Abstract
The present study employed mass spectrometry (ICP-MS) to measure the internal cadmium concentrations (Cdint) in Caenorhabditis elegans to determine Cd uptake from a Cd-containing environment as well as Cd release under Cd-free conditions. To analyze the functional role of several ATP binding cassette (ABC) transporters (e.g., HMT-1 and MRP-1) and phytochelatin synthase (PCS), we compared wild-type (WT) and different mutant strains of C. elegans. As a pre-test on selected mutant strains, several time-resolved experiments were performed to determine the survival rate and avoidance behavior of C. elegans under Cd stress, which confirmed the already known Cd sensitivity of the deletion mutants mrp-1Δ, pcs-1Δ, and hmt-1Δ. In addition, these experiments revealed flight reactions under Cd stress to be almost completely absent in mrp-1Δ mutants. The ICP-MS studies showed Cd uptake to be significantly higher in mrp-1Δ and WT than in hmt-1Δ. As Cd is ingested with food, food refusal due to very early Cd stress and its perception was likely the reason for the reduced Cd uptake of hmt-1Δ. Cd release (detoxification) was found to be maximal in mrp-1Δ, minimal in hmt-1Δ, and intermediate in WT. High mortality under Cd stress, food refusal, and minimal Cd release in the case of hmt-1Δ suggest a vital importance of the HMT-1/PCS-1 detoxification system for the survival of C. elegans under Cd stress. High mortality under Cd stress, absence of an avoidance behavior, missing food refusal, and maximal Cd release in the case of mrp-1Δ indicate that MRP-1 is less important for Cd detoxification under severe stress, but is probably important for Cd perception. Accordingly, our results suggest that the survival of WT under Cd stress (or possibly other forms of metal stress) primarily depends on the function of the HMT-1/PCS-1 detoxification system and the presence of a sensing mechanism to control the uptake of Cd (or other metals), which keeps internal Cd (or metal) concentrations under control, to some extent, for the timely mobilization of protection and repair systems.
Keywords: Applied sciences, Food science
1. Introduction
The physiological consequences of acute or chronic exposure to various metals, which originate from the earth’s crust or anthropogenic sources, are of general toxicological interest [1]. Cd, as a naturally occurring but non-essential metal, is particularly harmful because it impairs enzyme activities and DNA repair, alters protein binding, and evokes oxidative stress [2]. As an important part of evolutionary adaptation, organisms have developed protective mechanisms against this toxicant, including reduced dietary Cd intake mediated by some unknown sensory mechanisms [3], active Cd export, and the activation of various cellular stress responses [1]. The latter two responses include gene expression for ATP binding cassette (ABC) transporters, metallothioneins, enzymes of the glutathione (GSH) and phytochelatin (PC) pathways, antioxidant enzymes, and heat shock proteins [4, 5, 6].
As an in vivo system less complex than mammals, the model organism Caenorhabditis elegans provides the option to identify stress-protective mechanisms on genetic, molecular, and physiological levels [1]. These nematodes are free-living organisms in the interstitial (pore) water of soils and sediments, which contain Cd and other toxic substances in varying concentrations. These living conditions together with the genetic tractability of the worm lay the foundation for the use of C. elegans for studies on metal toxicity and gene-environment interactions [1]. As Cd uptake appears to take place via common intestinal transport mechanisms for divalent metal ions [7, 8], for example via the zinc transporters ZIP8, ZIP14, or DMT1 [9, 10], dietary Cd intake cannot be prevented, requiring the activation of intestinal stress-protective mechanisms. C. elegans has already been used several times for studies on the function of genes involved in heavy metal and Cd defense. Effects of these toxic substances on the survival rate, development, and reproduction were determined in WT, mutants, or worms treated with RNA interference (RNAi) [11, 12, 13]. These studies revealed, for instance, that protection from heavy metals relies on several ABC transporters, which were reported to be localized either at the apical (e.g., P-glycoprotein 1, PGP-1) or basolateral (multidrug-resistance associated protein 1, MRP-1) membranes of intestinal epithelia [14, 15, 16]. The ABC transporter MRP-4 is localized in membranes of intestinal granules and takes part in the formation of birefringent crystals there [17]. Metal-binding peptides such as GSH (γ-Glu-Cys-Gly) or cysteine-rich metallothioneins (MTLs) and PCs, which bind Cd ions via thiol groups [13, 18], are also important for Cd protection. GSH-bound Cd is exported by MRP-1 [19, 20], and the GSH pool is often regarded as short-term storage for metals. Metallothioneins function as long-term storage [21], but the exact roles of MTL-1 and MTL-2 in C. elegans are still a matter of debate [5]. PCs are enzymatically synthesized in several C. elegans tissues by PCS-1 and are oligomers of GSH ((γ-Glu-Cys)n-Gly, n ≤ 11) [22]. They can chelate and eliminate heavy metals via the intestinal half-molecule ABC transporter HMT-1 (heavy metal tolerance factor 1) [13, 23]. Cd exposure has been shown to result in an increase in the PC concentration via enhanced GSH synthesis [5].
To extend our knowledge on Cd protection in C. elegans, we measured internal Cd concentrations in WT and mutant strains by mass spectrometry (ICP-MS). Measurements were first taken during a period of Cd uptake at a specific ambient Cd level, and then, they were taken during a period of Cd release under Cd-free conditions. ICP-MS allows the determination of concentrations of metals (e.g., arsenic, cadmium, and copper) in biological samples with a detection limit below 0.001–0.1 μg/g of dry mass [24]. As pre-tests, survival rates and avoidance behaviors of these strains were determined at different ambient Cd levels. As the dry mass of worms proved to be invariant during ICP-MS measurements, we used it as reference point for data normalization (i.e., Cdint/dry mass). The results of this study suggest that HMT-1/PCS-1-mediated Cd detoxification and MRP-1-mediated Cd sensing are essential for the survival of C. elegans under Cd stress.
2. Materials and methods
2.1. Experimental organisms
C. elegans of the N2 Bristol variety (WT) and the deletion mutants VC252 hmt-1 (gk155) III, NL147 mrp-1 (pk89), VC712 mrp-4 (ok1095) X, VC128 mtl-2 (gk125) V, VF2 pcs–1 (tm1748) II, NL132 pgp-1 (pk17) IV, and VC134 pgp-2 (gk114) I as well as Escherichia coli OP50 were obtained from the Caenorhabditis Genetics Center (CGC) (https://www.cbs.umn.edu/research/resources/cgc). Worms were cultivated at 20 °C on nematode growth medium (NGM) plates with E. coli OP50 (OD600nm = 1-1.2) as the food source [25]. According to the German law, experiments carried out on the invertebrate C. elegans do not have to be announced or approved.
2.2. RNA interference
RNAi to knockdown specific C. elegans genes was applied by feeding E. coli HT115 strains, which expressed double-stranded RNA (dsRNA) against dpy-7 [F46C8.6] (phenotypic control to ensure the isopropyl-β-D-1-thiogalactopyranoside, IPTG, effect), mrp-1 [F57C12.5], mrp-4 [F21G4.2], and pcs-1 [F54D5.1] transcripts, with C. elegans fed E. coli HT115 (L4440, empty vector) as control animals. The bacterial strains were obtained from Source BioScience LifeSciences (Nottingham, UK) and grown overnight at 37 °C in lysogeny broth medium containing 100 μg/mL of ampicillin. Large (Ø 9.2 cm) NGM plates with 1 mmol/L of IPTG (Roth, Germany) were seeded with 1 mL of bacterial suspension (OD600nm = 1) and grown overnight at 20 °C to induce dsRNA expression. Synchronized L1 larvae of WT were then transferred to these plates for growth.
2.3. Testing the effects of Cd exposure
2.3.1. Avoidance behavior and survival of WT and mutants at 0–1 mmol/L of CdCl2
Young adult WT, hmt-1Δ, mrp-1Δ, mrp-4Δ, pcs-1Δ, and pgp-2Δ worms were transferred onto small (Ø 3.5 cm) NGM plates seeded with 0.2 mL of E. coli OP50 suspension and containing different cadmium chloride (CdCl2 × 2½ H2O; Sigma-Aldrich, Germany) concentrations (0–1 mmol/L). After incubation periods between 24 and 48 h, worms were screened for their specific position on the plate, with animals that had buried themselves into the NGM or climbed up the edge of the petri dish scored as worms having fled. Avoidance behavior was quantified on a scale from 0 (none of the worms fled) to 1 (all of the worms fled). Simultaneous with testing the avoidance behavior, the survival of worms was tested by applying weak mechanical stimuli to evoke body movements (touch response). During one series of experiments, WT (3–9 NGM plates with 15 worms each) and one of the mutant strains (9 NGM plates with 15 worms each) were tested. At each time point (24, 32, and 48 h), the effects of all six Cd concentrations were studied for the variables ‘avoidance behavior’ and ‘survival rate’. Five series of experiments were carried out, resulting in a mean ± SD from 21 (WT) or 9 (each mutant strain) tested groups.
2.3.2. Survival of WT and mutants at 2 mmol/L of CdCl2
Young adult WT, hmt-1Δ, mrp-1Δ, mrp-4Δ, mtl-2Δ, pcs-1Δ, and pgp-1Δ worms were incubated for periods between 0 and 96 h on medium-sized (Ø 6 cm) NGM plates seeded with 0.5 mL of E. coli OP50 suspension and containing 2 mmol/L of CdCl2. Flight behavior was prevented in this case by a thin layer of 10 mg/mL of palmitic acid (BioXtra; Sigma-Aldrich, Germany) around the edge of a petri dish [26]. Survival was tested by evoking the touch response. During one series of experiments, WT or one of the mutant strains (3 NGM plates with 10 worms each) was tested. For each time point (6, 24, 30, 48, 54, 72, 78, and 96 h), average values were calculated (i.e., mean number of surviving worms on 3 NGM plates). Per strain, 3–6 series of experiments were carried out, resulting in a mean ± SE from 3–6 tested groups.
2.3.3. Survival of RNAi-treated worms at 10 mmol/L of CdCl2
RNAi-treated young adult WT worms were incubated on medium-sized NGM plates seeded with 0.5 mL of E. coli HT115 suspension and containing either 0 or 10 mmol/L of CdCl2. As gene silencing by RNAi is frequently less effective than mutation, the Cd concentration was increased in these experiments. However, since 24 h on 10 mmol/L of CdCl2 were already sufficient to reduce the survival rate of control worms (WT fed with E. coli HT115 carrying the empty vector), we principally shortened the incubation period to 24 h to avoid too low survival rates. Survival was tested by evoking the touch response. Four treatments (control and three RNAi treatments) were tested at two Cd concentrations (0 and 10 mmol/L of CdCl2) (for each of the eight experimental conditions 3 NGM plates were used with 10 worms each), and average values were calculated (i.e., mean number of surviving worms on the 3 NGM plates). The 3–6 series of experiments resulted in a mean ± SE from 3–6 tested groups. As measurements at 0 mmol/L of CdCl2 always resulted in 100% survival, only data at 10 mmol/L of CdCl2 are shown.
2.4. Preparation of animals for ICP-MS measurements
Evenly sized small pieces of agar carrying many WT or mutant worms were transferred onto large NGM plates seeded with 1 mL of E. coli OP50 suspension and bred for 2–4 days at 20 °C. The resulting mixed worm populations were used for 24-h incubations at 2 mmol/L of CdCl2 (see below) and subsequent ICP-MS measurements (see 2.5). The use of synchronized worm populations was not possible in this case because the much higher time requirements for synchronized worm breeding would have far exceeded the time frame for the many ICP-MS experiments planned and performed. The breeding method used also delivered the exceptionally large worm numbers needed for the mass spectrometric method. Another positive effect of using mixed populations was that measured Cdint values were independent from the developmental state, as the large worm numbers in the populations guaranteed consistent age distributions. After harvesting worm populations by washing them down into micro centrifuge tubes with M9 buffer [25] and discarding the supernatants after the worms had settled, worm pellets (slightly diluted in a defined manner) were transferred to large NGM plates seeded with 1 mL of E. coli OP50 suspension and containing 2 mmol/L of CdCl2. After different incubation periods (up to 24 h) at 20 °C, worms were harvested (see procedure above). For comparability with subsequent experiments (i.e., identical harvesting, washing, and transfer steps), worm pellets were then transferred to large NGM plates seeded with 1 mL of E. coli OP50 solution (without CdCl2). After an incubation period of a few minutes, the worms were harvested again. To further remove external CdCl2 and CdCl2-containing bacteria, worms were cleaned by three centrifugation steps (2,700 × g; 1 min) using 1 mL of pure water for each, which served to minimize background noise for the ICP-MS measurements. As further active Cd export or Cd losses during harvesting and cleaning cannot be excluded, the measured Cdint values possibly underestimated the actual values. However, procedures were kept identical for all conditions and strains, which means that such deviations must have been similar in all cases. Worm pellets were then resuspended in 0.5 mL of pure water. Such samples were shock-frozen in liquid nitrogen and stored at −80 °C until use. To study Cd release after 24-h of exposure to 2 mmol/L of CdCl2, worms were harvested, with worm pellets transferred to large NGM plates seeded with 1 mL of E. coli OP50 solution (without CdCl2). After different periods (0–2 h), worms were harvested, cleaned by centrifugation steps using pure water, and shock-frozen (see above). Prior to ICP-MS measurements, samples were thawed at 4 °C and homogenized using small pestles (PELLET PESTLES®; Fisher Scientific, Germany). After determining the protein content by the method of [27], samples were acidified with 65% HNO3 and stored at −20 °C.
2.5. ICP-MS measurements of Cdint
The Cdint was quantified by measuring the cadmium isotopes 111Cd and 114Cd using an inductively coupled plasma mass spectrometer (ICP-MS 7700 Series; Agilent Technologies, Waldbronn, Germany) with an integration time of 0.1 s. The sample injection system consisted of an autosampler transferring the specimens into the Scott-type spray chamber and a conical nebulizer using a peristaltic pump. The aerosolized samples were supplied into the plasma (RF power: 1500 W) for ionization, with a carrier gas flow of 0.92 L/min and a dilution gas flow of 0.16 L/min (argon). Aliquots of all thawed and thoroughly mixed samples were diluted 1:30 in 10% HNO3 before measurements in helium, high helium or no gas modes were performed. As an internal standard, 103Rh was added to the samples during measurements using a peristaltic pump (stock solution of 50 μg/L rhodium [Merck, Germany] in 10% HNO3 [ROTIPURAN® Ultra; Carl Roth, Germany]). Calibration was performed by dilutions of a 1 g/L standard cadmium solution (CERTIPUR® Cadmium standard; Merck, Germany). As certified reference standards for trace elements, TM-15.2/TM-25.4 (1:10) and TMDA-53.3 (1:20) (Environment Canada, Gatineau, Canada) were used. Tuning was carried out before each measurement with a 10 μg/L in 10% HNO3 tuning solution (Li, Y, Tl, Co, and Ce) and P/A solution in 10% HNO3 (Agilent Technologies, Germany). According to the NORDTEST procedure [28], the measurement uncertainty for Cd in the sample matrix used was 3.55%, and the extended measurement uncertainty (k = 2) was 7.10%.
2.6. Subsequent measurements for data normalization
2.6.1. Measurements of the dry body mass of worms
To quantify the worm mass in the different samples for subsequent data normalization, we used thawed 50-μL aliquots of the samples, which were dried first at 95 °C and then at 120 °C in small aluminum bowls (ROTILABO® micro-aluminum dishes; Roth, Germany) for at least one day. Using an ultramicroscale (UMT2; Mettler-Toledo, Germany), the dry mass of the worms was determined with an accuracy of up to 0.1 μg. Measurements were carried out after removal of such a bowl, which was stored within a micro centrifuge tube, from a silica gel-filled desiccator using sterilized tweezers, with the tweezers grounded to prevent static charge of the aluminum bowl. Mass values were read out every 10 s over 80 s. The readings between 40 and 80 s were averaged, with the standard deviations always being below 0.3% of these average values. After subtracting the previously measured empty mass (without aliquot) of the respective bowl, the mass of a sample (dry mass of worms) resulted from a recalculation to a 0.5-mL sample volume. To check for mass contributions by dust, 50-μL samples of the solvent (pure water), in which worm pellets were resuspended (see section 2.4), were also tested in the manner described above. Although barely noticeable mass values were determined, we calculated the mean of these values and subtracted it from the dry mass of worms. Additional controls included repeated measurements of nine aluminum bowls without samples, which resulted in standard deviations below 1.1% of their masses, and measurements between different days, which showed variations below 0.6%.
2.6.2. Measurements of the quantity of worms
Additionally, to test for consistency in the worm number over the 2-h period, during which Cd release was determined, the experimental protocol as described above was exactly repeated using WT worms (24-h incubations at 2 mmol/L of CdCl2; identical harvesting and cleaning procedures). After resuspending the worm pellets in 500 μL of pure water, defined aliquots (maximum of 40 μL) were spread out onto small NGM plates (3–4 per time point) and dried. To immobilize the worms, the plates were incubated at approximately 90 °C for 10 min. Two people independently counted the number of worms on such a plate. Three different series of experiments were carried out in this manner. After recalculation to 500-μL sample volumes, single countings from a series of experiments were normalized in relation to the mean of all countings of this series, which was set to 1.
2.7. Statistical analysis
Data are given as a mean ± standard deviation (SD) or standard error of the mean (SE), with n indicating the number of tested animal groups. To test for significant differences between means of two samples, unpaired t-tests were used. To check for significant differences between curves, Kruskal-Wallis one-way analysis of variance (ANOVA) on Ranks was used in the case of non-normal distributions, and two-way ANOVA was used in the case of normal distributions. Subsequently, a multiple-comparison procedure (Student-Newman-Keuls method, SNK) was applied to identify differing curves. Linear regression analysis were carried out for trend analysis. SigmaPlot 11.0 (Systat Software, Germany) was used for graph preparations and statistical analyses.
3. Results
3.1. Effects of Cd exposure on the survival rate
3.1.1. Survival rate and avoidance behavior of WT and mutants at 0–1 mmol/L CdCl2
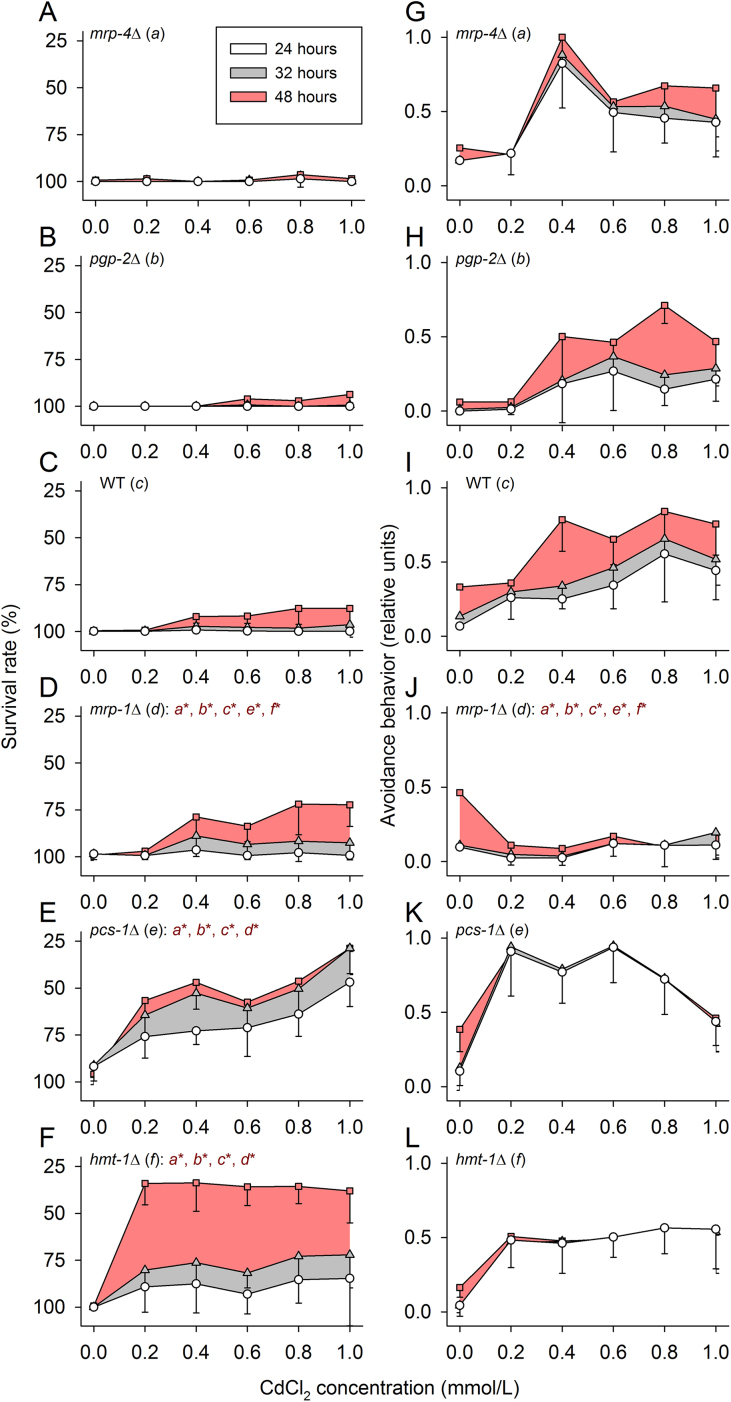
Testing survival rates of WT and different mutant strains after 24, 32, and 48 h on NGM plates containing different CdCl2 concentrations (0–1 mmol/L) revealed survival rates to be high in mrp-4Δ, pgp-2Δ, and WT, and the survival rates were low in pcs-1Δ and hmt-1Δ, with mrp-1Δ taking an intermediate position (Fig. 1A–F). As Cd avoidance was detected during these experiments, we also evaluated this type of behavioral response (Fig. 1G–L). Avoidance behavior was detected for each tested strain with the exception of mrp-1Δ (Fig. 1J), which showed almost no flight reaction under Cd stress.
Fig. 1.
Survival rates and avoidance behavior of WT and mutants at 0–1 mmol/L CdCl2. After incubation periods of 24 (white areas), 32 (gray areas), or 48 h (red areas) on NGM plates containing 0–1 mmol/L of CdCl2, (A-F) survival rates were maximal in mrp-4Δ, pgp-2Δ, and WT, minimal in pcs-1Δ and hmt-1Δ, and intermediate in mrp-1Δ. (G-L) Avoidance behavior was the lowest in mrp-1Δ. Statistical significance was tested by Kruskal-Wallis one-way ANOVA on ranks and subsequent SNK analyses (dark red letters*, P < 0.05). (Mean ± SD; per mean: n = 9-21 tested groups with 15 worms each.)
3.1.2. Survival rate of WT, mutants, or RNAi-treated worms at 2 or 10 mmol/L CdCl2
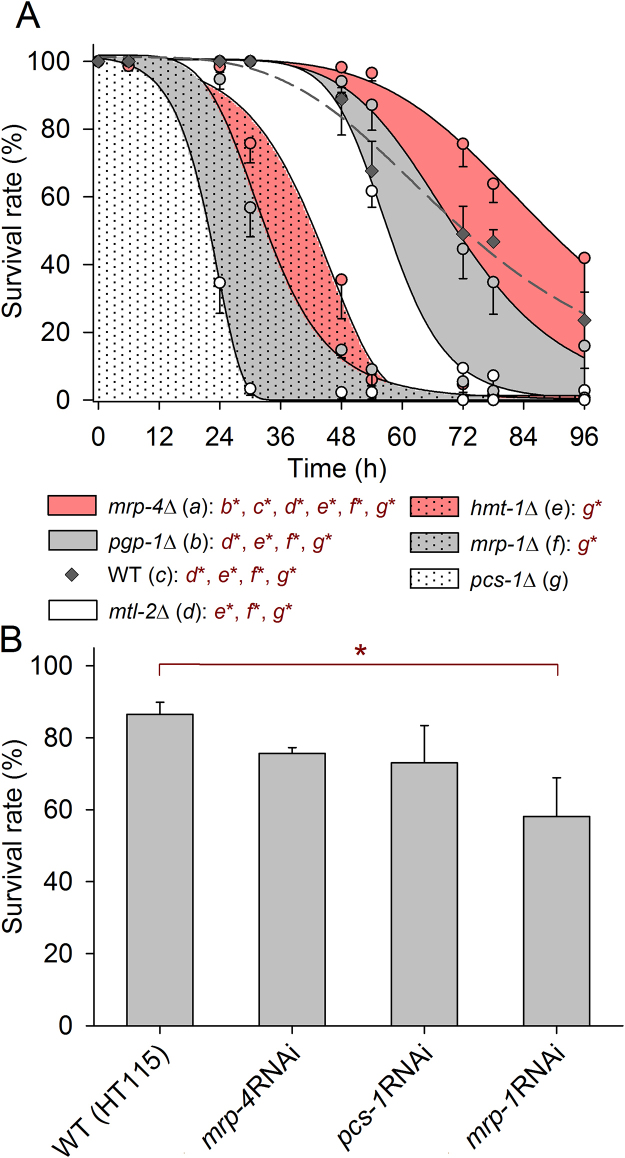
In the next experimental series, survival rates of WT and different mutant strains were studied over 96 h on NGM plates containing 2 mmol/L of CdCl2 (Fig. 2A). pgp-1Δ was tested instead of pgp-2Δ, as the latter strain had already shown high Cd resistance similar to mrp-4Δ (Fig. 1A, B), and mtl-2Δ was also included. Survival rates were now found to decrease in the following sequence: mrp-4Δ, pgp-1Δ and WT (no significant difference), mtl-2Δ, hmt-1Δ and mrp-1Δ (no significant difference), and pcs-1Δ, with WT differing from the mutant strains in curve progression (Fig. 2A). To check whether gene knockdown (RNAi) instead of gene knockout (mutation) also confers Cd sensitivity, RNAi treatment (feeding with E. coli HT115) was applied in WT (control and gene-specific RNAi; Fig. 2B) for genes that had been proven in tests on corresponding mutant strains (Fig. 2A) to be more (pcs-1, mrp-1) or less important (mrp-4) for the Cd resistance of worms. In comparison to control conditions (without CdCl2), at which all of the worms survived (100%), Cd stress always induced a reduction in the survival rate, which was significantly higher in mrp-1 RNAi-treated worms than in control worms (WT fed with E. coli HT115 carrying the empty vector) under the applied stress conditions (24 h on 10 mmol/L of CdCl2) (Fig. 2B).
Fig. 2.
Survival rates of WT, mutant, and RNAi-treated worms at 2 or 10 mmol/L CdCl2. (A) After increasing incubation periods (0, 6, 24, 30, 48, 54, 72, 78, and 96 h) on NGM plates containing 2 mmol/L of CdCl2, survival rates decreased significantly in the following sequence: mrp-4Δ, pgp-1Δ and WT (no significant difference), mtl-2Δ, hmt-1Δ and mrp-1Δ (no significant difference), and pcs-1Δ (mean ± SE; per mean: n = 3-6 tested groups with 30 worms each; data sets fitted by Gompertz or logistic sigmoidal equations with r2 values always above 0.99). Statistical significance was tested by Kruskal-Wallis one-way ANOVA on ranks and subsequent SNK analyses (dark red letters*, P < 0.05). (B) 24-h survival rates of control worms (WT fed with E. coli HT115, empty vector), or mrp-4, pcs-1, and mrp-1 RNAi-treated worms on NGM plates containing 10 mmol/L of CdCl2 (mean ± SE; per mean: n = 3-6 tested groups with 30 worms each). Survival rates of control and mrp-1 RNAi-treated worms differed significantly (t-test; *, P < 0.05).
3.2. Internal Cd concentration and parameters for data normalization
3.2.1. Measurements of Cd uptake and release, protein concentration, and dry body mass in WT and hmt-1Δ
Cd uptake and release was studied in C. elegans by measuring Cdint concentrations via mass spectrometry (ICP-MS) at two different ambient conditions. Cd uptake took place on CdCl2-containing NGM plates. To enable Cd release, the worms were subsequently transferred to CdCl2-free NGM plates. Based on survival experiments (Fig. 2A), which showed the survival rates of all strains (except for pcs-1Δ, but see below) to be higher than 90% during the first 24 h on 2 mmol/L CdCl2, we chose these incubation conditions to maximize Cd uptake. Consequently, we also used strains already tested under these Cd stress conditions (WT, mrp-4Δ, pgp-1Δ, hmt-1Δ, mrp-1Δ, and pcs-1Δ; Fig. 2A) for the ICP-MS experiments. To check for differences in Cd sensitivity between young adult worms (survival experiments) and mixed worm populations (ICP-MS experiments), we determined the survival rate of the most sensitive strain (pcs-1Δ) again and found Cd resistance to be significantly (P < 0.01) higher in the case of mixed worm populations than in the case of young adult worms (24-h survival at 2 mmol CdCl2/L: 92.3 ± 2.5% (n = 3 tested groups) vs. 34.6 ± 8.9% (n = 6 tested groups). This was advantageous from an experimental point of view and possibly explainable by a reduced amount of young adults in the population, which might have suffered from the detrimental effects of the Bag phenotype (i.e., hatching of embryos inside the body due to dysfunctional vulval muscles) [29].
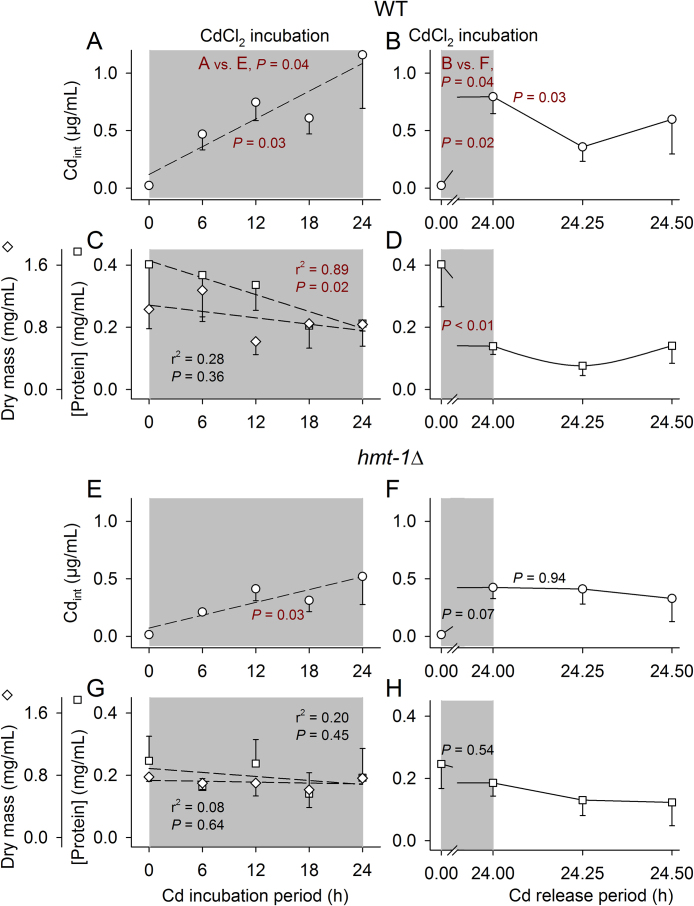
To gain experience with the new approach, we tested WT and a mutant (hmt-1Δ), which had shown low Cd resistance in several survival experiments (Fig. 1F, Fig. 2A). WT took up significantly more Cd than hmt-1Δ on the CdCl2-containing plates, with the final Cdint value higher than 1 μg/mL (approximately 10 μmol/L of Cd, which is 0.5% of the ambient Cd concentration) in WT (Fig. 3A) and only approximately 0.5 μg/mL in hmt-1Δ (Fig. 3E) after 24-h incubation periods. Concomitantly, the protein concentration of worms decreased significantly in WT (Fig. 3C) but not in the mutant strain (Fig. 3G). The measured dry body mass of worms did not show significant changes over time (Fig. 3C, G). Repeating a reduced version of this experiment (measurements after 0 and 24 h), with the main focus now on early Cd release, again showed Cd uptake to be significantly higher in WT than in hmt-1Δ (Fig. 3B, F) and thereafter, a significant early release of Cd in WT (Fig. 3B, 24 h vs. 24.25 h) but not in hmt-1Δ (Fig. 3F) on CdCl2-free plates. During Cd uptake, the protein concentration of worms again decreased significantly in WT (Fig. 3D) but not in hmt-1Δ (Fig. 3H).
Fig. 3.
Internal Cd concentration, protein concentration, and dry body mass of WT and hmt-1Δ first on CdCl2-containing and then on CdCl2-free NGM plates. Twenty-four hour incubations on NGM plates containing 2 mmol/L of CdCl2 caused the internal Cd concentration (Cdint) of worms to increase significantly more in (A) WT (more than 1 μg/mL after 24 h) than in (E) hmt-1Δ (approximately 0.5 μg/mL after 24 h). Concomitantly, the protein concentration of worms decreased significantly in (C) WT but not in (G) hmt-1Δ. (C, G) The dry body mass of worms proved to be invariant over time in WT and hmt-1Δ. (Mean ± SE; per mean: n = 3-4 tested groups with several hundreds of worms each.) Further experiments also showed Cdint to increase significantly more in (B) WT than in (F) hmt-1Δ after 24 h on CdCl2-containg plates, followed by a significant decrease in Cdint in (B) WT but not in (F) hmt-1Δ within the first fifteen minutes on CdCl2-free plates. The protein concentration decreased significantly in (D) WT but not in (H) hmt-1Δ on CdCl2-containing plates. (Mean ± SE; per mean: n = 3-13 tested groups with several hundreds of worms each.) r2 and P values were determined by linear regression analysis, and single P values were determined by either two-way ANOVA and subsequent SNK analyses (A vs. E) or t-tests, with dark red letters indicating statistical significance.
3.2.2. Testing parameters for data normalization
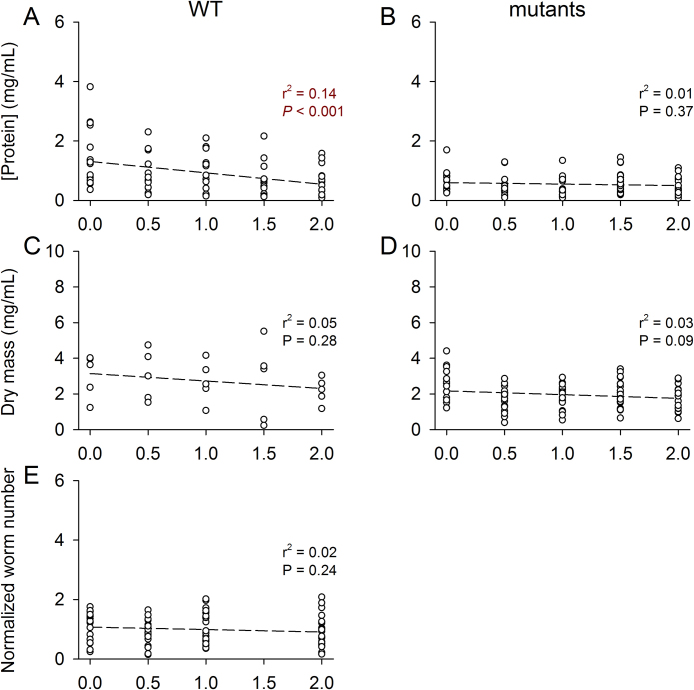
After establishing principles of the experimental protocol, we tested more intensely for suitable parameters for data normalization. For this, we carried out experiments on WT and mutant (mrp-4Δ, pgp-1Δ, hmt-1Δ, mrp-1Δ, and pcs-1Δ) strains under the experimental conditions of Cd release measurements (24-h incubations at 2 mmol CdCl2/L and subsequent transfer of worms to CdCl2-free NGM plates), with worm quantities on the NGM plates similar for each strain but higher than in previous experiments (Fig. 3). The measurement and evaluation of different parameters from WT and mutant strains, with all the data from the mutants evaluated in this case as a whole, showed the protein concentration of WT worms to decrease significantly (Fig. 4A), whereas that of all mutants (Fig. 4B), the dry body mass of WT and mutant worms (Fig. 4C, D), and the number of WT worms (Fig. 4E) did not change significantly during the first 2 h on CdCl2-free NGM plates. The detected changes in the protein concentration of WT under the experimental conditions of Cd uptake (Fig. 3C, D) and release (Fig. 4A) measurements excluded the use of the protein concentration as standard parameter for data normalization, and we decided therefore to use the invariant dry body mass as a suitable reference point.
Fig. 4.
Protein concentration, dry body mass, and normalized worm number of WT and mutants on CdCl2-free NGM plates after pre-incubation on CdCl2-containing plates for 24 h. After 24-h incubations at 2 mmol/L of CdCl2, the protein concentration decreased significantly on CdCl2-free NGM plates in (A) WT (n = 75 measurements on several hundreds of worms each) but not in (B) mutant worms (hmt-1Δ, mrp-1Δ, mrp-4Δ, pcs-1Δ, and pgp-1Δ) (n = 85 measurements on several hundred worms each). Significant changes in dry body mass, however, were not detected in (C) WT (n = 25 measurements on several hundred worms each) or in the (D) mutant worms (n = 85 measurements on several hundred worms each). Under identical experimental conditions, (E) the number of WT worms remained unchanged as well (n = 88 countings, with 28 worms actually counted in each on average). From these results, we decided to use the dry body mass as a reference point to normalize the data on Cdint obtained later. r2 and P values were determined by linear regression analysis, with dark red letters indicating statistical significance.
3.2.3. Changes of mass-specific Cdint in WT and mutants
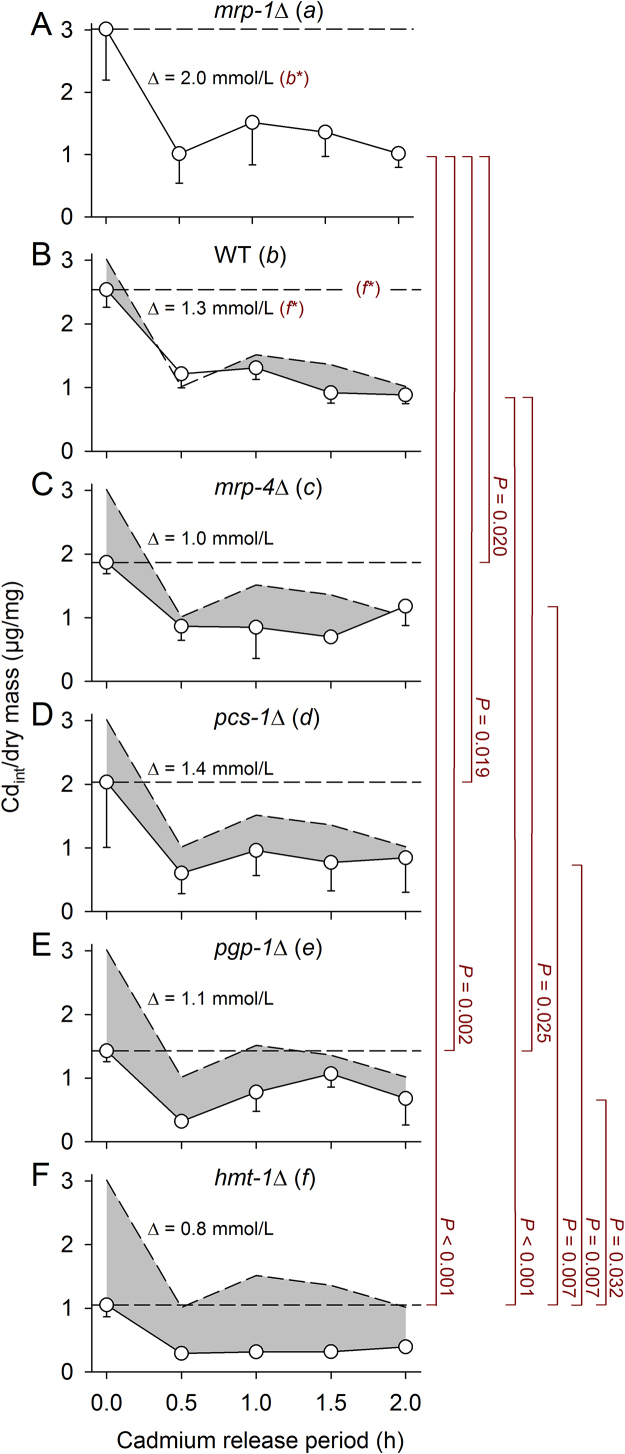
After 24-h incubations on CdCl2-containing NGM plates (2 mmol/L CdCl2), mass-specific Cd concentrations were measured in WT and mutant strains on CdCl2-free NGM plates over 2 h. Initial Cdint values (at 0 h) seemed to decrease in the following order: mrp-1Δ,WT, pcs-1Δ, mrp-4Δ, pgp-1Δ, and hmt-1Δ (Fig. 5, dashed lines), with statistical significance between WT and hmt-1Δ. Cd release between 0 and 0.5 h apparently declined in the following order: mrp-1Δ, pcs-1Δ, WT, pgp-1Δ, mrp-4Δ, and hmt-1Δ (Fig. 5, Δ values), with statistical significance between mrp-1Δ and WT as well as WT and hmt-1Δ. The Cdint values over the measurement period of 2 h were significantly 1) higher in mrp-1Δ than in mrp-4Δ, pcs-1Δ, pgp-1Δ, and hmt-1Δ; 2) higher in WT than in pgp-1Δ and hmt-1Δ; and 3) higher in mrp-4Δ, pcs-1Δ, and pgp-1Δ than in hmt-1Δ (Fig. 5).
Fig. 5.
Changes of mass-specific Cdint in WT and mutants over 2 h on CdCl2-free NGM plates after pre-incubation on CdCl2-containing plates for 24 h. After 24-h incubations at 2 mmol/L of CdCl2, changes in mass-specific Cdint in WT and mutants on CdCl2-free NGM plates (mean ± SE; per mean: n = 3-16 tested groups with several hundred worms each) were evaluated with regard to the initial Cdint values (at 0 h; dashed lines), absolute decreases in Cdint within the first half hour (Cd release; Δ values), and Cdint values over the 2-h measurement period. The initial Cdint values of (B) WT and (F) hmt-1Δ differed significantly (dark red letter*, P = 0.03). Cd release differed significantly between (A) mrp-1Δ and (B) WT (dark red letter*, P = 0.04) as well as between (B) WT and (F) hmt-1Δ (dark red letter*, P = 0.02). The Cdint values over 2 h differed significantly (vertical lines and P values) between (A) mrp-1Δ and (C-F) mrp-4Δ, pcs-1Δ, pgp-1Δ and hmt-1Δ; between (B) WT and (E-F) pgp-1Δ and hmt-1Δ; and between (C-E) mrp-4Δ, pcs-1Δ, pgp-1Δ and (F) hmt-1Δ. Gray areas show differences to the Cdint values of mrp-1Δ. Statistical significance was tested by two-way ANOVA and subsequent SNK analyses (Cdint values over 2 h) or t-tests, with dark red letters indicating statistical significance.
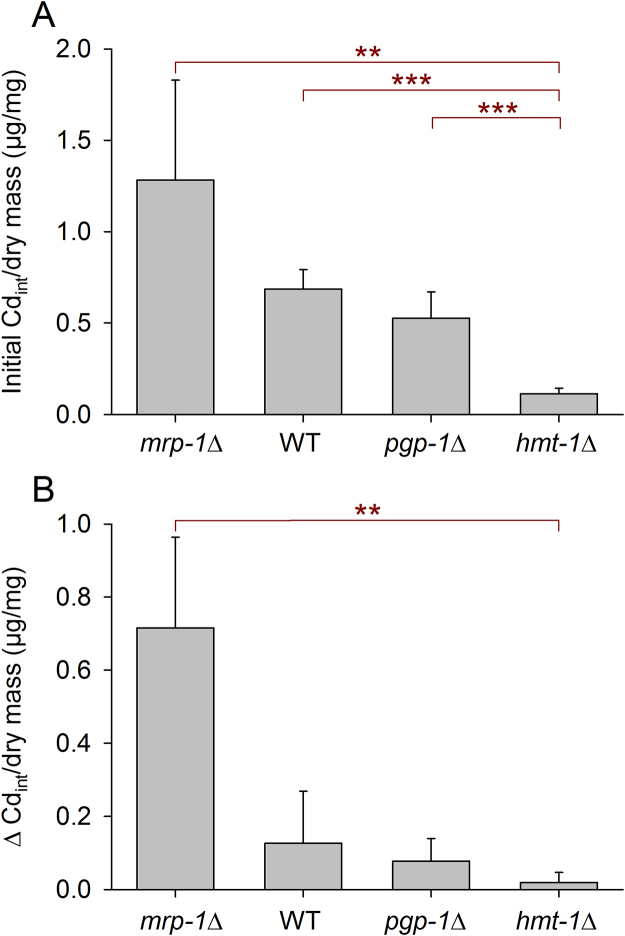
In the next experimental series, we focused on strains that took the two top (mrp-1Δ, WT) or lowest (pgp-1Δ, hmt-1Δ) positions for their Cdint values over the 2-h measurement period (Fig. 5). Mass-specific Cdint values were measured only after 0 and 0.5 h but under otherwise identical conditions. These experiments resulted in initial Cdint values (at 0 h) apparently decreasing in the sequence mrp-1Δ, WT, pgp-1Δ, and hmt-1Δ, with statistical significance between hmt-1Δ and mrp-1Δ, WT, or pgp-1Δ (Fig. 6A). Cd release between 0 and 0.5 h (Δ values) differed significantly between mrp-1Δ and hmt-1Δ (Fig. 6B).
Fig. 6.
Changes of mass-specific Cdint in WT and mutants over half an hour on CdCl2-free NGM plates after pre-incubation on CdCl2-containing plates for 24 h. After 24-h incubations at 2 mmol/L of CdCl2, changes in mass-specific Cdint in WT and mutants on CdCl2-free NGM plates (mean ± SE; per mean: n = 3-10 tested groups with several hundred worms each) were evaluated with regard to the initial Cdint values and absolute decreases in Cdint within half an hour. (A) Initial Cdint values differed significantly between mrp-1Δ, WT, or pgp-1Δ and hmt-1Δ, and (B) the release of Cd by mrp-1Δ and hmt-1Δ was also significantly different. Statistical significance was tested by t-tests (**, P < 0.01; ***, P < 0.001).
4. Discussion
The focus of the present study was the analysis of Cd uptake over 24 h at 2 mmol CdCl2/L and Cd release under CdCl2-free conditions in wild-type and mutant strains, with the latter carrying mutant genes for proteins already known to be involved in Cd resistance mechanisms. The Cd concentrations in worms were measured by ICP-MS, together with the dry body mass as a reference point for data normalization. As pre-tests, survival rates and avoidance behavior were determined in WT, mutant, and RNAi-treated worms.
The survival experiments were mostly carried out in a time-resolved manner, and worms were exposed to three ranges or values of CdCl2 concentration (0–1, 2, or 10 mmol/L). For strains selected for the ICP-MS studies, the overall conclusion of the survival experiments was that Cd resistance was significantly higher in mrp-4Δ, WT, and pgp-1Δ than in hmt-1Δ, mrp-1Δ, and pcs-1Δ (Fig. 1A–F, Fig. 2). This result fits quite well with previous studies [4, 13], which already showed highly negative effects of missing ABC transporter-associated mechanisms for the Cd resistance of C. elegans. In contrast to all other strains, mrp-1Δ did not show avoidance behavior under Cd stress (Fig. 1G–L). As Cd should not only paralyze mrp-1Δ but also mutant strains showing severely reduced survival rates under Cd stress (hmt-1Δ, pcs-1Δ), indirect or direct Cd sensing may be disturbed in mrp-1Δ resulting in a missing flight signal.
Cd uptake under severe Cd stress was significantly lower in hmt-1Δ than in WT, mrp-1Δ, and pgp-1Δ (Fig. 3A, B, E, F; Fig. 5, Fig. 6A). As Cdint depends on food intake, hmt-1Δ either has eaten (or reabsorbed) less food components (including Cd) than other strains. Food intake of C. elegans has been shown to be reduced at even sublethal concentrations of toxicants in food (e.g., above 200 μmol CdCl2/L), leading to a lower but still continuing food intake [30]. In the very beginning of CdCl2 exposure, the intestinal Cd level likely rose faster in hmt-1Δ than in WT and other strains as a consequence of the absence of intestinal HMT-1 [23] and impaired Cd detoxification (i.e., missing export of PC-bound Cd [5]). Therefore, a reduction in food intake has likely begun much earlier in hmt-1Δ. An intact Cd detoxification system, on the other hand, gave WT worms the chance to mobilize and adjust intestinal protection and repair mechanisms in response to rising Cdint values, which allowed them to tolerate higher internal Cd levels (Fig. 3A). As distinct morphological changes have been detected in hmt-1 RNAi-treated worms with Cd exposure (punctate inclusions near cell nuclei [13]), which indicate negative stress effects on intestinal tissue, the reabsorption of food (and Cd) may also be deteriorated in hmt-1Δ. Furthermore, the protein concentration decreased significantly in WT (Fig. 3C, D) but not in hmt-1Δ (Fig. 3G, H), during Cd stress, which might be related to the export of Cd-bound peptides (GSHs, PCs), with the export of PC-bound Cd requiring the presence of functional HMT-1 (in WT). Thus, WT indeed accumulated more Cd than hmt-1Δ over 24 h of Cd exposure, but it evidently had the chance to keep Cdint within controllable and tolerable levels (timely activation of protective mechanisms) by an intact Cd detoxification mechanism (and additional food refusal), whereas hmt-1Δ probably had only the option to reduce food intake even more to prevent Cdint from rising too fast.
The normalization of Cdint values required a reference point. For testing different parameters, we determined the protein concentration, dry body mass, and the relative number of WT worms over 2 h under CdCl2-free ambient conditions after 24 h of CdCl2 exposure. Since the protein concentration decreased significantly in WT (Fig. 4A), as was already the case during CdCl2 exposure (Fig. 3C, D), we decided to use the invariant dry body mass (Fig. 4C, D) as a reference point, which was additionally justified by the absence of changes in this parameter already during CdCl2 exposure (Fig. 3C, G).
As with any enzymatic reaction, Cd release under CdCl2-free conditions can vary with the amount of Cd accumulated during the 24 h of CdCl2 exposure (initial substrate concentration, initial Cdint) and the efficiency of the detoxification systems, which depends on enzyme characteristics or the presence/absence of specific enzymes or membrane transporters. The lowest initial Cdint was measured in hmt-1Δ (Fig. 5, Fig. 6A). The significant differences in Cdint values between strains over 2 h under CdCl2-free conditions (Fig. 5) indicate that the initial Cdint was actually also higher in mrp-1Δ than in mrp-4Δ, pcs-1Δ, pgp-1Δ, and hmt-1Δ; higher in WT than in pgp-1Δ and hmt-1Δ; and higher in mrp-4Δ, pcs-1Δ, and pgp-1Δ than in hmt-1Δ. Thus, the initial Cdint was likely the highest in mrp-1Δ, followed by WT, mrp-4Δ or pcs-1Δ, pgp-1Δ, and hmt-1Δ. As the initial Cdint likely decreases with a reduced rate of food intake during CdCl2 exposure (see above), mrp-1Δ has either suffered less from Cd toxicity (which is quite incomprehensible; Fig. 2A) or was not able to sense Cdint. Cd release during the first half hour under CdCl2-free conditions was maximal in mrp-1Δ, minimal in hmt-1Δ, and intermediate in WT (Fig. 5, Fig. 6B). However, it was not possible to differentiate between the effects of the substrate concentration and enzyme activity.
As the clearly lowest initial Cdint and Cd release values were measured in hmt-1Δ, HMT-1 is evidently most important for the detoxification of Cd in C. elegans, with phytochelatin (synthesized by PCS-1) required for Cd binding. Actually, hmt-1 RNAi-treated worms have already been reported to exhibit a Cd-hypersensitive phenotype, which was more pronounced than that shown by pcs-1 RNAi-treated worms, where intestinal tissue changes were also detected under Cd stress [13]. The superior role of HMT-1/PCS-1 in Cd detoxification also matches very well with the low survival rates of both mutant strains under Cd stress determined in the present study (Fig. 1E, F, Fig. 2A). As hmt-1 promoter activity was proven in intestinal cells as well as in head and tail neurons and pcs-1 promoter activity was only proven in the hypodermis, pharyngeal grinder, pharyngeal-intestinal valve, body wall, and vulval muscles [23], some yet unknown mechanism must exist to transport PC to HMT-1.
Concerning mrp-1Δ, there is the contradictory situation of high mortality under Cd stress (Fig. 1D, Fig. 2) combined with the highest initial Cdint and Cd release values (Fig. 5, Fig. 6) and a missing avoidance behavior under Cd stress (Fig. 1J). MRP-1, which was reported to be localized at the basolateral side of intestinal epithelia [15, 16], exports Cd bound to GSH [19, 20]. It would be possible that the high mortality of mrp-1Δ resulted from absent MRP-1-mediated Cd export, suggesting that MRP-1 plays an important role for Cd detoxification. However, this concept does not match the very high Cd release of mrp-1Δ. The basolateral MRP-1 likely exports ions (Cd2+) to the pseudocoelomic fluid for further processing by the coelomocytes, which endocytose this extracellular fluid [31] and have shown, as a unique exception, concurrent hmt-1 and pcs-1 promoter activities [23]. Liquid waste from the coelomocytes is excreted via the worm’s excretory or osmoregulatory system, the H-shaped excretory cell (H-cell) [32, 33]. Recalling the high initial Cdint of mrp-1Δ (Fig. 5, Fig. 6A), indicating less restriction in food intake under Cd stress than in other strains, as well as the absent avoidance behavior of mrp-1Δ being unique for all of the strains tested (Fig. 1G–L) and also considering the lack of direct neuronal connections to the intestine of C. elegans, one can suppose that MRP-1-mediated exports to the pseudocoelomic fluid primarily serve to inform some chemosensory system about ingested Cd or other metals for further neuronal processing (e.g., control of food intake), with these metals detoxified afterwards via the coelomocyte/H-cell-based excretory mechanism. A major role for Cd sensing instead of for Cd export could also explain the low efficiency of MRP-1-mediated Cd export (i.e., high Cd release in mrp-1Δ; Fig. 5, Fig. 6B) as well as the quite incomprehensible basolateral and non-apical localization of MRP-1, which would be much better suited for a mass export of Cd. Thus, MRP-1 activity did not result in the detoxification of high quantities of Cd, which is evidently more the task of HMT-1. This allocation of tasks is also reflected by the respective protein conjugate, as PC binds much more Cd than GSH, which likely accelerates Cd exports via HMT-1. Thus, we propose the hypothesis that the absence of MRP-1 leads to a non-controlled increase in Cdint, which, though counteracted by intact HMT-1-based Cd detoxification, is not properly sensed, resulting in an inadequate mobilization and adjustment of intestinal protection and repair mechanisms and in the end, a higher mortality.
Concerning other ABC transporters tested, MRP-4 is probably of minor importance for Cd detoxification because Cdint values over 2 h under CdCl2-free conditions were only slightly reduced in comparison to WT (Fig. 5), and survival rates under Cd stress were high (Fig. 1A, Fig. 2A). However, Cdint values over 2 h at CdCl2-free conditions were lower in pgp-1Δ than in WT (Fig. 5), but the survival rate was also high (Fig. 2A). The proper functioning of MRP-1- and HMT-1-based mechanisms in pgp-1Δ was probably responsible for food refusal and high survival under Cd stress. The low Cdint values suggest (such as in hmt-1Δ) a very early onset of food refusal due to a relatively fast increase in the intestinal Cd level. As MRP-1-mediated Cd exports into the pseudocoelomic fluid probably increased simultaneously, rising Cdint could be sensed by chemoreceptors. The even lower initial Cdint in hmt-1Δ (Fig. 5F, Fig. 6A) as well as the significantly lower survival rate of hmt-1Δ under Cd stress (Fig. 2A), however, suggest that the apical PGP-1 only supports HMT-1 during intestinal Cd detoxification. Thus, it is likely that a combination of food (Cd) refusal and HMT-1-based Cd detoxification caused the high survival rate of pgp-1Δ.
In conclusion, the ICP-MS measurements on internal Cd concentrations showed that HMT-1 (together with PCS-1) is of the upmost importance for the detoxification of Cd in C. elegans, with the knockout of hmt-1 or pcs-1 resulting in severely reduced survival rates under Cd stress. Although mrp-1Δ also showed a high Cd-induced mortality, it reduced food intake in the presence of CdCl2 less than other strains, and it showed the highest Cd release of all of the tested strains under CdCl2-free conditions. In contrast to all other strains, mrp-1Δ did not escape from Cd stress. These results suggest MRP-1 to be of minor importance for the detoxification of Cd under severe Cd stress. Its basolateral localization in the intestinal epithelium, together with the proven lack of Cd sensing in the mutant, suggest a role for this ABC transporter in the chemosensory perception of Cd (and possibly other metals) by exporting part of them into the pseudocoelomic fluid for further processing by sensory neurons.
Declarations
Author contribution statement
Sarah A. Winter, Ramona Dölling, Martha N. Mendelski: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Burkhard Knopf, Christoph Schäfers: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Rüdiger J. Paul: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Deutsche Forschungsgemeinschaft (Pa 308/13-1).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We appreciate the chance to use the microbalance at the Institute for Isotope Geochemistry, WWU Münster, and we thank Prof. Dr. Erik E. Scherer very much for his courtesy and cooperation. We also thank Karin Topp, Sabrina Ritz, Lisa Richter, and Katja Breuer (Institute of Zoophysiology, WWU Münster) for supporting measurements and data sets.
References
- 1.Martinez-Finley E.J., Aschner M. Revelations from the nematode Caenorhabditis elegans on the complex interplay of metal toxicological mechanisms. J. Toxicol. 2011;2011 doi: 10.1155/2011/895236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertin G., Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review) Biochimie. 2006;88:1549–1559. doi: 10.1016/j.biochi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Jones D., Candido E.P. Feeding is inhibited by sublethal concentrations of toxicants and by heat stress in the nematode Caenorhabditis elegans: relationship to the cellular stress response. J. Exp. Zool. 1999;284:147–157. doi: 10.1002/(sici)1097-010x(19990701)284:2<147::aid-jez4>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Broeks A., Gerrard B., Allikmets R., Dean M., Plasterk R.H. Homologues of the human multidrug resistance genes MRP and MDR contribute to heavy metal resistance in the soil nematode Caenorhabditis elegans. EMBO J. 1996;15:6132–6143. [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes S.L., Bundy J.G., Want E.J., Kille P., Stürzenbaum S.R. The metabolomic responses of Caenorhabditis elegans to cadmium are largely independent of metallothionein status, but dominated by changes in cystathionine and phytochelatins. J. Proteome Res. 2009;8:3512–3519. doi: 10.1021/pr9001806. [DOI] [PubMed] [Google Scholar]
- 6.Roh J.-Y., Lee J., Choi J. Assessment of stress-related gene expression in the heavy metal-exposed nematode Caenorhabditis elegans: a potential biomarker for metal-induced toxicity monitoring and environmental risk assessment. Environ. Toxicol. Chem. 2006;25:2946–2956. doi: 10.1897/05-676r.1. [DOI] [PubMed] [Google Scholar]
- 7.Valberg L.S., Sorbie J., Hamilton D.L. Gastrointestinal metabolism of cadmium in experimental iron deficiency. Am. J. Physiol. 1976;231:462–467. doi: 10.1152/ajplegacy.1976.231.2.462. [DOI] [PubMed] [Google Scholar]
- 8.Washko P., Cousins R.J. Role of dietary calcium and calcium binding protein in cadmium toxicity in rats. J. Nutr. 1977;107:920–928. doi: 10.1093/jn/107.5.920. [DOI] [PubMed] [Google Scholar]
- 9.Fujishiro H., Yano Y., Takada Y., Tanihara M., Himeno S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics. 2012;4:700–708. doi: 10.1039/c2mt20024d. [DOI] [PubMed] [Google Scholar]
- 10.Himeno S., Yanagiya T., Fujishiro H. The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie. 2009;91:1218–1222. doi: 10.1016/j.biochi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Anderson G.L., Boyd W.A., Williams P.L. Assessment of sublethal endpoints for toxicity testing with the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2001;20:833–838. [PubMed] [Google Scholar]
- 12.Harada H., Kurauchi M., Hayashi R., Eki T. Shortened lifespan of nematode Caenorhabditis elegans after prolonged exposure to heavy metals and detergents. Ecotoxicol. Environ. Saf. 2007;66:378–383. doi: 10.1016/j.ecoenv.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Vatamaniuk O.K., Bucher E.A., Sundaram M.V., Rea P.A. CeHMT-1, a putative phytochelatin transporter, is required for cadmium tolerance in Caenorhabditis elegans. J. Biol. Chem. 2005;280:23684–23690. doi: 10.1074/jbc.M503362200. [DOI] [PubMed] [Google Scholar]
- 14.Broeks A., Janssen H.W., Calafat J., Plasterk R.H. A P-glycoprotein protects Caenorhabditis elegans against natural toxins. EMBO J. 1995;14:1858–1866. doi: 10.1002/j.1460-2075.1995.tb07178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evers R., Zaman G.J., van Deemter L., Jansen H., Calafat J. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells. J. Clin. Invest. 1996;97:1211–1218. doi: 10.1172/JCI118535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J.J., Ann D.K., Kannan R., Lee V.H. Multidrug resistance protein 1 (MRP1) in rabbit conjunctival epithelial cells: its effect on drug efflux and its regulation by adenoviral infection. Pharm. Res. 2007;24:1490–1500. doi: 10.1007/s11095-007-9267-7. [DOI] [PubMed] [Google Scholar]
- 17.Currie E., King B., Lawrenson A.L., Schroeder L.K., Kershner A.M. Role of the Caenorhabditis elegans multidrug resistance gene, mrp-4, in gut granule differentiation. Genetics. 2007;177:1569–1582. doi: 10.1534/genetics.107.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chekmeneva E., Gusmão R., Díaz-Cruz J.M., Ariño C., Esteban M. From cysteine to longer chain thiols: thermodynamic analysis of cadmium binding by phytochelatins and their fragments. Metallomics. 2011;3:838–846. doi: 10.1039/c1mt00028d. [DOI] [PubMed] [Google Scholar]
- 19.Cole S.P., Deeley R.G. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol. Sci. 2006;27:438–446. doi: 10.1016/j.tips.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Leverrier P., Montigny C., Garrigos M., Champeil P. Metal binding to ligands: cadmium complexes with glutathione revisited. Anal. Biochem. 2007;371:215–228. doi: 10.1016/j.ab.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Freedman J.H., Ciriolo M.R., Peisach J. The role of glutathione in copper metabolism and toxicity. J. Biol. Chem. 1989;264:5598–5605. [PubMed] [Google Scholar]
- 22.Cobbett C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000;123:825–832. doi: 10.1104/pp.123.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz M.S., Benci J.L., Selote D.S., Sharma A.K., Chen A.G. Detoxification of multiple heavy metals by a half-molecule ABC transporter, HMT-1, and coelomocytes of Caenorhabditis elegans. PLoS One. 2016;5 doi: 10.1371/journal.pone.0009564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rüdel H., Kösters J., Schörmann J. Umweltprobenbank des Bundes (UPB) − Guidelines for sampling, transport, storage and chemical characterisation of environmental and human samples, Umweltbundesamt Berlin. Erich Schmidt Verlag; Berlin: 2011. Guidelines for chemical analysis: Determination of the elemental content of environmental samples using ICP-MS (version 2.1.0) [Google Scholar]
- 25.Stiernagle T. The C. elegans Research Community, WormBook. 2006. Maintenance of C. elegans. WormBook.http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller D.L., Roth M.B. C. elegans are protected from lethal hypoxia by an embryonic diapause. Curr. Biol. 2009;19:1233–1237. doi: 10.1016/j.cub.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 28.Magnusson B., Näykki T., Hovind H., Krysel M. Nordtest project 1589-02. 2012. Handbook for calculation of measurement uncertainty in environmental laboratories.http://www.nordtest.info/images/documents/nt-technical-reports/nt_tr_537_ed3_1_English_Handbook%20for%20Calculation%20of%20Measurement%20uncertainty%20in%20environmental%20laboratories.pdf [Google Scholar]
- 29.Hall J., Haas K.L., Freedman J.H. Role of MTL-1, MTL-2, and CDR-1 in mediating cadmium sensitivity in Caenorhabditis elegans. Toxicol. Sci. 2012;128:418–426. doi: 10.1093/toxsci/kfs166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd W.A., McBride S.J., Freedman J.H. Effects of genetic mutations and chemical exposures on Caenorhabditis elegans feeding: evaluation of a novel, high-throughput screening assay. PLoS One. 2007;2 doi: 10.1371/journal.pone.0001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altun Z.F., Hall D.H. WormAtlas. 2009. Pericellular structures. [Google Scholar]
- 32.Liégeois S., Benedetto A., Michaux G., Belliard G., Labouesse M. Genes required for osmoregulation and apical secretion in Caenorhabditis elegans. Genetics. 2007;175:709–724. doi: 10.1534/genetics.106.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altun Z.F., Hall D.H. WormAtlas. 2009. Excretory system. [Google Scholar]