Abstract
Nanotechnology has revolutionized science and consumer products for several decades. Most recently, its applications to the fields of medicine and biology have improved drug delivery, medical diagnostics, and manufacturing. Recent research of this modern technology has demonstrated its potential with novel forms of disease detection and intervention, particularly within orthopedics. Nanomedicine has transformed orthopedics through recent advances in bone tissue engineering, implantable materials, diagnosis and therapeutics, and surface adhesives. The potential for nanotechnology within the field of orthopedics is vast and much of it appears to be untapped, though not without accompanying obstacles.
Keywords: Nanotechnology, Nanomedicine, Orthopedics
1. What is nanotechnology?
Nanotechnology refers to the engineering of matter at a very small, molecular scale.1 Specifically, nanotechnology deals with “dimensions and tolerances of less than 100 nanometers” and particularly with the “manipulation of individual atoms and molecules.”2 The field of nanotechnology has existed for several centuries but has expanded prolifically with the emergence of the Information Age.2 In fact, early applications of nanotechnology can be traced back all the way to pre-modern times beginning with the crafting of glass for ceramic antiques.3, 4 By the mid-twentieth century, its use became integral in various fields of science, serving as the basis for instruments such as the field ion and atomic microscopes.3 The field especially grew in breadth and recognition throughout the twentieth century. Of note, Richard Feynman's description of the field during a historic 1959 talk highlighted the immense capacity and potential of nanotechnology, leading to further exploration of its applications.5, 6
“Nanotech,” as it is often called, found its way into the marketplace by the early 2000s, allowing manufacturers to improve materials such as sunscreens, tennis rackets, and display screens for electronic devices.3 In practice, the altercation of tiny molecules can make large changes; for instance, changing the girth of a guitar or tennis string can change the sounds and power of the instrument.7 Today, nanotechnology remains present in various avenues of daily life and has also grown into an increasingly important role within medical research and practice.
2. Nanomedicine
The application of nanotechnology to medicine, commonly referred to as “nanomedicine,” has been hailed as nothing short of revolutionary.8 As most biological molecules are on the nanoscale, this type of technology has had much success and room for growth within the medical field. Nanotechnology has been used in its active state to change the mechanisms by which drugs are delivered and is being explored for its potential to serve as a scaffold for nerve regeneration, among many other applications.8 Thus far, nanotechnology has proven particularly successful in transforming drug delivery and manufacturing as well as medical diagnostic tools.9 As researchers learn more about the mechanisms and characteristics of medical nano-particles, these molecules have become increasingly known for their pharmacologic potential in improving drug synthesis and carriers as well as optimizing materials and reducing toxicity.9
Some benefits of nanotechnology that have already become apparent in medicine include permanent implantation of small devices, semi-automation of diagnosis and treatment, the quick suspension of new diseases, cheaper surgical tools, and the ability to replace certain organs.10 Within cancer biology, nanotech has been used to deliver drugs such as doxorubicin in a way that shuts off cancer genes that normally allow cells to escape the drug.11 Nano-equipped breathalyzers for diabetics have been developed to measure acetone levels in one's breath, providing an alternative to traditional finger-prick glucose testing.12 Eye surgery has been robotized by nanomedicine, such as by the creation of a magnetically guided robot that allows for greater precision in surgery and dosage of drug delivery.12 Nanotechnology has also been shown to be useful in detection of cardiac arrest, serving as the basis for a sensor that detects heart attacks via screening blood cells.12 These examples are diverse but yet only represent a small fraction of the capabilities of nanomedicine, which has found a place in nearly every branch of medicine.
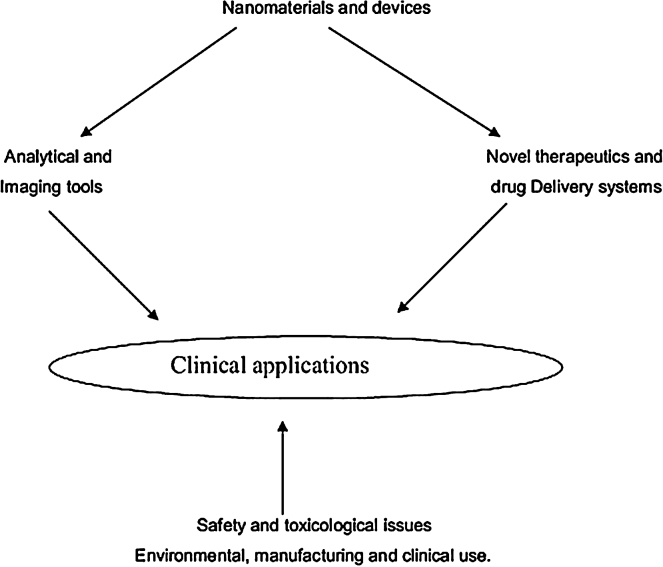
Nanotechnology has infiltrated a particularly successful niche within orthopedic surgery, where bench and translational research have uncovered several applications for manipulating nano-particles.13 Through many different avenues, these innovations allow for improved clinical capabilities. The main dimensions through which nanotechnology can be employed to make an impact in medicine and orthopedic surgery are displayed in Fig. 1.14
Fig. 1.
The main dimensions in which nanotechnology can impact clinical practice.
Source: J Indian Soc Periodontol. 2008 May–Aug; 12(2): 34–40 (http://creativecommons.org/licenses/by-nc/3.0/).
3. Current applications to orthopedic surgery
3.1. Nanotechnology for bones: implants and scaffolds
Implantable biomaterials have become essential components of orthopedic surgery, largely due to their ability to provide for osteointegration and to better stimulate healthy bone processes, especially in comparison to their standard material counterparts.15 These essential improvements come at a time of great importance, as the aging population demands an increasing amount of orthopedic implants. For instance, the use of joint implants in the US is estimated to be over 600,000 per annum and growing.16 Implants in orthopedics are used in a variety of ways across many areas of the body, but across the board, the functioning and purpose of implants are well served by the addition of nanomaterials.
Older methods of bone defect treatment, such as bone allografts and autografts, are still often employed, estimated to compose nearly 80% of surgeries related to bone defects.17 However, these techniques are accompanied by many risks including infection, rejection by the immune system, and lengthy times for complete repair, especially for minor defects.17 Implants using nanomaterials have improved upon many of those risks, but still, are not without fail. Implants derived from nanomaterials are not yet able to provide restoration of full functionality, nor do they often have longevity beyond a decade or two, at best.16 Complete failure of implants may occur and can be particularly challenging, requiring extensive and expensive re-operations.16
Nevertheless, nanotechnology has proven incredibly beneficial for use in orthopedic implants, improving the treatment of many types of bone defects and orthopedic traumas. Several materials have been investigated and applied, leading to the use of a wide array of potential materials with their own unique properties and benefits. Examples of materials include gelatin, bioactive ceramics, biodegradable polymers, and polysaccharides such as agarose.17 These nanomaterials are able to work well within the human body, as their physical properties and nanoscale features allow them to promote cell growth and tissue regeneration. The ability of these nanomaterials to mimic cellular environment is key in replicating mechanisms of cells, which also have nanometer dimensions and come together to form extra-cellular matrices.17 Furthermore, implants with nanomaterials are able to form a greater surface area, which helps cultivate a healthy environment for bone growth and reduce infection rates.17
Oftentimes, the use of nanomaterials for implants will involve a coating to provide for scaffolding. Extracellular adhesion proteins have been shown to better interact with nanophase implant scaffolds than conventional implant surfaces. Greater absorption of these proteins provides an environment that is well-suited for osteoblast adhesion, as well as for bone formation and for fusion between implant and bone.17 Furthermore, the use of nanotechnology for implants has been demonstrated to have many positive effects on clinical outcome, including decreasing the likelihood of infection and improving scar appearance.18, 19
There are many instances in which nanomaterials have been shown to work effectively. For instance, they have been integral in total joint replacements (TJRs), for which aseptic loosening is a major cause of failure.13 Nanotextured material has been shown to reduce this risk, particularly through improving osteoblast adhesion and osteointegration. Similarly, implants and scaffolds made of nanomaterials have shown to be effective across the body for bone defects. One study by Kon et al. demonstrated the effectiveness of nanocomposite implants in the treatment of osteochondral knee defects.20 Scaffolds from nanomaterials have also been used to improve treatment of peripheral nerve injuries. Collagen scaffolds that are impregnated with silver are used to increase the amount of absorbed proteins that are useful for nerve healing, which ultimately speeds up the rate of nerve regeneration.21 A study comparing the silver (nanotechnology) scaffolds to standard collagen scaffolds found that the use of nanotechnology increased the thickness of myelin sheaths and bettered nerve conduction.21
Nanomaterials have a wide array of uses for implants and scaffolding in orthopedics, ultimately contributing to faster recoveries, decreasing risks of surgery, and improving overall health of the affected area. However, many potential uses are yet to be investigated and there is still a lack of clarity surrounding long-term safety and clinical benefits.
4. Diagnosis
Another major use of nanotechnology in orthopedics is in the realm of detection. Specifically, nanotechnology has been used to diagnose bone diseases, such as Paget's disease, renal osteodystrophy, and osteoporosis.15, 22 This is often done using biosensors. These sensors are available in many designs and forms and can be implanted. Often, biosensors employ carbon nanotubes (CNTs), as their unique properties make the sensors strong and electrically conductive.24
There is a diversity of detection products employing nanotechnology and revolutionizing the field of orthopedics. For instance, for osteoporosis, techniques for diagnosis have great importance in providing precise data detection in a timely, affordable, and non-invasive manner. Prior to techniques employing nanomaterials, there were few reasonable options for detection. However, new methods using nanotechnology allow for detection of osteoporosis with a handheld device. Specifically, research has led to the development of novel biochip that uses gold nanoparticles to detect a protein that is indicative of osteoporosis.25 It has been shown to effectively evaluate bone conditioning and provide accurate detection and identification of the degree of damage to bones.25
In addition, nanotechnology in orthopedics has also been utilized to monitor orthopedic therapies and inform the course of treatment.15 Some sensors have even been equipped so that they are able to detect bone growth and lack thereof and dispense additional therapeutic drug as needed.15
5. Delivery of drugs
Nanotechnology has revolutionized therapeutics, allowing for greater precision of drug delivery, proving to be especially beneficial in the field of orthopedics. One way this is done, as mentioned previously, is through coupling drug delivery to nanosensors. Alternatively, nanophase delivery systems can be used for drug delivery without an accompanying sensor.
Precise delivery of drugs using nanotechnology through using gold nanoparticles has shown particularly promising results. Preclinical, animal studies have shown the promise of gold in effectively delivering iontophoresis to treat tendinopathy, or the disease and injury of a tendon.6, 26 In rats, the nanophase gold has also been shown to improve effectiveness of certain anti-inflammatory agents.26 Other modes of drug delivery, including poly-l-lactic-acid (PLLA), have also shown promise in their ability to deliver drugs at the nanoscale. PLLA has been used to delivery bone morphogenetic protein (BMP), which has in turn improved the time of closing for certain large bone defects.27
Nanophase drug delivery is being investigated for its potential application to TJR. In one study by Li et al., a nanofilm made of a biodegradeable polypeptide was used to deliver cefazolin; the results of this study demonstrated that nanoscale drug delivery for TJR may help decrease risk of infection in addition to improving osteoblast recruitment and bone growth.6, 28 This system draws special attention as it may also be able to alter the pharmacokinetics of dispensing antibiotics and target release such that it occurs during the most critical period, immediately after implantation.28
Drug delivery using nanotechnology is also relevant to cancer therapy, and specifically to bone cancer. Osteosarcoma is a commonly occurring form of cancer, and bone is a primary area of cancer metastases.29 Recent research has uncovered nanomaterials that are able to identify and deliver drug therapies to cancerous bones in a targeted manner.30 Some examples of these new nanotherapies include a magnetite-enriched collagen hydroxyapatite biocomposite for bone grafting material, a three dimensional nanomagnetite-chitosan rod for local hypothermia, and a magnetite-hydroxyapatite composite that aids direct bonding to bones.31, 32, 33
Nanotechnology for targeted drug therapy originally began through the use of large particles (i.e. growth factors) and has more recently incorporated smaller particles (i.e. silver) into nanostructured materials.34, 35, 36 Using nanotechnology for drug delivery improves precision and also serves a vital purpose in reducing bacterial growth, and thus, the risk of infection.
6. Future directions
Nanotechnology is a relative newcomer to the forefront of orthopedic research, diagnostics, and treatment. However, in the limited amount of time it has been studied and implemented, nanotech has been able to revolutionize the science and practice of orthopedic treatment. Many conventional therapies are being replaced, as nanotechnology provides ways to treat the human body in ways that are more precise, better for bone growth, and theoretically safer, at least in terms of infection rates and necessity of re-operations.
Though promising in its infancy, nanotechnology is no panacea to many of the field of orthopedic surgery's problems. Before its acceptance and acclaim can be widened, questions related to its long-term clinical safety must be answered. The risks of lung cytotoxicity and inflammation of internal organs that have been hypothesized through early research6, 37, 38 are in dire need of further investigation, and if needed, remedy.
To better understand the role of nanotechnology in the future, its long-term effects, both positive and harmful, must be better understood. Questions surrounding viability and toxicity of nanoparticles and sensors also need answers. In addition, more head-to-head comparisons of nanomaterials with traditional materials will better elucidate the value proposition of nanotechnology and direct future research.
In addition to increasing research on current processes, benefits, and risks of nanotechnology, issues surrounding regulatory, manufacturing, and cost barriers should be investigated and improved. Manufacturing of nanotech products is difficult, given the nature and complexity of the products. The high price of these products can reduce accessibility, and current regulatory processes can be enduring, limiting the immediacy of translating research into practice. Mitigating these concerns will increase the accessibility of nanomaterials and better promote its use within the field of orthopedics.
Finally, further and continuous thought should be given to the quality of current biomaterials and to the search to identify and mold even better nanoparticles. The average lifetime of current nanomaterials hovers in between one to two decades and certainly has room for improvement.
The safety and regulatory challenges surrounding nanotechnology will likely persist as issues hindering its widespread and rapid acceptance and use, but with more investment and research, it appears that nanotechnology has growing niche within the field of orthopedic surgery.
Funding
No funding was received for this work.
Conflicts of interest
The authors have none to declare.
References
- 1.http://www.crnano.org/whatis.htm.
- 2.http://www.oxforddictionaries.com/us/definition/american_english/nanotechnology.
- 3.http://www.nano.gov/timeline.
- 4.The Lycurgus Cup — A Roman Nanotechnology (Ian Freestone, Nigel Meeks, Margaret Sax, Catherine Higgitt).
- 5.Feynman R. There's plenty of room at the bottom. Eng Sci. 1960;23:22–36. [Google Scholar]
- 6.Nanotechnology: Current Concepts in Orthopaedic Surgery and Future Directions. (Sullivan). [DOI] [PubMed]
- 7.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2813556/.
- 8.http://www.nanotechproject.org/inventories/medicine/.
- 9.http://www.research.umd.edu/sites/default/files/documents/brochures/nanotechnology-medical-applications.pdf.
- 10.http://www.crnano.org/medical.htm.
- 11.http://news.mit.edu/2013/one-two-punch-knocks-out-aggressive-tumors-1021.
- 12.http://www.qmed.com/mpmn/article/10-nanotech-breakthroughs-you-should-know-about-updated.
- 13.Nanotechnology: current concepts in orthopaedic surgery and future directions. J Indian Soc Periodontol. 2008;12(May–August (2)):34–40. [Google Scholar]
- 14.J Indian Soc Periodontol. 2008;12(May-Aug 2):34–40. doi: 10.4103/0972-124X.44088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4592034/“state of the art”.
- 16.Nanobiomaterial Applications in Orthopedics (authors: Christensen, anseth, etc). [DOI] [PubMed]
- 17.How Nanotechnology can Really Improve the Future of Orthopedic Implants and Scaffolds for Bone and Cartilage Defects.
- 18.Webster T.J., Ergun C., Doremus R.H., Siegel R.W., Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21:1803–1810. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 19.Shirwaiker R.A., Samberg M.E., Cohen P.H., Wysk R.A., Monteiro-Riviere N.A. Nanomaterials and synergistic low-intensity direct current (LIDC) stimulation technology for orthopedic implantable medical devices. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:191–204. doi: 10.1002/wnan.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kon E., Delcogliano M., Filardo G. A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury. 2010;41:693–701. doi: 10.1016/j.injury.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Ding T., Luo Z.J., Zheng Y., Hu X.Y., Ye Z.X. Rapid repair and regeneration of damaged rabbit sciatic nerves by tissue-engineered scaffold made from nano-silver and collagen type I. Injury. 2010;41:522–527. doi: 10.1016/j.injury.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Yun Y.H., Eteshola E., Bhattacharya A., Dong Z., Shim J.S., Conforti L., Kim D., Schulz M.J., Ahn C.H., Watts N. Tiny medicine: nanomaterial-based biosensors. Sensors (Basel) 2009;9(11):9275–9299. doi: 10.3390/s91109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y., Taylor S., Li H. Advances toward bioapplications of carbon nanotubes. J Mater Chem. 2004;14(4):527–541. [Google Scholar]
- 25.Early Detection Techniques for Osteoporosis Kanika Singh1,2 and Kyung Chun Kim2 1KHAN Co, Ltd, Aju-dong, Geoje-do, 2Pusan National University, Busan, Republic of Korea.
- 26.Dohnert M.B., Venâncio M., Possato J.C. Gold nanoparticles and diclofenac diethylammonium administered by iontophoresis reduce inflammatory cytokines expression in Achilles tendinitis. Int J Nanomed. 2012;7:1651–1657. doi: 10.2147/IJN.S25164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schofer M.D., Roessler P.P., Schaefer J. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PLoS ONE. 2011;6:25462. doi: 10.1371/journal.pone.0025462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Ogle H., Jiang B., Hagar M., Li B. Cefazolin embedded biodegradable polypeptide nanofilms promising for infection prevention: a preliminary study on cell responses. J Orthop Res. 2010;28:992–999. doi: 10.1002/jor.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kansara M., Thomas D.M. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007;26(January (1)):1–18. doi: 10.1089/dna.2006.0505. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed M., Borchard G., Jordan O. In situ forming implants for local chemotherapy and hyperthermia of bone tumors. J Drug Deliv Sci Technol. 2012;22:393–408. [Google Scholar]
- 31.Andronescu E., Ficai M., Voicu G., Ficai D., Maganu M., Ficai A. Synthesis and characterization of collagen/hydroxyapatite: magnetite composite material for bone cancer treatment. J Mater Sci Mater Med. 2010;21(July (7)):2237–2242. doi: 10.1007/s10856-010-4076-7. [DOI] [PubMed] [Google Scholar]
- 32.Hu Q., Chen F., Li B., Shen J. Preparation of three-dimensional nanomagnetite/chitosan rod. Mater Lett. 2006;60(3):368–370. [Google Scholar]
- 33.Murakami S., Hosono T., Jeyadevan B., Kamitakahara M., Ioku K. Hydrothermal synthesis of magnetite/hydroxyapatite composite material for hyperthermia therapy for bone cancer. J Ceram Soc Jpn. 2008;116(1357):950–954. [Google Scholar]
- 34.Pleshko Nancy. PhD On the Horizon From the ORS. J Am Acad Orthop Surg. 2012;20:60–62. doi: 10.5435/JAAOS-20-01-060. [DOI] [PubMed] [Google Scholar]
- 35.Wei G., Jin Q., Giannobile W.V., Ma P.X. Nano-fibrous scaffold for controlled delivery of recombinant human PDGFBB. J Control Release. 2006;112(1):103–110. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing Z.C., Chae W.P., Huh M.W. In vitro anti-bacterial and cytotoxic properties of silver-containing poly(l-lactide-co-glycolide) nanofibrous scaffolds. J Nanosci Nanotechnol. 2011;11(1):61–65. doi: 10.1166/jnn.2011.3551. [DOI] [PubMed] [Google Scholar]
- 37.Polyzois I., Nikolopoulos D., Michos I., Patsouris E., Theocharis S. Local and systemic toxicity of nanoscale debris particles in total hip arthroplasty. J Appl Toxicol. 2012;32:255–269. doi: 10.1002/jat.2729. [DOI] [PubMed] [Google Scholar]
- 38.Sato M., Webster T.J. Nanobiotechnology: implications for the future of nanotechnology in orthopedic applications. Expert Rev Med Devices. 2004;1:105–114. doi: 10.1586/17434440.1.1.105. [DOI] [PubMed] [Google Scholar]