Abstract
Epigenetic control of gene expression in adult tissues is crucial to maintain organ function and homeostasis. A report in this issue of The EMBO Journal (Chiacchiera et al, 2016b), together with another one published in Gastroenterology (Koppens et al, 2016), unveils how chromatin repressive complex PRC2 controls the equilibrium between secretory and absorptive fates in the intestine. PRC2 controls proliferation of cells within the crypt and at the same time represses the transcription factor Atoh1, thus favoring the generation of enterocytes versus secretory cell types in the adult intestine.
Subject Categories: Chromatin, Epigenetics, Genomics & Functional Genomics; Stem Cells
Understanding the control of gene expression is one of the major challenges to understanding living organisms. In any given adult organism, nearly all somatic cells share an identical genomic sequence, which implies that different layers of control establish which set of genes are expressed in a given cell type, ultimately specifying cell function. To achieve this high level of control, epigenetics factors contribute to regulating gene expression by a plethora of mechanisms, such as covalent modifications of DNA and histone proteins. Further, it is critical that gene expression is regulated in a spatiotemporal fashion during both development and homeostasis. Epigenetic machineries are often multiprotein complexes that are able to introduce, read, or erase covalent modifications on chromatin, which in turn affect the transcription level of adjacent or associated genes. Two examples of such complexes are the Polycomb repressive complex 1 (PRC1), which catalyzes the monoubiquitination of histone H2A on lysine 119 (H2AK119ub1), and PRC2, which catalyzes the methylation of histone H3 on the lysine 27 (H3K27me). Both histone modifications are correlated with chromatin compaction and gene silencing (reviewed in Aranda et al, 2015). Much of our knowledge about function of these complexes is based on studies performed in embryonic stem (ES) cells.
Adult organisms contain stem cells, which are present in many organs and confer the capacity to maintain and repair the tissue in which they reside. However, as these stem cells are technically more difficult to obtain and maintain in the laboratory than ES cells, our knowledge about the epigenetic players that govern their function is still limited, although it is possible that the same chromatin remodelers characterized in ES cells play important roles also in adult stem cells. Two new works, one from the Pasini laboratory published in this issue (Chiacchiera et al, 2016b) and another from the van Lohuizen laboratory (Koppens et al, 2016), describe the role of PRC2 in one of the organs with the highest self‐renewal capacity: the intestine. Intestinal stem cells (ISCs) are located at the bottom of the crypts (Fig 1). ISCs continuously proliferate, both to maintain their own stem cell pool (self‐renew) and to give rise to all differentiated cell types within crypts and villi (i.e., enterocytes and secretory cells, including Paneth, goblet, and enteroendocrine cells). Since PRC2‐deficient mice display an early embryonic lethal phenotype (Faust et al, 1995), both studies take advantage of conditional, intestine‐specific knockout mouse models. Deletion of EZH2, the enzymatic subunit of PRC2, only results in a minor phenotype (Koppens et al, 2016). This is likely due to the compensation by the paralog member EZH1, which is mutually exclusive with EZH2 (Di Croce & Helin, 2013). These data are in agreement with depletion of EZH2 in other tissues such as the skin, where double knockout of Ezh1/2 needs to be performed to observe a strong phenotype that is similar to that observed upon Eed and Suz12 knockouts (Dauber et al, 2016). Consequently, in these two studies, both groups focused their effort on conditional deletion of EED, a core component required for PRC2 stability, in order to achieve a complete PRC2 loss of function in the intestine.
Figure 1. Role of Polycomb repressive complexes in shaping the adult intestine.
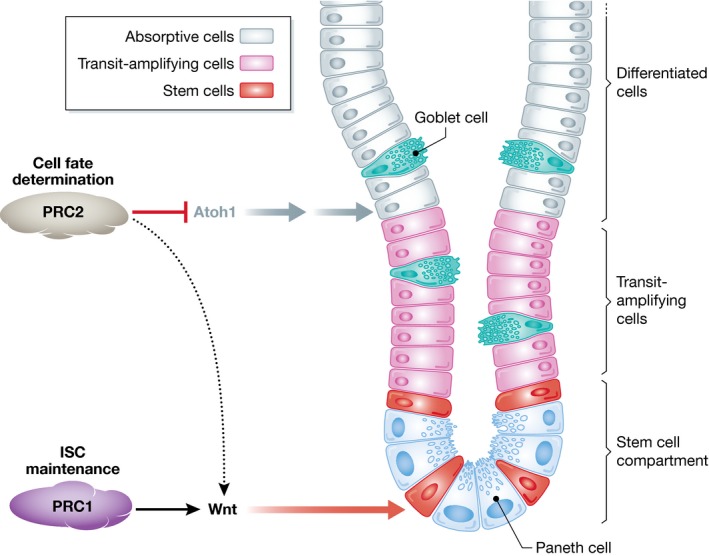
Maintenance of the stem cell compartment relies on the activity of PRC1, while PRC2 controls cell fate decisions. Both complexes control the expression of key transcription factors and signaling pathways to maintain the intestine homeostasis.
A previous report from the Pasini laboratory (Chiacchiera et al, 2016a) indicated that loss of PRC1 impairs ISC function. The loss of H2Aub that accompanied PRC1 deletion did not affect H3K27 methylation, indicating independent functions of both complexes in this context. Inversely, deletion of PRC2 exclusively affects H3K27 methylation, which intriguingly does not seem to affect ISC themselves, but rather the proliferation of transient amplifying cells of the crypt (Chiacchiera et al, 2016b). Accordingly, although the architecture of the crypt is affected, the animals are healthy. Conversely, van Lohuizen and colleagues report a significant loss of animal weight in intestine‐specific Eed‐depleted mice, and a degeneration of the stem cell compartment. Since both laboratories used the same animal model (Xie et al, 2014), the discrepancy might be due to the way in which the animals were housed, since Eed −/− mice have a compromised response to stress, and this might affect the ISC compartment in the long run. Both studies, however, coincide in the most striking result: Loss of PRC2 provokes an accumulation of secretory lineage cells, resulting in an imbalance of these with respect to enterocytes. Additionally, the stress‐induced regeneration of ISCs is impaired in PRC2‐depleted intestines (Chiacchiera et al, 2016b). These data indicate a direct role of PRC2 in cellular plasticity. Furthermore, both studies demonstrate that the effect on cell fate determination is independent of the proliferation defects caused by reactivation of the PRC2 target gene Cdkn2a.
What mechanism is responsible for skewing the cell fate? Both studies report the pattern of H3K27me3 by chromatin immunoprecipitation (ChIP) experiments in entire crypts and correlate its enrichment with the changes in transcriptome that occur upon PRC2 depletion. While some discrepancies between the two studies are evident, especially regarding the expression of Wnt signaling family members, both studies share a key finding: Depletion of PRC2 causes derepression of Atoh1 (also known as Math1), a well‐known master regulator of lineage fate in the crypt, which is required for secretory commitment downstream of Notch signaling (Yang et al, 2001). Indeed, Atoh1 is a PRC2 target, leading to the hypothesis that PRC2 may cooperate with Notch signaling to maintain Atoh1 in a silenced state. Interestingly, it has also been recently reported in skin that after PRC2 depletion, the derepression of Atoh1 (among others) also regulates cell fate, in this case the formation of Merkel cell formation (Perdigoto et al, 2016).
Both studies are good examples of the effort that needs to be done in order to fully understand the epigenetic control of transcription in adult organs. Yet, the results also highlight unsolved questions related to the mechanisms by which the PRC2 and PRC1 complexes maintain transcriptional repression independently of each other at target genes. Moreover, these studies reinforce the need to understand how specific recruitment of both complexes is achieved and regulated in distinct cellular/environmental contexts. Thus, investigating the role of epigenetic factors in homeostasis is crucial for understanding how epigenetic deregulation may lead to human diseases (Fig 1).
See also: F Chiacchiera et al (November 2016)
References
- Aranda S, Mas G, Di Croce L (2015) Regulation of gene transcription by Polycomb proteins. Sci Adv 1: e1500737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiacchiera F, Rossi A, Jammula S, Piunti A, Scelfo A, Ordonez‐Moran P, Huelsken J, Koseki H, Pasini D (2016a) Polycomb complex PRC1 preserves intestinal stem cell identity by sustaining Wnt/beta‐catenin transcriptional activity. Cell Stem Cell 18: 91–103 [DOI] [PubMed] [Google Scholar]
- Chiacchiera F, Rossi A, Jammula S, Zanotti M, Pasini D (2016b) PRC2 preserves intestinal progenitors and restricts secretory lineage commitment. EMBO J 35: 2301–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauber KL, Perdigoto CN, Valdes VJ, Santoriello FJ, Cohen I, Ezhkova E (2016) Dissecting the roles of Polycomb repressive complex 2 subunits in the control of skin development. J Invest Dermatol 136: 1647–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Croce L, Helin K (2013) Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol 20: 1147–1155 [DOI] [PubMed] [Google Scholar]
- Faust C, Schumacher A, Holdener B, Magnuson T (1995) The eed mutation disrupts anterior mesoderm production in mice. Development 121: 273–285 [DOI] [PubMed] [Google Scholar]
- Koppens MA, Bounova G, Gargiulo G, Tanger E, Janssen H, Cornelissen‐Steijger P, Blom M, Song JY, Wessels LF, van Lohuizen M (2016) Deletion of Polycomb repressive complex 2 from mouse intestine causes loss of stem cells. Gastroenterology 151: 684–697.e12 [DOI] [PubMed] [Google Scholar]
- Perdigoto CN, Dauber KL, Bar C, Tsai PC, Valdes VJ, Cohen I, Santoriello FJ, Zhao D, Zheng D, Hsu YC, Ezhkova E (2016) Polycomb‐mediated repression and sonic hedgehog signaling interact to regulate merkel cell specification during skin development. PLoS Genet 12: e1006151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Xu J, Hsu JH, Nguyen M, Fujiwara Y, Peng C, Orkin SH (2014) Polycomb repressive complex 2 regulates normal hematopoietic stem cell function in a developmental‐stage‐specific manner. Cell Stem Cell 14: 68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY (2001) Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294: 2155–2158 [DOI] [PubMed] [Google Scholar]


