Abstract
How the B‐cell antigen receptor (BCR) is activated upon interaction with its cognate antigen or with anti‐BCR antibodies is not fully understood. We have recently shown that B‐cell activation is accompanied by the opening of the pre‐organized BCR oligomers, an observation that strengthens the role of receptor reorganization in signalling. We have now analysed the BCR oligomer opening and signalling upon treatment with different monovalent stimuli. Our results indicate that monovalent antigens are able to disturb and open the BCR oligomer, but that this requires the presence and activity of the Src family kinase (SFK) Lyn. We have also shown that monovalent Fab fragments of anti‐BCR antibodies can open the BCR oligomers as long as they directly interact with the antigen‐binding site. We found that monovalent antigen binding opens both the IgM‐BCR and IgD‐BCR, but calcium signalling is only seen in cells expressing IgM‐BCR; this provides a molecular basis for IgM‐ and IgD‐BCR functional segregation.
Keywords: B‐cell antigen receptor, dissociation activation, monomeric antigen
Subject Categories: Immunology
Introduction
B cells play a central role in the adaptive immune response and signalling through the B‐cell antigen receptor (BCR) complex that controls B‐cell proliferation and differentiation (Reth, 1992). The BCR is composed of a membrane‐bound form of immunoglobulin (mIg) comprising two identical heavy chains (HC) and light chains (LC) and a non‐covalently associated signalling subunit, the CD79a/CD79b heterodimer, also referred to as Igα/Igβ (Hombach et al, 1990). The exact conformation of the BCR on the B‐cell surface is still a matter of controversy. We found that on mature resting B cells, both IgM‐BCR and IgD‐BCR form auto‐inhibited oligomers (Yang & Reth, 2010a). This oligomeric organization of the BCR has now been detected by several methods, including bimolecular fluorescence complementation (BiFC), super resolution microscopy and electron microscopy and by a Fab‐based proximity ligation assay (Fab‐PLA) (Mattila et al, 2013; Kläsener et al, 2014; Maity et al, 2015). The latter method has also shown that B‐cell activation is accompanied by an opening of the BCR oligomers, as suggested by the dissociation activation model (DAM) (Yang & Reth, 2010b).
The cytoplasmic tail of the BCR signalling proteins Igα and Igβ each carries an immunoreceptor tyrosine‐based activation motif (ITAM) that plays a central role in B‐cell activation. The specific binding of an antigen to the BCR results in the activation of two different cytosolic protein tyrosine kinases, namely the Src family kinase (SFK) Lyn and the spleen tyrosine kinase (Syk) (Schmitz et al, 1996). By phosphorylating and binding to the two ITAM tyrosines, Syk plays a crucial role in the initiation and amplification of BCR signalling (Pao et al, 1998). While the binding of Syk to the phosphorylated ITAM tyrosines of Igα and Igβ is necessary to open BCR oligomers upon the exposure to multivalent antigens, Lyn facilitates the opening, but is not absolutely required for this process (Kläsener et al, 2014). In addition, Syk phosphorylates the adaptor protein SLP‐65, also known as BLNK (Fu et al, 1998; Wienands et al, 1998). Once phosphorylated, SLP‐65 organizes a calcium signalosome complex containing BTK and PLCγ (Takata & Kurosaki, 1996), which in turn generates the second messenger inositol‐3 phosphate (IP3) that mediates the release of calcium ions from the endoplasmic reticulum (ER). The depletion of the calcium stores of the ER results in the opening of calcium channels in the plasma membrane and the drastic increase in the intracellular calcium concentration associated with B‐cell activation (Baba & Kurosaki, 2016).
B lymphocytes can be activated by different forms of antigens. Numerous studies in the mouse have shown that polyvalent antigens are more efficient than monovalent antigens in inducing an antibody response (Puffer et al, 2007). B cells have also been shown to be more efficiently activated by membrane‐bound than by soluble antigens (Carrasco & Batista, 2006). However, small soluble antigens such as hen egg lysozyme (HEL) or ovalbumin (OVA) are also able to activate B cells in vivo (Schelling & Silverman, 1968; Benjamin et al, 1980). In general, polyvalent antigens such as NIP15‐BSA are more potent than monovalent antigens in inducing BCR signalling in cultured B cells, as indicated by the increased ITAM phosphorylation or calcium response (Kim et al, 2006; Minguet et al, 2010; Mukherjee et al, 2013; Avalos & Ploegh, 2014; Ubelhart et al, 2015). Nevertheless, several groups have shown that monovalent antigens can activate B cells, also in vitro (Kim et al, 2006; Mukherjee et al, 2013; Avalos et al, 2014).
B cells can also be activated by exposure to diverse anti‐Ig antibodies. In fact, the classical cross‐linking model of B‐cell activation is based on the observation that only bivalent F(ab')2 fragments, but not monovalent Fab fragments, of anti‐Ig antibodies can efficiently activate the BCR (Woodruff et al, 1967). The cross‐linking model proposes that the surface of resting B cells carries signalling inert BCR monomers and the cross‐linking of which leads to B‐cell activation. However, this model is in conflict with the finding that many monovalent antigens can activate the BCR although they cannot cross‐link this receptor (Kim et al, 2006; Mukherjee et al, 2013; Avalos et al, 2014). Using a Fab‐based proximity ligation assay (Fab‐PLA), we monitored the nanometre conformation of the BCR before and after the exposure of polyvalent or monovalent reagents. We find that on the surface of resting B cells, the IgM‐BCR and IgD‐BCR form oligomers that are opened upon the exposure of the B cell to either monovalent antigens or an anti‐Ig Fab fragment that interacts directly with the antigen‐binding site.
Results
Opening and activation of BCR oligomers by monovalent reagents
To study the activation of the BCR by monovalent antigens, splenic B cells specific for the hapten 4‐hydroxy‐3‐iodo‐5‐nitrophenylacetyl (NIP) were isolated from κ‐deficient B1‐8 transgenic mice (Sonoda et al, 1997). The B cells were first left in culture medium for a minimum of 2 h and then loaded with the calcium indicator indo‐1. The B cells were then exposed to different forms of antigens or anti‐BCR antibodies at pM to nM concentrations and their calcium response was monitored by flow cytometry. The multivalent antigen NIP15‐BSA induced a strong calcium flux in the splenic B cells (Fig 1A). For monovalent antigens, we employed either 1NIP‐pep carrying a single NIP hapten group covalently attached to an 8‐amino acid peptide or 1NIP‐DNA with the NIP hapten attached to a double‐strand 19‐nucleotide DNA oligomer (Fig EV1A and B). These monovalent antigens also induced a calcium response (Fig EV1C and E), which was weaker than that observed with the multivalent antigen NIP15‐BSA (Fig 1A).
Figure 1. Monovalent antigen binding opens BCR oligomers and induces a calcium flux in splenic B cells.
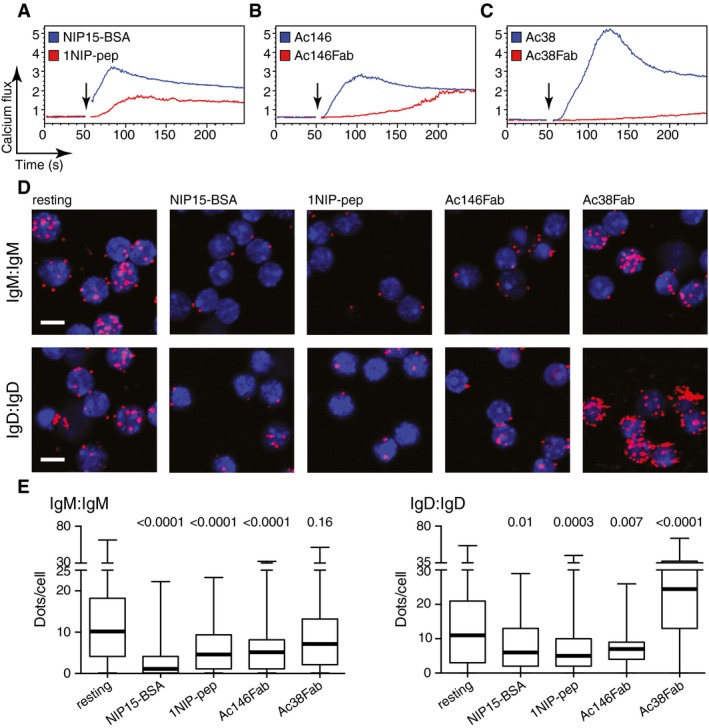
-
A–CCalcium flux measured by FACScan for splenic B cells isolated from B1‐8 transgenic mice after stimulation with (A) NIP15‐BSA (30 pM) or 1NIP‐pep (see Fig EV1, 80 nM); (B) Ac146 antibody (12.5 nM) or Ac146Fab (25 nM); (C) Ac38 antibody (12.5 nM) or Ac38Fab (25 nM). The addition of the stimuli to the cells is indicated by arrows.
-
DRepresentative microscopic images showing Fab‐PLA results measuring the BCR proximity for the IgM‐BCR (upper) and the IgD‐BCR (lower) on untreated or treated B1‐8 splenic B cells. PLA signals are shown as red dots, and nuclei were visualized by DAPI staining. Scale bar: 5 μm.
-
EThe Fab‐PLA results are quantified by BlobFinder software and presented as box plots. The median values are highlighted as thick lines, and the whiskers represent the minimum and maximum value. PLA signals (dots/cells) were counted from at least 100 cells for each sample. Data from the treated samples were compared with data from the resting cells; P‐values were calculated by Kruskal–Wallis one‐way analysis of variance (ANOVA).
Figure EV1. Synthetic monovalent antigens activate B1‐8 splenic B cells.
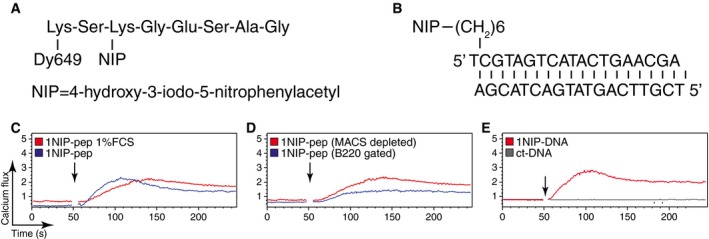
-
A, BSchematic illustration of the synthetic monovalent antigens.
-
C–ECalcium flux measured by FACScan for splenic B cells isolated from B1‐8 transgenic mice after stimulation with (C) 1NIP‐pep (80 nM) with or without 1% FCS in the medium; (D) 1NIP‐pep (80 nM) (with or without the staining of anti‐B220); (E) 1NIP‐DNA (80 nM) and control DNA (same sequence, without NIP, 80 nM). The addition of the stimuli to the cells is indicated by arrows. Data are representative of three independent experiments.
It has previously been reported that divalent anti‐Ig antibodies can activate the BCR, whereas monovalent Fab fragments derived from these anti‐Ig antibodies fail to do so (Woodruff et al, 1967). To test whether this behaviour also holds true for anti‐idiotypic antibodies, we used the monoclonal antibodies Ac146 and Ac38 that bind with similar affinity to different epitopes in the variable region of the B1‐8 BCR (Reth et al, 1979). The binding of Ac146 to the B1‐8 BCR competes with the free hapten NIP‐ε‐aminocaproic acid (NIP‐cap), suggesting that this antibody directly interacts with the antigen‐binding site. In contrast, the binding of Ac38 is only inhibited by larger antigens such as NIP15‐BSA, but not by the free hapten, suggesting that Ac38 binds close to, but not directly to, the paratope of the B1‐8 BCR (Reth et al, 1979). Both anti‐idiotypic antibodies are able to induce a calcium flux in B1‐8 splenic B cells, but only the Fab fragment of Ac146 (Ac146Fab), and not the Fab fragment of Ac38 (Ac38Fab), can stimulate B1‐8 B cells to mobilize calcium (Fig 1B and C). The purity of our Fab preparations was verified by SDS–PAGE (Appendix Fig S1). B1‐8 B cells responded to the Ac38Fab only after further treatment of the B cells with a secondary anti‐κ antibody (Appendix Fig S2A), and the same holds true for Fab fragments derived from a monoclonal anti‐λ light chain antibody (Appendix Fig S2B).
The DAM hypothesis proposes that BCR signalling is initiated by the opening of auto‐inhibited BCR oligomers (Yang & Reth, 2010b). The finding that monovalent antigens are able to elicit a calcium flux in B1‐8 splenic B cells prompted us to examine whether BCR oligomers open when bound by monovalent reagents. For this, we monitored and quantified the proximity of BCR on the surface of resting and activated B1‐8 splenic B cells using Fab‐PLA (Kläsener et al, 2014) (Fig 1D and E). As a positive control, we stimulated the B1‐8 B cells for 1 min with the polyvalent antigen NIP15‐BSA. Compared with resting B cells, the close proximity of BCR monomers was lost for both the IgM‐BCR and the IgD‐BCR oligomers upon contact with the polyvalent antigen, as previously reported (Kläsener et al, 2014). A reduced BCR proximity was also found for both BCR classes after the exposure of B1‐8 B cells to the monovalent reagent 1NIP‐pep or Ac146Fab. The Ac38Fab, as well as the anti‐λ Fab, however, did not reduce the BCR proximity, indicating that the binding of these reagents did not open the BCR oligomer (Fig 1D and E, Appendix Fig S3). In comparison with polyvalent antigens and anti‐BCR antibodies, the monovalent reagents, however, only weakly increased the phosphorylation of BCR signalling elements such as Igα, ERK and AKT (Fig EV2). Together, these data show that monovalent antigens and anti‐Ig Fab fragments that directly engage the antigen‐binding site can open the BCR oligomers and activate the calcium signalling pathway inside the B cell. Furthermore, the finding that a reagent such as Ac38Fab which fails to open the BCR oligomer is unable to induce downstream calcium signals supports the DAM hypothesis.
Figure EV2. Monovalent antigens induce weak phosphorylation of AKT, ERK and Igα.

Western blot analysis of AKT, ERK and Igα phosphorylation for B1‐8 splenic B cells upon 1‐ and 5‐min treatment with different stimuli. Western blot analysis of Syk and β‐actin expression functions as a loading control. Data are representative of three independent experiments.Source data are available online for this figure.
Lyn is required for sensing monovalent reagents by the BCR
We have recently shown that the binding of Syk to the phosphorylated ITAM tyrosines of Igα and Igβ is necessary for the opening of BCR oligomers on B cells exposed to multivalent antigens, while a SFK such as Lyn enhances but is not absolutely required for this process (Kläsener et al, 2014). This is in agreement with earlier reports showing that the BCR can signal independently of SFKs, but strictly requires Syk activity (Takata et al, 1994). Interestingly, it was recently found that in the presence of SFK inhibitors, B cells with a HEL‐specific BCR cannot be stimulated by monomeric soluble HEL, whereas multimeric HEL still elicits a response in the absence of SFK activity (Mukherjee et al, 2013). We therefore asked whether Lyn, the dominant SFK in B cells, is involved in sensing monovalent reagents by the BCR. We first interbred B1‐8 transgenic and Lyn‐deficient mice (Hibbs et al, 1995). B cells isolated from the spleen of Lyn‐deficient B1‐8 mice (Appendix Fig S4) were exposed to different antigens and antibodies and analysed as described above. The Lyn‐deficient B1‐8 B cells showed a strong calcium signal upon binding of NIP15‐BSA (Fig 2A), although, in comparison with B1‐8 B cells, the peak of the response was delayed (Fig EV3A). The 1NIP‐pep, however, failed to induce a calcium release in these B cells (Figs 2A and EV3B). Furthermore, the Lyn‐deficient B cells responded only to the complete anti‐idiotypic antibodies Ac146 and Ac38, but not to Fab fragments derived from these antibodies (Fig 2B and C). Both anti‐idiotypic Fab fragments required the binding of secondary anti‐κ antibodies to induce a calcium response (Appendix Fig S5). The analysis of the BCR proximity by Fab‐PLA confirmed the results of the calcium assay. Only the polyvalent NIP15‐BSA, but none of the monovalent reagents, opened the IgM‐BCR or IgD‐BCR on Lyn‐deficient B1‐8 B cells (Fig 2D and E).
Figure 2. Lyn is indispensable for the opening and signalling of the BCR upon monovalent antigen binding.
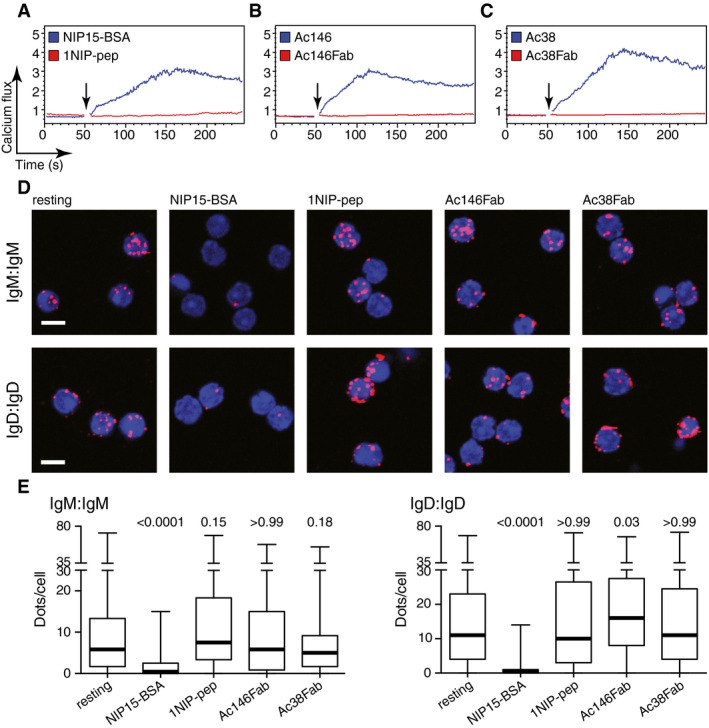
-
A–CCalcium flux measured by FACScan for splenic B cells isolated from Lyn‐deficient B1‐8 transgenic mice after stimulation with (A) NIP15‐BSA (30 pM) or 1NIP‐pep (80 nM); (B) Ac146 antibody (12.5 nM) or Ac146Fab (25 nM); (C) Ac38 antibody (12.5 nM) or Ac38Fab (25 nM). Arrows indicate the addition of the stimuli to the cells.
-
DRepresentative microscopic images showing Fab‐PLA results monitoring the BCR proximity for the IgM‐BCR (upper) and the IgD‐BCR (lower) on untreated or treated B1‐8 splenic B cells. PLA signals are shown as red dots, and nuclei were visualized by DAPI staining. Scale bar: 5 μm.
-
EQuantified Fab‐PLA results are presented as box plots, where the median values are highlighted as thick lines and the whiskers represent the minimum and maximum value. PLA signals (dots/cells) were counted from at least 100 cells for each sample; P‐values were calculated by Kruskal–Wallis one‐way ANOVA.
Figure EV3. Monovalent antigen‐induced BCR signalling is Lyn dependent.
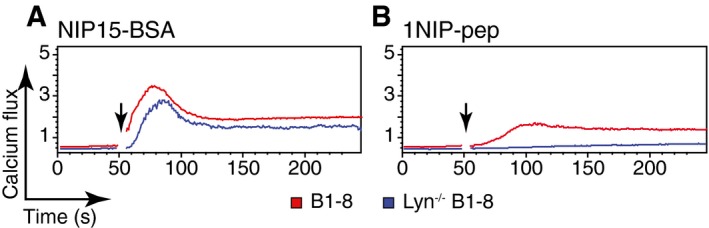
-
A, BCalcium flux measured by FACScan for splenic B cells isolated from B1‐8 and Lyn‐deficient B1‐8 mice upon stimulation with (A) NIP15‐BSA (30 pM) or (B) 1NIP‐pep (80 nM). Stimuli were added at time points indicated by the arrows. Data are representative of three independent experiments.
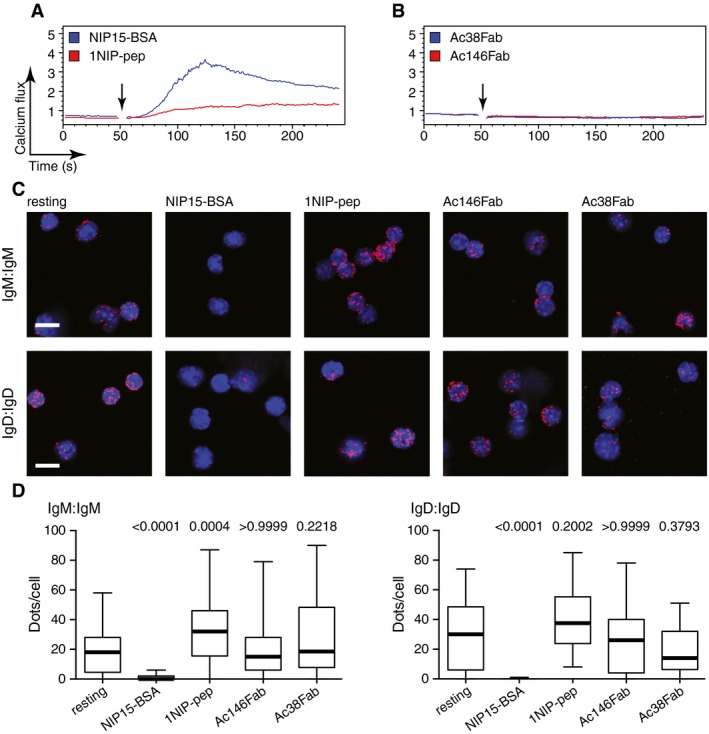
We next treated B1‐8 splenic B cells for 45 min with 1 μM of the SFK inhibitor PP2 to inhibit the kinase activity of Lyn. Similar to the Lyn‐deficient B cells, the PP2‐treated B cells did not open the IgM‐BCR and IgD‐BCR and hardly mobilize calcium upon the exposure to monovalent reagents (Fig EV4). Together, these results suggest that Lyn plays a crucial role in the sensing of monovalent reagents.
Figure EV4. The kinase activity of Lyn is crucial for monovalent antigen‐induced calcium response and BCR opening.
-
A, BCalcium flux measured by FACScan for splenic B cells isolated from B1‐8 transgenic mice after the stimulation with NIP15‐BSA (30 pM), 1NIP‐pep (80 nM), Ac146Fab (25 nM) or Ac38Fab (25 nM) after 45‐min incubation with 1 mM PP2. Arrows indicate the addition of the stimuli to the cells.
-
CRepresentative microscopic images showing Fab‐PLA results monitoring the BCR proximity for IgM‐BCR and IgD‐BCR on untreated or treated cells. PLA signals are shown as red dots and nuclei were visualized by DAPI staining. Scale bar: 5 μm.
-
DQuantified PLA results presented as box plots. The median values are highlighted as thick lines and the whiskers represent the minimum and maximum value. PLA signals (dots/cells) were counted from at least 100 cells for each sample. P‐values were calculated by Kruskal–Wallis one‐way analysis of variance (ANOVA).
The IgD‐BCR opens, but fails to signal upon exposure to monovalent reagents
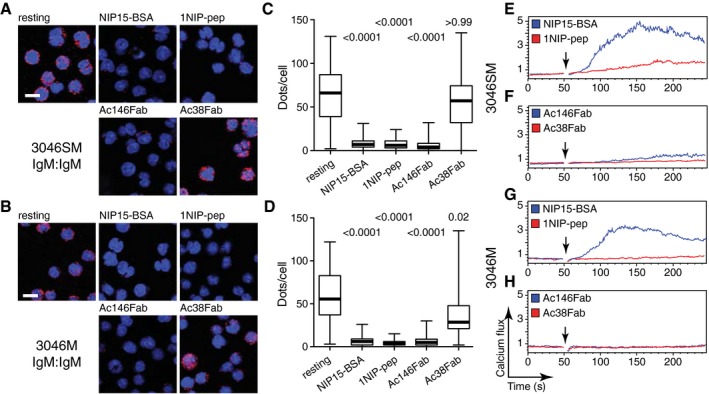
B1‐8 splenic B cells co‐express IgM‐BCR and IgD‐BCR oligomers on their surface. It is thus not possible to determine the individual contribution of each receptor class to the antigen‐induced calcium response of splenic B cells. To address this question, we expressed NIP‐specific IgM‐BCR or IgD‐BCR separately on the surface of the SLP65/Igα‐double‐deficient pro‐B‐cell line 3046 transfected with expression vectors for Igα and the Bcr‐Abl oncogene (Yang & Reth, 2010a). Although the 3046 cells produce the surrogate light chain components VpreB and λ5, they express after their transfection with λ LC and HC vectors mostly a BCR instead of a pre‐BCR on the cell surface (Appendix Figs S6 and S7). As SLP65 is an important part of the calcium signalosome of mature B cells (Fruman et al, 2000), we also transfected the 3046 pro‐B cells with a SLP65 expression vector to generate the 3046S cell line. The expression of the mIg chains at similar levels and their similar 1NIP‐pep binding capacity on the different mIg transfectants of 3046 and 3046S cells were assessed by flow cytometry (Appendix Fig S7). The cells were then exposed for 1 min to different antigens and antibodies and analysed for BCR oligomer opening and calcium mobilization (Fig 3). The IgM‐BCR was opened upon the exposure of 3046SM or 3046M B cells to either the polyvalent antigen NIP15‐BSA or the monovalent reagent 1NIP‐pep or Ac146Fab, whereas Ac38Fab again failed to alter the BCR conformation (Fig 3A–D). The monovalent reagents that opened the IgM‐BCR also induced a calcium response in 3046SM, albeit not in the SLP65‐deficient 3046M B cells (Figs 3E–H and EV5A–C). In contrast, the polyvalent antigen NIP15‐BSA induced a calcium release in both IgM‐BCR‐positive 3046 B‐cell lines (Fig 3E and G). These data show that the calcium response induced by binding of monovalent reagents to the IgM‐BCR is dependent on SLP65.
Figure 3. Monovalent antigen binding to IgM‐BCR induces calcium signalling in a SLP‐65 dependent manner.
-
A–DProximity between IgM‐BCR on the surface of 3046SM (A, C) or 3046M (B, D) cells before and after a 1‐min stimulation with the indicated reagents, assayed by Fab‐PLA. Results are presented as representative microscopic images (A, B) and box plots after quantification (C, D). PLA signals are shown as red dots, and nuclei were visualized by DAPI staining. Scale bar: 5 μm. In the box plots, the median values are highlighted as thick lines and the whiskers represent the minimum and maximum value. PLA signals (dots/cells) were counted from at least 100 cells for each sample, and P‐values were calculated by Kruskal–Wallis one‐way ANOVA.
-
E–HCalcium flux measured by FACScan for 3046SM (E, F) and 3046M (G, H) cells after stimulation with NIP15‐BSA (30 pM), 1NIP‐pep (80 nM), Ac146Fab (25 nM) or Ac38Fab (25 nM). Arrows indicate the addition of the stimuli to the cells.
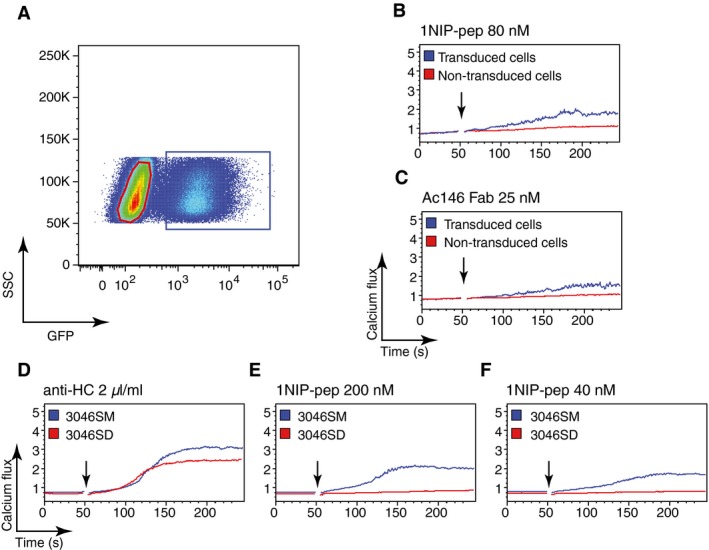
Figure EV5. Monovalent antigen induced calcium signalling only in IgM‐BCR‐expressing cells.
-
AFACScan analysis of 3046S cells transduced with IgM‐BCR components. The GFP‐positive gate represents the transduced cells (3046SM).
-
B, CCalcium flux measured by FACScan for the transduced 3046SM cells upon stimulation with (B) 1NIP‐Pep (80 nM) or (C) Ac146 Fab (25 nM). The non‐transduced cells in the same tube function as internal control.
-
D–FCalcium flux measured by FACScan for 3046SM and 3046SD cells upon stimulation with (D) anti‐HC (2 μl/ml), or (E) 200 nM or (F) 40 nM of 1NIP‐pep.
We next analysed the conformation and signalling function of the IgD‐BCR expressed on 3046SD or 3046D B cells (Fig 4). The binding of either NIP15‐BSA or the monovalent reagents 1NIP‐pep and Ac146Fab to the IgD‐BCR on these two B‐cell lines resulted in the opening of receptor oligomers, whereas the Ac38Fab failed to do so (Fig 4A–D). Thus, in terms of receptor opening, the IgD‐BCR and IgM‐BCR behave similarly. The two BCR classes differ, however, in their requirements for calcium signalling. Only the polyvalent NIP15‐BSA, but none of the monovalent reagents, induced a calcium release and that held true even in 3046SD B cells expressing the SLP65 adaptor (Figs 4E–H and EV5D–F). These results are in agreement with the recent finding that antigens with low valency are not able to trigger IgD‐BCR signalling (Ubelhart et al, 2015). Together, our data show that a calcium signal is always associated with BCR opening, but that this opening does not always result in a calcium response.
Figure 4. Monovalent antigen binding opens IgD‐BCR oligomers, but fails to induce a calcium response.

-
A–DProximity between IgD‐BCRs on the surface of 3046SD (A, C) or 3046D (B, D) cells before and after a 1‐min stimulation, assayed by Fab‐PLA. Results are presented as representative microscopic images (A, B) and box plots after quantification (C, D). PLA signals are shown as red dots and nuclei were visualized by DAPI staining. Scale bar: 5 μm. In the box plots, the median values were highlighted as thick lines and the whiskers represent the minimum and maximum value. PLA signals (dots/cells) were counted from at least 100 cells for each sample, and P‐values were calculated by Kruskal–Wallis one‐way ANOVA.
-
E–HCalcium flux measured by FACScan for 3046SD (E, F) and 3046D (G, H) cells after stimulation with NIP15‐BSA (30 pM), 1NIP‐pep (80 nM), Ac146Fab (25 nM) or Ac38Fab (25 nM). Arrows indicate the addition of the stimuli to the cells.
A monovalent antigen enhances BCR signalling induced by anti‐Ig antibody
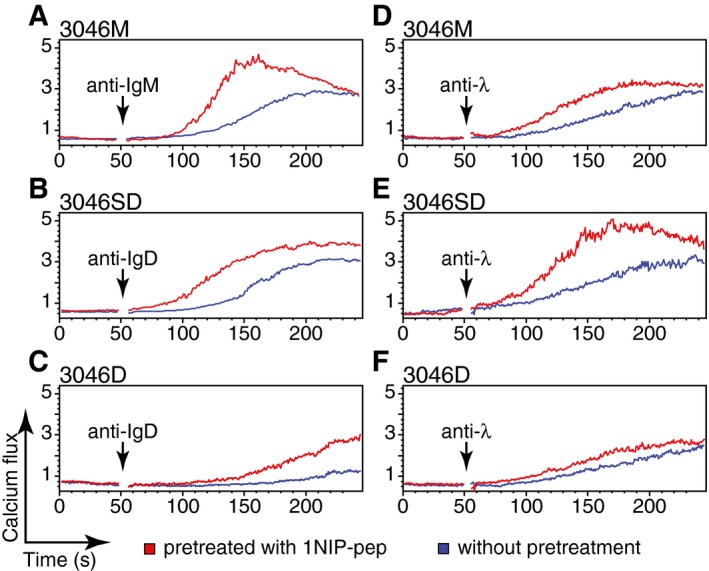
The surprising finding that the monovalent antigen 1NIP‐pep can open the BCR but fails to induce a calcium flux in the 3046M, 3046SD and 3046D B‐cell lines prompted us to examine whether the opened BCR oligomers showed a different signalling behaviour when exposed to different anti‐Ig antibodies. For this, we used flow cytometry to monitor the anti‐Ig‐induced calcium response of the different IgM‐BCR or IgD‐BCR‐expressing B‐cell lines, with or without 1NIP‐pep pretreatment (Fig 5). All the B cells pretreated with the 1NIP‐pep showed a faster and stronger calcium flux after the addition of anti‐Ig antibodies, compared with untreated B cells. This was seen after the stimulation of pretreated B cells with anti‐HC (Fig 5A–C) or anti‐λ LC antibodies (Fig 5D–F), showing that the additional aggregation of the BCR is required for the observed signal augmentation, regardless of which epitope is targeted by the anti‐Ig antibody binding. For the two IgD‐BCR‐expressing B‐cell lines 3046SD and 3046D, SLP65 expression is associated with an increased calcium response, in agreement with the role of SLP65 as part of the B‐cell calcium signalosome (compare Fig 5B and E with 5C and F). The pretreatment of the B cells with the monovalent Ac38Fab reagent, however, did not boost the calcium response after anti‐Ig stimulation (Appendix Fig S8). Taken together, these studies suggest that BCR opening and BCR aggregation are two different aspects of B‐cell activation.
Figure 5. Monovalent antigen‐prestimulated BCRs are more responsive to anti‐Ig antibody stimulation.
-
A–FCalcium flux measured by FACScan for 3046M (A, D), 3046SD (B, E) and 3046D (C, F) cells stimulated with anti‐HC (2 μl/ml) (A, B, C) or anti‐λ (2 μl/ml) (D, E, F) antibodies with or without 1‐min prestimulation with 1NIP‐pep (80 nM). The addition of antibodies is indicated by black arrows. The 1NIP‐pep was added immediately before recording. Data are representative of three independent experiments.
CD19 enhances the BCR response to monovalent antigen
It is well known that the co‐aggregation of the BCR with the co‐receptor CD19 augments the BCR signal 100‐ to 1,000‐fold (Carter et al, 1991). Increased BCR signalling was also observed when the CD19 co‐receptor was engaged by anti‐CD19 antibodies on B cells exposed to either their cognate antigen or anti‐Ig antibodies (Fearon & Carter, 1995). We thus asked whether an anti‐CD19 antibody could also augment the calcium response of the NIP‐specific B cells stimulated by the monovalent antigen 1NIP‐pep. We exposed B1‐8 splenic B cells and the different 3046 B‐cell transfectants expressing IgM‐BCR or IgD‐BCR either to 1NIP‐pep, to the anti‐CD19 antibody or to a combination of both reagents (Fig 6). The exposure of B1‐8 B cells to anti‐CD19 antibody alone induced only a weak and late calcium response. However, in combination with 1NIP‐pep, the anti‐CD19‐induced calcium release is more rapid and stronger than the response seen in B1‐8 B cells exposed to each reagent alone (Fig 6A). The synergistic effect of the combination of 1NIP‐pep and the anti‐CD19 antibody on the calcium response was also seen with 3046SM B cells (Fig 6B), but to a much lesser extent in the SLP65‐deficient 3046M or the IgD‐BCR‐expressing 3046 B cells (Fig 6C–E), although these cells carry similar amounts of CD19 on their cell surface (Appendix Fig S9). The latter B cells, however, did not respond at all when exposed to either 1NIP‐pep or the anti‐CD19 antibody alone.
Figure 6. Stimulation of the B cell with anti‐CD19 enhances its response to monovalent antigen.
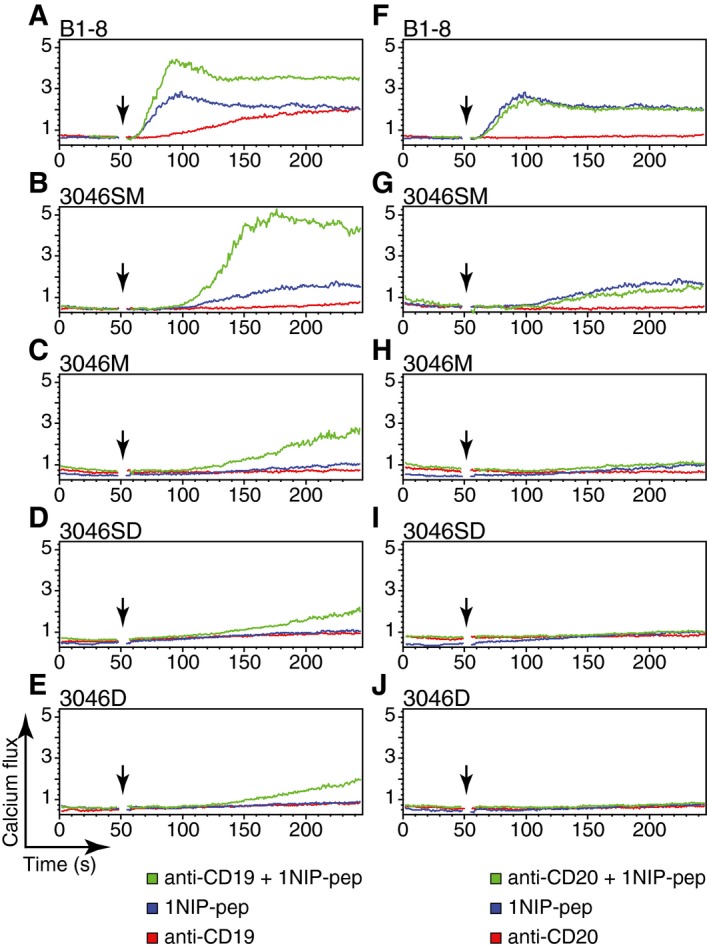
-
A–EB1‐8 B cells, 3046SM cells, 3046M cells, 3046SD cells or 3046D cells were stimulated with 1NIP‐pep (80 nM), or anti‐CD19 antibody (62 nM), or co‐stimulated with 1NIP‐pep and anti‐CD19 antibody. The calcium fluxes were measured by FACScan.
-
F–JB1‐8 B cells, 3046SM cells, 3046M cells, 3046SD cells or 3046D cells were stimulated with 1NIP‐pep (80 nM), or anti‐CD20 antibody (62 nM), or co‐stimulated with 1NIP‐pep and anti‐CD20 antibody. The calcium fluxes were measured by FACScan.
On resting B cells, CD19 is co‐localized with the tetraspanin CD20 in an IgD‐BCR‐containing protein island (Kläsener et al, 2014). We thus asked whether co‐stimulation of B1‐8 splenic B cells, or the various 3046 B‐cell transfectants, with 1NIP‐pep and anti‐CD20 antibody also resulted in an increased calcium response and found that this was not the case (Fig 6F–J). Antibodies to other B‐cell surface proteins, including CD21, CD22, CD81 and MHC class II, were also unable to enhance the calcium response of the B cells exposed to the monovalent antigen 1NIP‐pep (data not shown). The co‐stimulatory effect is thus a unique feature of anti‐CD19 antibodies.
Discussion
We here used the Fab‐PLA technique to compare the conformational alterations of the IgM‐BCR and IgD‐BCR at 10–20 nanometre distances with the signalling output, when B cells are exposed to monovalent antigens or anti‐BCR Fab fragments. We found that the calcium response is always associated with a loss of the Fab‐PLA signal indicating BCR opening. However, the opening of the BCR oligomers did not always induce a calcium response. Furthermore, we found that Lyn is required for the opening of the BCR by monovalent reagents and that co‐ligation with anti‐Ig or anti‐CD19 antibodies can augment BCR signals induced by the binding to these monovalent reagents.
Previously, only complete, divalent anti‐BCR antibodies, but not monovalent Fab preparations of these antibodies, were reported to activate the BCR (Woodruff et al, 1967). This observation was interpreted to mean that a “cross‐linking” of two monomeric BCR complexes is required for B‐cell activation, thereby supporting the cross‐linking model. However, in disagreement with this model, monovalent antigens such as HEL or OVA are able to efficiently activate the BCR (Kim et al, 2006; Mukherjee et al, 2013; Avalos et al, 2014). These monovalent antigens can bind to, but never directly cross‐link, the BCR. Furthermore, the recent finding that the numerous BCR complexes on the surface of resting B cells are forming oligomers that are concentrated inside dense nanoclusters or protein islands (Yang & Reth, 2010a; Fiala et al, 2013; Mattila et al, 2013; Kläsener et al, 2014; Maity et al, 2015) is also in conflict with the cross‐linking model predicting a random distribution of BCR monomers.
When the cross‐linking model was formulated, it was not possible to directly monitor the conformation of the BCR at nanometre distances. With the Fab‐PLA technique, we are, for the first time, able to monitor the nanoscale conformation of the BCR before and after its exposure to either anti‐BCR antibodies or different forms of antigens. In agreement with previous data, we found that, in most cases, only full‐length, divalent anti‐BCR antibodies, but not their Fab fragments, can activate the B cells. However, with Fab‐PLA, we found that this B‐cell activation is not accompanied by the cross‐linking of two monomeric BCR complexes, but rather the dissociation of preformed BCR oligomers. An interesting exception to the failure of anti‐BCR Fab fragments to activate the BCR is the anti‐idiotypic Ac146Fab. This reagent opens and activates the IgM‐BCR oligomer to a similar extent as the monovalent antigen 1NIP‐pep, whereas the Ac38Fab fails, to do this. Of the two anti‐idiotypic antibodies, only Ac146 directly interacts with the anti‐NIP binding site and competes with the free hapten NIP‐cap for binding to the B1‐8 antibody (Reth et al, 1979).
The different reactivity of the two anti‐idiotypic Fab fragments suggests that the antigen‐binding region is a privileged site for the opening of the BCR oligomer. It is thus feasible that within the BCR oligomer, the antigen‐binding regions are closely clustered together. In this way, tiny alterations of the binding site conformation that are induced by occupancy with a monovalent antigen could disturb the clusters of antigen‐binding regions in a way that results in BCR oligomer dissociation. The Fab fragments of other anti‐idiotypic antibodies, which bind outside the binding site, or of anti‐Ig antibodies binding to the constant part of the HC or the LC would not be able to do so. These reagents are only active as a part of a full‐length antibody that, with its bivalent binding mode, can directly pull apart two neighbouring BCR molecules, thereby disturbing and activating the BCR oligomer.
Interestingly, the monovalent reagent 1NIP‐peptide or Ac146Fab can no longer open and activate the BCR oligomer in the absence or upon the inhibition of the SFK Lyn (Figs 2 and EV4). In accordance with this, Mukherjee et al (2013) found that soluble HEL does not activate HEL‐specific B cells exposed to the SFK inhibitor PP2. Similarly, it was also found that PP2 blocks BCR signalling induced by antigen, but not by anti‐BCR antibodies (Stepanek et al, 2013). Furthermore, PP2 also inhibits the conformational opening of the IgM‐BCR monitored by a live imaging FRET assay (Tolar et al, 2005). These findings suggest that SFK plays an important role in the antigen sensing process. Currently, the role of SFK such as Lyn in BCR signalling has only been discussed in the context of its ability to directly phosphorylate and interact with the first ITAM tyrosine of the BCR signalling subunit Igα. However, SFKs are also involved in cytoskeletal regulation (Playford & Schaller, 2004). In resting B cells, the cytoskeleton limits BCR diffusion, whereas B‐cell activation is accompanied by a rapid actin depolymerization and increased BCR diffusion (Treanor et al, 2010, 2011). Furthermore, the disruption of the actin cytoskeleton by the actin depolymerization agent latrunculin A results in an opening of the BCR oligomer and B‐cell signalling (Kläsener et al, 2014; Maity et al, 2015). Together, these data suggest that the cortical actin cytoskeleton supports the oligomeric conformation of the BCR and sets the critical thresholds for BCR activation. It is thus feasible that on resting B cells, the BCR oligomers are connected to Lyn via the actin cytoskeleton. Small conformational alterations induced by the binding of monovalent antigens may induce Lyn to promote actin depolymerization in the vicinity of the BCR, thus increasing the accessibility of the ITAM tyrosines so that Syk can efficiently open the BCR oligomer. This scenario would also explain the delayed kinetics of BCR opening in Lyn‐deficient B cells exposed to multivalent antigens (Mukherjee et al, 2013; Kläsener et al, 2014).
It is well known that Lyn‐deficient mice develop B‐cell autoimmunity (Hibbs et al, 1995; Nishizumi et al, 1995). This phenotype was explained by the finding that inhibitory receptors such as CD22 are less active without Lyn, thus resulting in a prolonged B‐cell activation in these mice. Indeed, CD22 requires phosphorylation by Lyn for coupling to the protein tyrosine phosphatase SHP‐1, which counteracts Syk activity (Cornall et al, 1998). However, our finding that Lyn is critically involved in antigen sensing suggests that an alteration of the self‐/non‐self‐selection process for B cells may also contribute to the autoimmune phenotype of Lyn‐deficient mice. The increased threshold of BCR activation of Lyn‐deficient B cells may allow autoreactive B cells to pass this checkpoint and develop into mature B cells that then promote the disease.
Although monovalent antigens such as 1NIP‐pep can open the BCR oligomers as efficiently as the polyvalent antigen NIP15‐BSA, only the latter one induced a stronger calcium response in naïve B1‐8 B cells. Similarly, Ac146Fab is more efficient in calcium signalling when further aggregated by anti‐κ antibodies (Appendix Fig S5). Thus, in terms of signalling, BCR aggregation provides an additional quality beyond BCR opening. We think that this phenomenon can be explained by the recent finding that the B‐cell plasma membrane is highly compartmentalized. All receptors, including the BCR, are probably concentrated inside distinct protein islands or nanoclusters (Mattila et al, 2013; Kläsener et al, 2014). For example, we recently found that the IgM‐BCR and the IgD‐BCR are localized inside different protein islands with different dimensions and composition. Interestingly, upon B‐cell activation, the IgM and IgD protein islands move closer together and this may allow the spreading and amplification of the BCR signal (Maity et al, 2015). Similarly, on the T‐cell surface, the TCR complex and the transmembrane adaptor LAT are localized on distinct protein islands that concatenate upon T‐cell activation (Lillemeier et al, 2010). This aggregation or concatenation may facilitate the interaction of the BCR with the relevant calcium channels that so far have not been localized at a particular protein island.
We think that the observed differences in the calcium response of B cell exposed to either monovalent or polyvalent antigens may reflect signalling events occurring only inside a specific protein island or between protein islands. In this way, the monovalent antigen 1NIP‐pep may open the BCR oligomers only within specific BCR islands, whereas polyvalent antigens could induce BCR clusters, thereby promoting the concatenation of many different protein islands and the amplification of the BCR signal. This scenario is supported by our finding that the monovalent reagent 1NIP‐pep or Ac146Fab opens the IgM‐BCR on SLP‐65‐deficient 3046M B cells without inducing a calcium response. In pre‐B‐cell lines such as 3046, the transmembrane adaptor LAT is expressed and can partially substitute for the missing SLP‐65 calcium signalosome (Su & Jumaa, 2003). Similar to what has been found in T cells, LAT may also be localized on the B‐cell surface in distinct protein islands. Thus, only polyvalent reagents would induce larger BCR aggregates required for the concatenation of the LAT and BCR protein islands and efficient calcium signalling.
That the opening of BCR oligomers and BCR aggregation are two different B‐cell signalling modes is also indicated by our finding that a prestimulation of 3046 transfectants with the monovalent antigen 1NIP‐pep can increase the calcium response induced by low amounts of anti‐Ig antibodies. This cooperative behaviour between receptor conformation and aggregation has also been found in a study of TCR signalling (Minguet et al, 2007).
The isotype‐specific compartmentalization of the B‐cell plasma membrane may also be the reason for the different signalling output of the IgM‐BCR and IgD‐BCR when exposed to monovalent antigens. Ubelhart et al (2015) found that soluble HEL does not induce a calcium flux in HEL‐specific B cells expressing only an IgD‐BCR. The authors assumed that the highly flexible hinge region of the IgD‐BCR prevented opening and activation of the IgD‐BCR oligomer by monovalent antigens. In our Fab‐PLA studies, we found, however, that monovalent antigens are able to open the IgD‐BCR just as well as the IgM‐BCR oligomer, thus disproving this assumption. It may be the different nanoenvironments inside the IgM and IgD protein islands that render the opened, but not aggregated, IgD‐BCR signalling inert. On the surface of resting B cells, the IgD‐BCR is found in close proximity to CD19 and several tetraspanins such as CD81 and CD20, whereas the IgM‐BCR gains access to these proteins only after the B‐cell activation (Kläsener et al, 2014). Inside the IgD protein islands, the conformation of CD19 may be such that it inhibits rather than promotes BCR signalling. Once bound by anti‐CD19 antibodies, however, CD19 may switch from a negative to a positive signalling mode and this could explain why these antibodies increase the signalling efficiency of monovalent antigens.
In summary, we here provide direct evidence that the exposure of B cell to monovalent antigens can alter the conformation of the BCR. The increased potency of polyvalent antigens for B‐cell activation could be due to the fact that these reagents not only alter the BCR conformation but also the nanoscale organization of the B‐cell membrane.
Materials and Methods
Antibodies
For FACS analysis of splenic B cells or 3046 cells, the following fluorophore‐conjugated anti‐mouse antibodies were used: anti‐IgM‐efluor‐660 (II/41, eBioscience), anti‐IgD‐AlexaFluor‐647 (11‐26c.2a, BioLegend), anti‐CD19‐eFluor‐660 (eBio1D3, eBioscience), anti‐CD20‐PE (AISB12, eBioscience), anti‐λ‐biotin (goat anti‐mouse, polyclonal antiserum, Southern Biotech) and streptavidin‐APC (BD Pharmingen) and anti‐λ5‐PE (lm34, homemade). For stimulating cells and measuring Ca2+ response, the following antibodies were used: anti‐IgD (goat anti‐mouse, polyclonal antiserum, eBioscience), anti‐IgM (goat anti‐mouse, polyclonal antiserum, Southern Biotech), anti‐λ (goat anti‐mouse, polyclonal antiserum, Southern Biotech), anti‐CD19 (eBio1D3, eBioscience), anti‐CD20 (AISB12, eBioscience), anti‐MHCII (NIMR‐4, eBioscience), anti‐CD21 (7E9, BioLegend), anti‐CD22 (OX‐97, BioLegend), anti‐CD81 (JS‐81, BD Pharmingen), Ac146 and Ac38 (both prepared from in‐house hybridoma). Ac146 Fab, Ac38 Fab and anti‐λ Fab were prepared from the corresponding antibodies using Pierce Fab Micro preparation kit following the manufacturer's instructions. For cross‐linking Ac146 Fab or Ac38 Fab, anti‐κ‐PE (187.1, BD Pharmingen) was used.
For preparing PLA probes, the following antibodies were used: anti‐IgD (11‐26c, Southern Biotech) and anti‐IgM (1B4B1, Southern Biotech). The following Abs were used for Western blotting: anti‐phospho‐Igα‐Y182 (polyclonal, Cat# 5173, Cell Signaling), anti‐phospho‐AKT‐S473 (D9E XP, Cell Signaling), anti‐phospho‐ERK1/2‐T202/Y204 (D13.14.4E XP, Cell Signaling), anti‐Syk (D3Z1E XP, Cell Signaling) and anti‐β‐actin (I‐19, Santa Cruz).
Hapten, antigen and chemicals
NIP15‐BSA was purchased from Biosearch Technologies, USA. 1NIP‐peptide was custom‐synthesized from IRIS Biotech, Germany. Components for 1NIP‐DNA were ordered from Purimex, Germany.
Isolation of mouse spleen B cells
Total splenocytes were isolated from 8‐ to 12‐week‐old κ‐deficient B1‐8 or Lyn−/− κ‐deficient B1‐8 transgenic mice harbouring a NIP‐specific BCR. Splenic B cells were enriched by MACS depletion of CD43+ cells using anti‐CD43 magnetic beads (Miltenyi Biotech) according to the manufacturer's instructions. The purified B cells were left for a minimum of 2 h at 37°C in 7.5% CO2, in Iscove's medium (Biochrom, with stable glutamine) supplemented with 10 units/ml penicillin/streptomycin, 50 μM β‐mercaptoethanol (Sigma) and 10% FCS (PAN Biotech). The B cells were then further analysed for BCR surface expression and calcium flux.
Cell culture and transfection
The Igα−/− and SLP65−/− double‐deficient pro‐B‐cell line (3046) was cultured at 37°C in 7.5% CO2, in Iscove's medium (Biochrom, with stable glutamine) supplemented with 10 units/ml penicillin/streptomycin, 0.4 ng/ml mouse recombinant IL‐7 (all Invitrogen), 50 μM β‐mercaptoethanol (Sigma) and 10% FCS (PAN Biotech).
Retroviral transductions were performed as previously described (Storch et al, 2007). In brief, Phoenix cells were transfected using PolyJet DNA in vitro transfection reagent following the manufacturer's protocol (SignaGen Laboratories). Retrovirus‐containing supernatants were collected 48 h after transfection and used for transduction.
Calcium measurement and flow cytometry
Calcium measurements were performed as previously described (Storch et al, 2007). In brief, 1 × 106 cells were loaded with indo‐1 (Molecular Probes) following the manufacturers' instructions and resuspended in 500 μl Iscove's medium supplemented with 1% FCS. Before analysis, the cells were pre‐warmed for 5 min at 37°C. Stimuli were added after 1 min of baseline recording. Calcium flux was measured with an LSR II (BD).
For FACS analysis of BCR surface expression in 3046 cells, 5 × 105 cells were stained with anti‐IgM‐efluor‐660 (1:100) or anti‐IgD‐AlexaFluor‐647 (1:100) for the corresponding HC, anti‐λ‐biotin/streptavidin‐APC (1:200) for λ LC, 1NIP peptide (0.2 μM) for NIP‐specific BCR, and measured with LSR II (BD). For FACS analysis of CD19, CD20 and pre‐BCR surface expression, 5 × 105 cells were stained with anti‐CD19‐eFluor‐660 (1:100) or anti‐CD20‐PE (1:100) for the corresponding co‐receptor, and anti‐λ5‐PE (1:100) for the pre‐BCR, and measured with LSR II (BD). Data were exported in FCS‐3 format and analysed with FlowJo software (TreeStar).
PLA probe preparation and experiment
For Fab‐PLA, Fab fragments were prepared from corresponding antibodies using Pierce Fab Micro preparation kit (Thermo Fisher Scientific) according to the manufacturer's protocol. After desalting (Zeba™ spin desalting columns, Thermo Fisher Scientific), Fab fragments were coupled with PLA Probemaker Plus or Minus oligonucleotides according to the manufacturer's protocol (Olink Bioscience) to generate Fab‐PLA probes.
For in situ PLA experiments, the cells were settled on polytetrafluoroethylene (PTFE)‐coated slides (Thermo Fisher Scientific) for 30 min at 37°C. After treatment, non‐stimulated and stimulated cells were fixed for 15 min with 2% paraformaldehyde, containing 0.02% glutaraldehyde, in PBS. PLA was performed as previously described (Kläsener et al, 2014). In brief, after incubation with a blocking solution containing 25 μg/ml sonicated salmon sperm DNA and 250 μg/ml BSA in PBS, the cells were incubated with Fab‐PLA probes in Probemaker diluent. PLA signal amplification was performed following the manufacturer's protocol. Resulting samples were directly mounted on slides with DAPI‐Fluoromount‐G (Southern Biotech) to visualize the PLA signals in relation to the nucleus.
Imaging and image analysis
All microscopic images were acquired using a Zeiss 780 Meta confocal microscope (Carl Zeiss), equipped with a Zeiss Plan‐Apochromat 63× oil immersion objective lens. For each sample, several images were captured from randomly chosen regions. All recorded images were analysed with BlobFinder software (Centre for Image Analysis, Uppsala University). PLA signals (dots/cells) were counted from at least 100 cells for each sample.
Data processing and statistical analysis
Raw data produced by BlobFinder were exported to Prism software (GraphPad, La Jolla, CA). Since most of the data did not pass the D'Agostino–Pearson omnibus normality test, box plots were chosen to present the data and P‐values were obtained by Kruskal–Wallis one‐way analysis of variance (ANOVA).
Western blot for protein phosphorylation analysis
About 2 × 106 isolated B1‐8 splenic B cells were resuspended in 500 μl Iscove's medium supplemented with 1% FCS and equilibrated at 37°C for 10 min. The cells were then stimulated with NIP15‐BSA (30 pM), 1NIP‐pep (80 nM), Ac146 Fab (25 nM), Ac38 Fab (25 nM) or anti‐IgM antiserum (2 μl/ml) for the indicated time and immediately lysed on ice in lysis buffer containing 1% Triton X. Cleared lysates were subjected to 12% SDS–PAGE and the subsequent immunoblotting.
Author contributions
The experiments were planned by JY and MR The experiments were conducted by CV, NB and MB. The Lyn‐deficient B1‐8 mice were generated by EH. Manuscript preparation was done by JY and MR with the help of CV.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Acknowledgements
We thank Peter Nielsen and Lise Leclercq for critical reading of this manuscript. We thank Hassan Jumaa for the 3046 pro‐B‐cell line. We also thank Klaus Rajewsky and Margaret Hibbs for the B1‐8 transgenic mice and the Lyn‐deficient mice, respectively. This study was supported by the Excellence Initiative of the German Federal and State Governments (EXC 294), by ERC Grant 322972, by the Deutsche Forschungsgemeinschaft through TRR130 and by the Freiburg Institute for Advanced Studies (FRIAS) and the University of Strasbourg Institute for Advanced Study (USIAS) through the Joint Fellowship Programme Strasbourg/Freiburg.
The EMBO Journal (2016) 35: 2371–2381
Contributor Information
Jianying Yang, Email: yang@immunbio.mpg.de.
Michael Reth, Email: Michael.Reth@bioss.uni-freiburg.de.
References
- Avalos AM, Bilate AM, Witte MD, Tai AK, He J, Frushicheva MP, Thill PD, Meyer‐Wentrup F, Theile CS, Chakraborty AK, Zhuang X, Ploegh HL (2014) Monovalent engagement of the BCR activates ovalbumin‐specific transnuclear B cells. J Exp Med 211: 365–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos AM, Ploegh HL (2014) Early BCR events and antigen capture, processing, and loading on MHC class II on B cells. Front Immunol 5: 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Kurosaki T (2016) Role of calcium signaling in B cell activation and biology. Curr Top Microbiol Immunol 393: 143–174 [DOI] [PubMed] [Google Scholar]
- Benjamin CD, Miller A, Sercarz EE, Harvey MA (1980) A predominant idiotype on anti‐hen egg white lysozyme antibodies from diverse mouses strains. J Immunol 125: 1017–1025 [PubMed] [Google Scholar]
- Carrasco YR, Batista FD (2006) B cell recognition of membrane‐bound antigen: an exquisite way of sensing ligands. Curr Opin Immunol 18: 286–291 [DOI] [PubMed] [Google Scholar]
- Carter RH, Tuveson DA, Park DJ, Rhee SG, Fearon DT (1991) The CD19 complex of B lymphocytes. Activation of phospholipase C by a protein tyrosine kinase‐dependent pathway that can be enhanced by the membrane IgM complex. J Immunol 147: 3663–3671 [PubMed] [Google Scholar]
- Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC (1998) Polygenic autoimmune traits: Lyn, CD22, and SHP‐1 Are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity 8: 497–508 [DOI] [PubMed] [Google Scholar]
- Fearon DT, Carter RH (1995) The CD19/CR2/TAPA‐1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol 13: 127–149 [DOI] [PubMed] [Google Scholar]
- Fiala GJ, Kaschek D, Blumenthal B, Reth M, Timmer J, Schamel WWA (2013) Pre‐clustering of the B cell antigen receptor demonstrated by mathematically extended electron microscopy. Front Immunol 4: 427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Satterthwaite AB, Witte ON (2000) Xid‐like phenotypes: a B cell signalosome takes shape. Immunity 13: 1–3 [DOI] [PubMed] [Google Scholar]
- Fu C, Turck CW, Kurosaki T, Chan AC (1998) BLNK: a central linker protein in B cell activation. Immunity 9: 93–103 [DOI] [PubMed] [Google Scholar]
- Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR (1995) Multiple defects in the immune system of Lyn‐deficient mice, culminating in autoimmune disease. Cell 83: 301–311 [DOI] [PubMed] [Google Scholar]
- Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M (1990) Molecular components of the B‐cell antigen receptor complex of the IgM class. Nature 343: 760–762 [DOI] [PubMed] [Google Scholar]
- Kim YM, Pan JYJ, Korbel GA, Peperzak V, Boes M, Ploegh HL (2006) Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation. Proc Natl Acad Sci USA 103: 3327–3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kläsener K, Maity PC, Hobeika E, Yang J, Reth M (2014) B cell activation involves nanoscale receptor reorganizations and inside‐out signaling by Syk. elife 3: e02069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillemeier BF, Mörtelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM (2010) TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol 11: 90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity PC, Blount A, Jumaa H, Ronneberger O, Lillemeier BF, Reth M (2015) B cell antigen receptors of the IgM and IgD classes are clustered in different protein islands that are altered during B cell activation. Sci Signal 8: ra93 [DOI] [PubMed] [Google Scholar]
- Mattila PK, Feest C, Depoil D, Treanor B, Montaner B, Otipoby KL, Carter R, Justement LB, Bruckbauer A, Batista FD (2013) The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor‐mediated signaling. Immunity 38: 461–474 [DOI] [PubMed] [Google Scholar]
- Minguet S, Swamy M, Alarcón B, Luescher IF, Schamel WWA (2007) Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity 26: 43–54 [DOI] [PubMed] [Google Scholar]
- Minguet S, Dopfer E‐P, Schamel WWA (2010) Low‐valency, but not monovalent, antigens trigger the B‐cell antigen receptor (BCR). Int Immunol 22: 205–212 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Zhu J, Zikherman J, Parameswaran R, Kadlecek TA, Wang Q, Au‐Yeung B, Ploegh H, Kuriyan J, Das J, Weiss A (2013) Monovalent and multivalent ligation of the B cell receptor exhibit differential dependence upon Syk and Src family kinases. Sci Signal 6: ra1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T (1995) Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn‐deficient mice. Immunity 3: 549–560 [DOI] [PubMed] [Google Scholar]
- Pao LI, Famiglietti SJ, Cambier JC (1998) Asymmetrical phosphorylation and function of immunoreceptor tyrosine‐based activation motif tyrosines in B cell antigen receptor signal transduction. J Immunol 160: 3305–3314 [PubMed] [Google Scholar]
- Playford MP, Schaller MD (2004) The interplay between Src and integrins in normal and tumor biology. Oncogene 23: 7928–7946 [DOI] [PubMed] [Google Scholar]
- Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL (2007) Activating B cell signaling with defined multivalent ligands. ACS Chem Biol 2: 252–262 [DOI] [PubMed] [Google Scholar]
- Reth M, Imanishi‐Kari T, Rajewsky K (1979) Analysis of the repertoire of anti‐(4‐hydroxy‐3‐nitrophenyl)acetyl (NP) antibodies in C 57 BL/6 mice by cell fusion. II. Characterization of idiotopes by monoclonal anti‐idiotope antibodies. Eur J Immunol 9: 1004–1013 [DOI] [PubMed] [Google Scholar]
- Reth M (1992) Antigen receptors on B lymphocytes. Annu Rev Immunol 10: 97–121 [DOI] [PubMed] [Google Scholar]
- Schelling ME, Silverman PH (1968) The effect of route of injection upon the development of circulating antibody in response to a variety of antigens. Immunology 14: 781–785 [PMC free article] [PubMed] [Google Scholar]
- Schmitz R, Baumann G, Gram H (1996) Catalytic specificity of phosphotyrosine kinases Blk, Lyn, c‐Src and Syk as assessed by phage display. J Mol Biol 260: 664–677 [DOI] [PubMed] [Google Scholar]
- Sonoda E, Pewzner‐Jung Y, Schwers S, Taki S, Jung S, Eilat D, Rajewsky K (1997) B cell development under the condition of allelic inclusion. Immunity 6: 225–233 [DOI] [PubMed] [Google Scholar]
- Stepanek O, Draber P, Drobek A, Horejsí V, Brdicka T (2013) Nonredundant roles of Src‐family kinases and Syk in the initiation of B‐cell antigen receptor signaling. J Immunol 190: 1807–1818 [DOI] [PubMed] [Google Scholar]
- Storch B, Meixlsperger S, Jumaa H (2007) The Ig‐alpha ITAM is required for efficient differentiation but not proliferation of pre‐B cells. Eur J Immunol 37: 252–260 [DOI] [PubMed] [Google Scholar]
- Su Y‐W, Jumaa H (2003) LAT links the pre‐BCR to calcium signaling. Immunity 19: 295–305 [DOI] [PubMed] [Google Scholar]
- Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T (1994) Tyrosine kinases Lyn and Syk regulate B cell receptor‐coupled Ca2+ mobilization through distinct pathways. EMBO J 13: 1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M, Kurosaki T (1996) A role for Bruton's tyrosine kinase in B cell antigen receptor‐mediated activation of phospholipase C‐gamma 2. J Exp Med 184: 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar P, Sohn HW, Pierce SK (2005) The initiation of antigen‐induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat Immunol 6: 1168–1176 [DOI] [PubMed] [Google Scholar]
- Treanor B, Depoil D, Gonzalez‐Granja A, Barral P, Weber M, Dushek O, Bruckbauer A, Batista FD (2010) The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity 32: 187–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor B, Depoil D, Bruckbauer A, Batista FD (2011) Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J Exp Med 208: 1055–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubelhart R, Hug E, Bach MP, Wossning T, Dühren‐von Minden M, Horn AHC, Tsiantoulas D, Kometani K, Kurosaki T, Binder CJ, Sticht H, Nitschke L, Reth M, Jumaa H (2015) Responsiveness of B cells is regulated by the hinge region of IgD. Nat Immunol 16: 534–543 [DOI] [PubMed] [Google Scholar]
- Wienands J, Schweikert J, Wollscheid B, Jumaa H, Nielsen PJ, Reth M (1998) SLP‐65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J Exp Med 188: 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff MF, Reid B, James K (1967) Effect of antilymphocytic antibody and antibody fragments on human lymphocytes in vitro. Nature 215: 591–594 [DOI] [PubMed] [Google Scholar]
- Yang J, Reth M (2010a) Oligomeric organization of the B‐cell antigen receptor on resting cells. Nature 467: 465–469 [DOI] [PubMed] [Google Scholar]
- Yang J, Reth M (2010b) The dissociation activation model of B cell antigen receptor triggering. FEBS Lett 584: 4872–4877 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Source Data for Expanded View
Review Process File


