 200 |
| Blood meal | 50 |
| Tapioca | 150 |
| Wheat flour | 150 |
| Mineral mix | 20 |
Leaves of P. niruri were purchased from a local traditional medicinal plant vendor. Aqueous extract of P. niruri was prepared according to our earlier protocol (Logambal et al., 2000 ▶).
Sheep red blood cells (SRBC) were used as the antigen for the studies on antibody response. Blood was collected from the jugular vein of a sheep and SRBC was prepared according to our earlier protocol (Logambal et al., 2000 ▶). Heat aggregated-bovine serum albumin (HA-BSA) was used for neutrophil activation assay and prepared according to the protocol of Nakano (1976) ▶.
Fishes were administered intraperitoneally with 0.2 ml of saline containing five different doses of the aqueous extract of P. niruri with 10-fold dilution that corresponds to 20 mg to 2 µg (w/v) dose range. All the injections were made with 1 ml tuberculin syringes fitted with 24 gauge needle. Control group received 0.2 ml saline.
Two days after the administration of plant extracts, experimental fishes were primed with 0.1 ml of 5% SRBC intraperitoneally. After three days, a booster dose of 0.1 ml of 25% SRBC was administered. To investigate secondary response, the same priming and booster doses were administered intraperitoneally after sixty days post primary challenge.
Fishes were bled (0.1-0.2 ml) repetitively from common cardinal vein with an interval of five days for 85 days after antigen priming (Michael et al., 1994 ▶). Serum was separated and complement was inactivated as described elsewhere (Logambal et al., 2000 ▶) and stored at -20°C until used. Primary and secondary antibody responses were measured according to our earlier protocols (Logambal et al., 2000 ▶).
To estimate the number of activated neutrophils, another set of six groups (n=8 per group) of fishes were intraperitoneally administered the same doses of the aqueous extract as that of the previous experiment, two days prior to antigen challenge. Untreated control group received 0.2 ml of saline. One hundred μL of prepared HA-BSA (5 mg) was injected intraperitoneally to elicit neutrophil activation. 0.5 ml of blood samples were withdrawn from the common cardinal vein using 1 ml tuberculin syringe containing 0.5 ml heparinized saline (40 IU heparin/ml) at 2 days intervals after the challenge. Activated neutrophils were counted using the method described previously (Venkatalakshmi and Michael, 2001 ▶).
Results
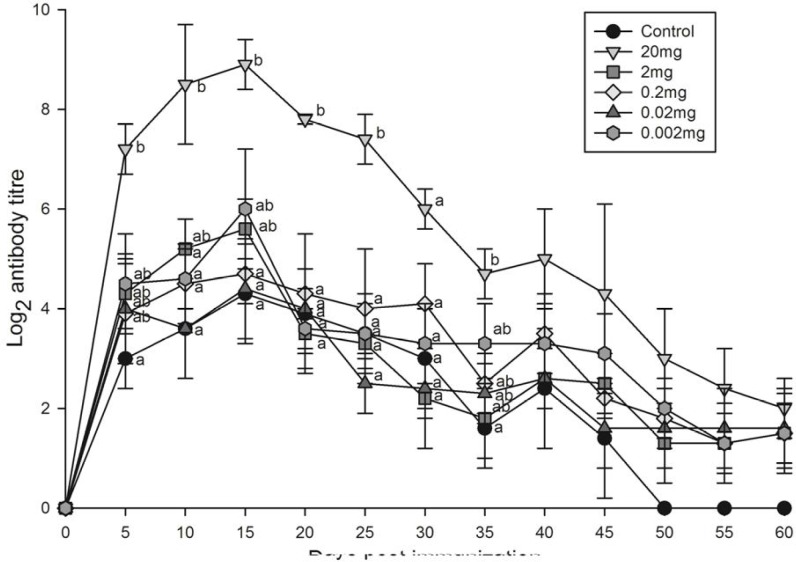
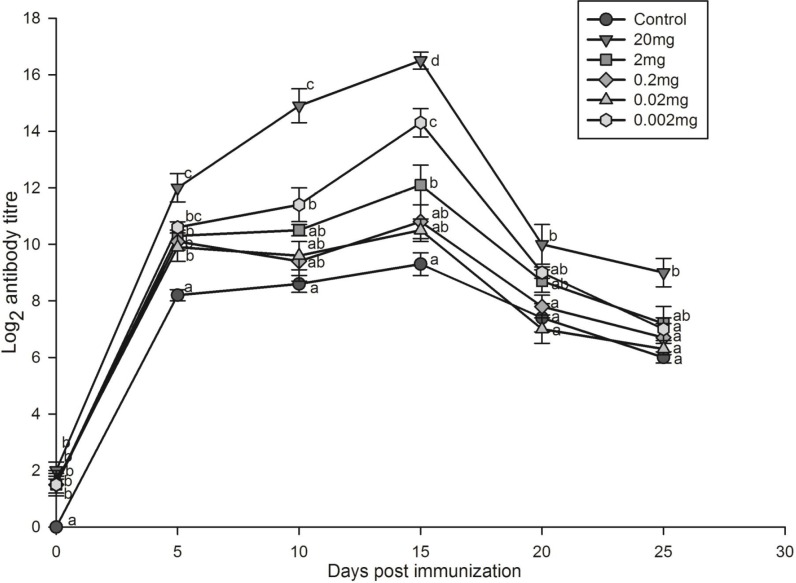
Only the highest dose of 20 mg leaf extract was able to elicit significantly higher primary (Fig. 1) antibody titres compared to those of other doses and untreated control group. The secondary antibody response (Fig. 2) on the peak day (day 15) was significantly enhanced by different doses of P. niruri aqueous leaf extract (20, 2, and 0.002 mg) compared to untreated control group.
Fig. 1.
Primary antibody response of Oreochromis mossambicus treated with various doses of Phyllanthus niruri aqueous leaf extract against SRBC. Each point represents mean±SE of eight fishes; a posteriori Tukey comparison of control and treated groups on particular days shown with different alphabets represents significant difference (P<0.05
Fig. 2.
Secondary antibody response of Oreochromis mossambicus treated with various doses of Phyllanthus niruri aqueous leaf extract against SRBC. Each point represents mean±SE of eight fishes; a posteriori Tukey comparison of control and treated groups on particular days shown with different alphabets represents significant difference (P<0.05)
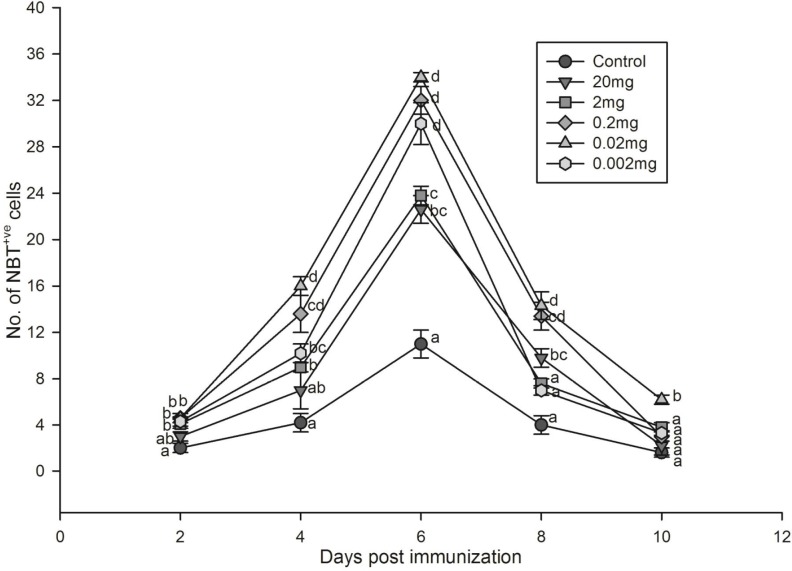
Neutrophil activity was enhanced several fold by all doses of the extract tested (Fig. 3). The highest number of activated neutrophils was observed in fishes administered with 0.02 mg extract followed by 0.2 and 0.002 mg.
Fig. 3.
Effect of Phyllanthus niruri aqueous leaf extract on neutrophil activity of Oreochromis mossambicus as evidenced by the number of NBT positive cells. Each point represents mean±SE of eight fishes; a posteriori Tukey comparison of control and treated groups on particular days shown with different alphabets represents significant difference (P<0.05
Discussion
Our study shows that the primary and secondary antibody responses of O. mossambicus were enhanced with aqueous extracts of P. niruri. This is in agreement with another study where the methanol extracts of P. niruri showed increased primary and secondary antibody titres of rats against SRBC (Eze, 2014 ▶). Aqueous extract of P. niruri is a potent murine lymphocyte mitogen (Nworu et al., 2010b ▶) and improved the antigen presentation capability of dendritic cells (Nworu et al., 2010a ▶). This may be an explanation of the increased production of antibodies by aqueous extract of P. niruri. Phyllanthus niruri also activated neutrophils at all the doses tested. Lower doses caused significantly higher activity than that of the higher doses which is similar to the study conducted with Levamisole (Kajita et al., 1990 ▶).
Though proved to be an immunostimulant in other species, serious studies on the effect of P. niruri on fish immunity are lacking. Phyllanthus niruri has been shown to possess antimicrobial activity against the dominant fish pathogen, Vibrio harveyi (Punitha et al., 2008 ▶). Preliminary studies of P. niruri in O. niloticus showed antihyperglycemic properties (Ibrahim et al., 2015 ▶) and improved haematological parameters in Labeo rohita (Annalakshmi et al., 2013 ▶).
This stimulation of immune mechanisms in fish by P. niruri most likely protects them from infection from pathogens. However, studies on other non-specific and specific parameters and also on functional immunity/ disease resistance involving pathogen challenge experiments may bring out the efficacy of this traditional medicinal plant and its applicability in aquaculture.
References
- Anderson, DP. Immunostimulants, adjuvants, and vaccine carriers in fish: applications to aquaculture. Annu. Rev. Fish Dis. 1992;2:281–307. [Google Scholar]
- Annalakshmi, T, Syed Ali Fathima, KM, Xavier Innocent, B, Sivagurunathan, A. Evaluation of immuno-stimulatory potential of Phyllanthus amarus in labeo rohita infected with Aeromonas hydrophila: haematological assessment. Int. J. Res. Ayurveda Pharm. 2013;4:96–100. [Google Scholar]
- Bagalkotkar, G, Sagineedu, SR, Saad, MS, Stanslas, J. Phytochemicals from Phyllanthus niruri Linn and their pharmacological properties: a review. J. Pharm. Pharmacol. 2006;58:1559–1570. doi: 10.1211/jpp.58.12.0001. [DOI] [PubMed] [Google Scholar]
- Boim, MA, Heilberg, IP, Schor, N. Phyllanthus niruri as a promising alternative treatment for nephro-lithiasis. Int. Braz. J. Urol. 2010;36:657–664. doi: 10.1590/s1677-55382010000600002. (discussion 664) [DOI] [PubMed] [Google Scholar]
- Eze, C. Immunomodulatory activities of methanol extract of the whole aerial part of Phyllantus niruri L. J. Pharmacognosy Phytother. 2014;6:41–46. [Google Scholar]
- Gabriel, NN, Qiang, J, He, J, Ma, XY, Kpundeh, MD, Xu, P. Dietary Aloe vera supplementation on growth performance, some haemato-biochemical para-meters and disease resistance against Streptococcus iniae in tilapia (GIFT) Fish Shellfish Immunol. 2015;44:504–514. doi: 10.1016/j.fsi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Ibrahim, M, Khan, M, Rinard, J, Mustafa, A. Determination of effective dosage of Phyllanthus niruri to modulate stress in tilapia, Oreochromis niloticus. Bioeng. Biosci. 2015;3:68–71. [Google Scholar]
- Kajita, Y, Sakai, M, Atsuta, S, Kobayashi, M. The immunomodulatory effects of levamisole on Rainbow trout, Oncorhynchus mykiss. Fish Pathol. 1990;25:93–98. [Google Scholar]
- Logambal, SM, Venkatalakshmi, S, Dinakaran Michael, RD. Immunostimulatory effect of leaf extract of Ocimum sanctum Linn in Oreochromis mossambicus (Peters) Hydrobiologia. 2000;430:113–120. [Google Scholar]
- Michael, RD, Srinivas, SD, Sailendri, K, Muthukkaruppan, VR. A rapid method for repetitive bleeding in fish. Indian J. Exp. Biol. 1994;32:838–839. [Google Scholar]
- Nakano, K. Studies on the role of macrophages in the antibody response of mice: the relationship between the immunogenicity of different forms of antigen and the mode of antigen handling by macrophages. J. Reticuloendothel Soc. 1976;19:361–374. [PubMed] [Google Scholar]
- Narendra, K, Swathi, J, Sowjanya, K, Satya, AK. Phyllanthus niruri: a review on its ethno botanical, phytochemical and pharmacological profile. J. Pharm. Res. 2012;5:4681–4691. [Google Scholar]
- Nworu, CS, Akah, PA, Okoye, FB, Esimone, CO. Aqueous extract of Phyllanthus niruri (Euphorbiaceae) enhances the phenotypic and functional maturation of bone marrow-derived dendritic cells and their antigen-presentation function. Immunopharmacol. Immunotoxicol. 2010a;32:393–401. doi: 10.3109/08923970903463939. [DOI] [PubMed] [Google Scholar]
- Nworu, CS, Akah, PA, Okoye, FB, Proksch, P, Esimone, CO. The effects of Phyllanthus niruri aqueous extract on the activation of murine lymphocytes and bone marrow-derived macrophages. Immunol. Invest. 2010b;39:245–267. doi: 10.3109/08820131003599585. [DOI] [PubMed] [Google Scholar]
- Punitha, SMJ, Babu, MM, Sivaram, V, Shankar, VS, Dhas, SA, Mahesh, TC, Immanuel, G, Citarasu, T. Immunostimulating influence of herbal biomedicines on nonspecific immunity in Grouper Epinephelus tauvina juvenile against Vibrio harveyi infection. Aquacult. Int. 2008;16:511–523. [Google Scholar]
- Reverter, M, Bontemps, N, Lecchini, D, Banaigs, B, Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture. 2014;433:50–61. [Google Scholar]
- Vaseeharan, B, Thaya, R. Medicinal plant derivatives as immunostimulants: an alternative to chemo-therapeutics and antibiotics in aquaculture. Aquacult. Int. 2014;22:1079–1091. [Google Scholar]
- Venkatalakshmi, S, Michael, RD. Immuno-stimulation by leaf extract of Ocimum sanctum Linn in Oreochromis mossambicus (Peters) J. Aquacult. Trop. 2001;16:1–10. [Google Scholar]
- Venkateswaran, PS, Millman, I, Blumberg, BS. Effects of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses: in vitro and in vivo studies. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:274–278. doi: 10.1073/pnas.84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]