Abstract
Objective
The desired therapeutic effect of Arthrokinex™ autologous conditioned serum (ACS) is facilitated by the ability of IL-1-Ra to limit the destructive inflammatory intra-articular (IA) actions of IL-1β. Previous studies have proven the capacity of Arthrokinex™ (ACS) to induce the anti-inflammatory cytokine, IL-1-Ra. The primary purpose of this retrospective study was to investigate the effect of Arthrokinex™ (ACS) to reduce pain, improve joint function and enhance quality of life in patients with knee osteoarthritis.
Methods
Venous blood from 100 patients with symptomatic knee osteoarthritis (KOA) was conditioned and injected into the affected joint in this treatment protocol. Each patient received a total of six ultrasound-guided IA injections at day 0, 7, 14, 90, 180, and 270 and followed for up to one year. Treatment outcome measures were assessed by three different patient-administered surveys at each visit. Using the Visual Analog Pain Scale (VAS), participants were asked to classify pain in the previous 24 h. The Extra Short Musculoskeletal Functional Assessment (XSMFA-D) survey is a series of 16 questions designed to determine the functionality of the OA-affected joint. Finally, the patient completed a patient global impression of change (PGIC) survey to assess their individual level of satisfaction with the treatment regimen.
Results
Compared to baseline, a total of 84% of patients reported better pain control at 6 months with 91% reporting improvement at 12 months. A robust and statistically significant improvement in each XSMFA-D subscale was observed in KOA patients over 12 months. The overall reduction of pain and enhanced joint function was observed within 1 week and sustained 3, 6 and even 12 months after the initial injection. In addition to symptomatic control of OA, 92% of patients reported satisfaction with the treatment regimen 12 months after the initial injection.
Conclusion
Given the favorable safety profile, reduction in pain and enhanced quality of life experienced by patients enrolled in this joint health program, Arthrokinex™ (ACS) has the potential to offer an alternative, chondroprotective, natural, molecular approach to treating pain and functionality in patients with mild, moderate or severe knee osteoarthritis.
Keywords: Autologous conditioned serum, Knee osteoarthritis, IL-1-Ra, IL-1
1. Introduction
Osteoarthritis (OA) of the knee is a painful, progressively debilitating disease that affects 15.1 million individuals in the United States.1 Traditionally considered a disorder of the aging, one in eight (1.7 million) of those individuals are less than 45 years of age. Unfortunately, many of these individuals will develop advanced disease and require surgery since the current spectrum of treatment options do not address the underlying disease pathology. The incidence of total knee replacement (TKR) surgeries in the United States has increased each year from 1991 to 2008 and is expecting to continue to increase. In 2005, almost 500,000 TKR were performed in the United States at an estimated cost of $11 billion.2 By the year 2030, it is estimated that 3.48 million operations will be performed.3 After adjusting for comorbidities, a recently diagnosed 45 year old non-Hispanic white woman has a 61% lifetime risk of undergoing a TKR and a 7% lifetime risk of needing a revision TKR.1
OA was previously characterized as a mechanical, non-inflammatory disease associated with overuse and often described as “degenerative arthritis”. Heightened interest has led to a broader understanding of the inflammatory process that causes synovitis, subchondral bone remodeling and hyaline cartilage destruction, the central pathobiologic feature of OA. Interleukin 1 (IL-1) has been implicated in many human pathological conditions4 and is the chief mediator of OA-associated inflammation. Under non-disease conditions, IL-1 is regulated by Interleukin 1 receptor antagonist (IL-1-Ra or IRAP). IL-1-Ra is a soluble, naturally occurring 22 kd glycosylated protein that inhibits IL-1.5, 6, 7, 8 IA injections of IL-1-Ra or transfection of IL-1-Ra gene has been shown to reduce the progression of experimental OA in animal models9 as well as provide clinical benefits in horses10 and humans.11, 12, 13, 14
The American College of Rheumatology (ACR) recommends several non-pharmalogic and pharmalogic options to treat KOA. The goal of treatment is to provide analgesic relief and decrease inflammation. While acetaminophen is the most cost effective and continues to be the first line analgesic agent recommended to treat KOA, many providers choose non-steroidal anti-inflammatory drugs (NSAIDs) in lieu of acetaminophen because of the superior pain relief.15 However, these medications have serious gastrointestinal and cardiovascular side effects and are often associated with low compliance. The addictive and often abused opioid analgesics are commonly prescribed. Treatment of KOA with opioids represents a dangerous option as the death toll associated with opioid overdose continues to increase despite a marked effort to reduce the number of opioids prescribed. Intra-articular (IA) injections of corticosteroids are also recommended. However, while effective at reducing inflammation, IA corticosteroid injections are short acting, prone to adverse effects and unable to modify hyaline cartilage destruction. Another therapy that failed to provide significant clinical benefit was the use of hyaluronic acid (HA).16, 17 ACR currently offers no recommendations for the use of HA to treat OA. Thus, conventional management options that are short term and largely ineffective, coupled with the increased burden of KOA, illustrate the need for innovative disease modifying treatment strategies.
Regenerative therapies have emerged as alternative strategies to treat OA. Platelet rich plasma (PRP) has garnered much attention lately and gained a significant share of the market.18 PRP is very similar to Arthrokinex™ and other ACS products with one notable difference, the PRP manufacturing process does not include the final steps to induce IL-1-Ra. Initially, PRP was portrayed as the “wonder drug,” but recent trials have failed to provide evidence for the efficacy of PRP to treat KOA.19 Furthermore, PRP injections are being produced without a standardized protocol creating heterogeneous blood products, inconsistent platelet concentrations and trials that provide varying numbers of injections.19 Another option that is being explored to treat OA is the harvesting of adult mesenchymal stem cells (MSC). MSCs can be isolated from a variety of human tissues for the purpose of chondrocyte differentiation. This technology, while promising, is relatively new and can be affected by age20 and smoking.21 Currently, the capacity of MSCs to provide clinical benefits is unclear.22
Our novel autologous conditioning processing, Arthrokinex™ (ACS), and subsequent standardized protocol, was developed following the success of treatment strategies such as Orthokine and Arthrex that induced IL-1-Ra from whole blood.10, 11 Arthrokinex™ (ACS) offers an interventional orthopedic treatment plan at the molecular level and it incorporates the inhibition of IL-1β through the rapid induction of IL-1-Ra. We have previously demonstrated that our novel formulation produces IL-1-Ra levels that are consistently adequate to block the IA destructive effects of IL-β.23 After a brief interaction with medical grade concentrator beads, post-conditioned serum IL-1-Ra level are, on average, 999 times greater than serum IL-β concentration.23 This ratio is dramatically higher than the 10–100 IL-1-Ra to IL-1β ratio necessary to reverse the equilibrium imbalance of OA-affected joints.24 In addition to IL-1-Ra, the biochemical architecture of ACS contains a mixture of PRP and several growth factors present in the α granules of platelets.25 These growth factors, which include transforming growth factor beta 1 (TGF-β1), platelet-derived GF (PDGF), insulin-like GF-I (IGF-I), basic fibroblast GF (bFGF) and vascular endothelial GF (VEGF),26, 27 have been shown to stimulate chondrocyte proliferation and augment articular cartilage metabolism.27, 28, 29 The purpose of this retrospective chart review analysis is to determine if Arthrokinex™ (ACS) significantly reduced pain and increased function in patients with symptomatic KOA.
2. Methods
2.1. Study design
Treatment and data collection was conducted in two separate locations in Oklahoma City, OK, USA and one location in Austin, TX, USA. Each participant agreed to receive IA joint injections of Arthrokinex™ (ACS) conditioned serum and be followed for a period of one year. The first patient was recruited in March 2014 and the final patient completed the trial in July 2016. All aspects of this retrospective chart review were extensively reviewed and approved by IntegReview Institutional Review Board (IRB) as being considered exempt from requiring IRB approval as it met all requirements outlined in 45 CFR 46.101(b)(4), specifically (1) the research involved only the collection or study of pre-existing data, documents, records, pathologic specimens or diagnostic specimens and (2) the information was recorded in such a manner that the subjects could not be identified directly or through identifiers linked to the subjects.
2.2. Participants
A total of 100 patients with symptomatic KOA met the American College of Rheumatolgy (ACR) inclusion criteria for analysis. According to the ACR criteria, patients with at least three of the six primary clinical features were diagnosed with KOA. These symptoms include age >50, morning stiffness <30 min duration, crepitus on active range of motion, tenderness of the bony margins of the joint, bony enlargement noted on examination and a lack of palpable warmth of the synovium. Exclusion criteria included: patient charts of those in generally poor health; drug dependent (chronic opioid use, alcohol, etc.); undergone surgery or treatment of the affected joint within the last 3 months; systemic disease of the musculoskeletal system; bone cancer; metastasis or tumor-like lesions in the immediate proximity to the treated joint; fracture in the last 3 months; acute bacterial infection; blood clotting disorders; continuous corticoid or NSAID therapy due to other diseases. Informed consent was obtained from each participant and all work was performed in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki, 1975 revised 2008).
2.3. Intervention
Venous blood was extracted from all patients currently enrolled in the Arthrokinex™ (ACS) joint health program. Each blood sample was conditioned to induce IL-1-Ra using the Arthrokinex™ (ACS) procedure. Using aseptic techniques, 60 mL of whole blood from the median cubital vein was harvested into a sterile 60 mL syringe containing 3 mL of anticoagulant citrate dextrose (ACD) solution and centrifuged (3200 rpm, 15 min). The resultant Platelet Rich Plasma (PRP) and Platelet Poor Plasma (PPP) were then extracted and the remaining layer, containing erythrocytes, was discarded. Both the PRP and PPP were transferred to a specialized, closed-system, centrifuge tube containing medical grade concentrator beads, mixed and allowed to incubate for 30 min at ambient temperature. After the short incubation period, centrifuge filtration (2000 rpm, 3 1/2 min) through a sterile 0.45 μm filter was completed and the resulting sterile filtrate was slowly drawn into 1 mL syringes. The 1 mL syringes could be used immediately for IA injections or stored at −20 °C for future use.
Each patient received a total of six IA injections (day 0, 7, 14, 90, 180, 270) containing 1 cc of autologous conditioned serum each. Injections were prepared in a 3 cc syringe with a 23 gauge, 1.5 in. needle attached. Using aseptic techniques, an ultrasound guided injection of conditioned serum was administered in the supra-patellar pouch of the affected knee using a superolateral approach. Before each injection, the participants were asked to rate their level of pain (on a scale of 0–10) and to complete the other treatment outcome measures. Participants were instructed to contact the clinical coordinator immediately if any adverse events occurred following the injection. Similar to any joint injection, potential adverse events include but may not be limited to joint swelling, redness, warmth and fever. Each participant agreed to complete all outcome measures in an office visit or phone interview at day 0, 7, 14, 90, 180, 270 and 365.
2.4. Outcome measures
Treatment outcome measures were assessed by three different patient-administered surveys at each visit. Using the Visual Analog Pain Scale (VAS), participants were asked to classify pain in the previous 24 h. A score of zero would indicate no pain and a score of ten would indicate the most severe pain. The Extra Short Musculoskeletal Functional Assessment (XSMFA-D) survey is a series of 16 questions designed to determine the functionality of the OA-affected knee. The XSMFA-D has four subscales designed to assess (1) knee function, (2) knee activity, (3) bothersome index and (4) knee mobility. A higher score represents a poorer outcome. Finally, the patients completed a patient global impression of change (PGIC) survey to assess their individual level of satisfaction with the treatment regimen.
2.5. Statistical analysis
Statistical Packages for the Social Sciences (SPSS) 12.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The non-parametric Wilcoxon Signed Rank test was performed to analyze the statistical difference between baseline outcome measures versus early (0 vs 3 months), middle (0 vs 6 and 0 vs 9 months) and late (0 vs 12 months) treatment effects. Since our main clinical outcome was to determine the clinical benefit of Arthrokinex™ (ACS) after one year, p values are only reported at this time point. Statistical analysis at each time point was performed and followed the same trend as the data presents at one year. All results shown are the mean ± standard error of the mean (SEM) of two or more experiments. P values less than or equal to 0.05 were considered significant.
3. Results
Table 1 summarizes patient demographics and arthritis scores of participants in this trial. The average participant in this trial was greater than 60 years old and had an obese body mass index. In fact, only 13 patients were classified as normal weight (BMI < 25 kg/cm2) and 15 were classified as overweight (BMI 25–29.9 kg/cm2). Of those remaining, 31 patients had a BMI that is described as class I obese (30–34.9 kg/cm2), 24 as class II obese (35–39.9 kg/cm2) and 17 as class III obese (≥40 kg/cm2). Overall, the vast majority of patients (75%) in this trial were obese. Participants reported high pain scores (81% reported a baseline pain score of 5 or greater), significant dysfunction, decreased activity, decreased mobility and a high bothersome index. In short, patients were severely affected by the disease and had to modify daily activities of living (ADL).
Table 1.
Participant demographics and baseline clinical disease scores.
| n | 100 |
| Age (years) | 61.2 ± 1.2 |
| Male/female | 32/68 |
| Weight (kg) | 96.9 ± 2.1 |
| BMI (kg/cm2) | 33.8 ± 1.4 |
| BMI classification | |
| Normal weight (<25 kg/cm2) | 13/100 (13%) |
| Overweight (25–29.9 kg/cm2) | 15/100 (15%) |
| Obese class I (30–34.9 kg/cm2) | 31/100 (31%) |
| Obese class II (35–39.9 kg/cm2) | 24/100 (24%) |
| Obese class III (≥40 kg/cm2) | 17/100 (17%) |
| VAS pain | 5.8 ± 0.6 |
| XSFMA-D dysfunction score | 29.3 ± 1.1 |
| XSFMA-D activity score | 12.4 ± 0.9 |
| XSFMA-D mobility score | 12.3 ± 1.2 |
| XSFMA-D bothersome index | 10.6 ± 1.1 |
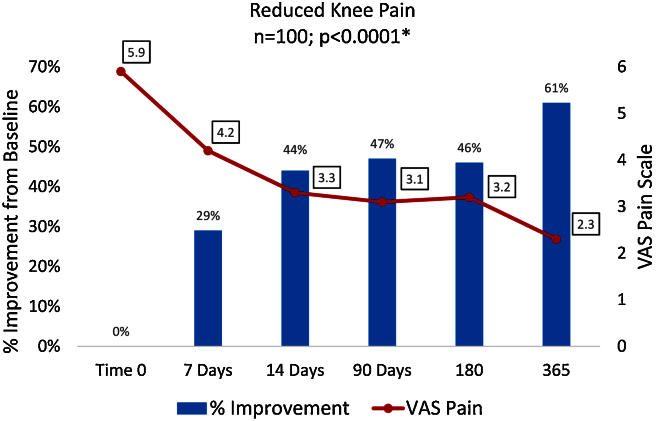
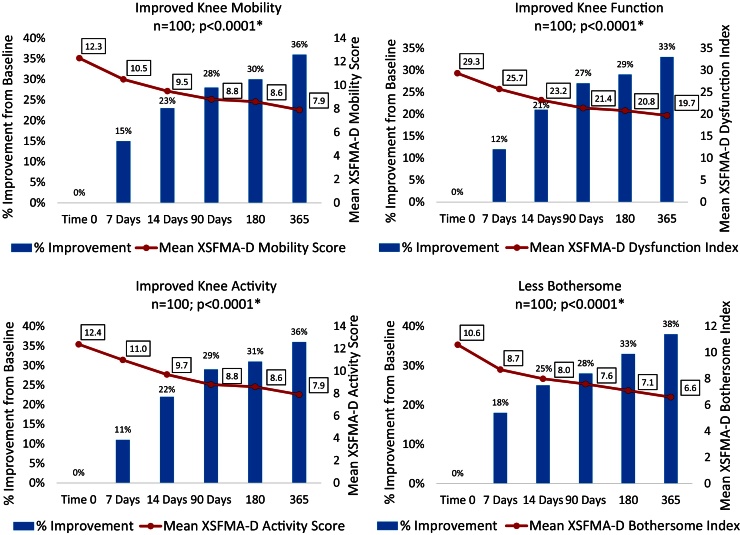
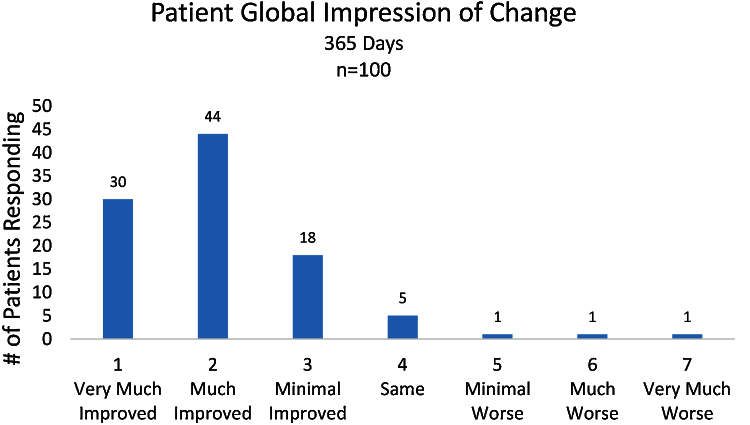
The series of Arthrokinex™ (ACS) injections significantly reduced pain at each time point and sustained for at least one year (p < 0.0001) (Fig. 1). Participants reported pain relief after the first injection. A total of 65 (65%) patients had a reduction in pain at their second office visit, one week after the initial injection. Participants had a 47% reduction in pain at 3 months, 46% reduction at 6 months and a 61% reduction in pain at 1 year. One participant reported a 10/10 for pain at baseline. Following the Arthrokinex™ treatment, pain was significantly reduced in this individual to a 3/10 after the first injection and the reduced pain was maintained for the entire year. In addition to decreased pain, patients reported a significantly enhanced quality of life in all XSFMA-D criteria. On average, patients reported a 33% increase in knee function, 36% increase in knee activity, 36% increase in knee mobility and 38% improvement in the amount of time bothered by the disease over a one year period (Fig. 2). Each participant was asked to complete the patient global impression of change survey at the conclusion of this trial. Patients in this trial reported their overall satisfaction with the treatment regimen. Of the 100 participants, 92 (92%) reported an improvement in symptoms following the IA injections of Arthrokinex™ at the one year time point (Fig. 3).
Fig. 1.
VAS Pain Scales illustrating pain reported by patients and the % improvement from baseline. *Nonparametric Wilcoxon Sign Rank Test comparing baseline vs values reported at one year.
Fig. 2.
Extra Short Musculoskeletal Functional Assessment (XSMFA-D) values reported and % improvement from baseline. *Nonparametric Wilcoxon Sign Rank Test comparing baseline vs values reported at one year.
Fig. 3.
Patient global impression of change after completing 12 months of the Arthrokinex™ (ACS) protocol.
4. Discussion
Our analysis indicates that inhibition of IL-1β through a series of Arthrokinex™ (ACS) injections can ameliorate pain and enhance joint function in OA-affected knees. Pain, one of the most bothersome symptoms associated with KOA, was decreased at each time point. A clinically significant reduction in pain was characterized as a 50% decrease from baseline to one year. Some patients (26%) reported a 50% reduction in pain after the first injection (t = one week). A greater number of individuals (48%) reported the same clinically significant reduction in pain after two injections (t = two weeks) and an even greater number of patients (54%) reported a 50% reduction in pain after the third injection (t = three weeks). A total of 66% of patients achieved this 50% reduction in pain at the one year mark. Of all 100 patients, a mean reduction in pain of 61% was reported at the one year mark when compared to baseline. Additionally, patients reported improved knee function, activity, mobility and were less bothered by their knee symptoms. Since the XSFMA-D scale has a strong correlation with the Functional Western Ontario and McMaster University (WOMAC) Osteoarthritis Index,30 the WOMAC metric for clinically meaningful difference in patient reported outcomes can be directly applied to activities of daily living (ADL) assessed by the XSFMA-D scale. A minimum of 9–12 mm difference in WOMAC reported values is considered clinically meaningful.31 We observed a mean improvement of 22.5 points in XSMFA-D scores at the one year time point which is well above the minimum change needed to determine clinical benefit. Another important parameter assessed was the patient's perception of symptomatic improvement following the series of injections. Patients had an overwhelmingly positive response to therapy with a 92% rate of satisfaction reported. Overall, IA injections of Arthrokinex™ (ACS) have the capacity to quickly alleviate pain (even after one week) and restore loss of function to OA-affected joints.
Previously, Baltzer et al.11 reported the effectiveness of Orthokine to treat mild to moderate knee pain. One of the clinical outcome benchmarks assessed by the authors was also a >50% reduction in VAS pain scores. At the 3 month time point, 71% of the participants had met this benchmark and 67% at the 6 month time point. WOMAC scores were also significantly improved in the treatment arm versus hyaluronate and the treatment arm versus placebo. Interestingly, these clinical effects persisted for at least 2 years. We cannot accurately state the duration of our treatment effect beyond one year but it seems reasonable to conclude that the effects of our formulation could persist 2 years as well. Another trial involving 167 patients, demonstrated the superiority of Orthokine to treat KOA.12 Orthokine significantly reduced KOOS symptoms and KOSS sports parameters versus participants only receiving placebo. Orthokine clinically improved WOMAC scores but the difference did not reach statistical significance.
Chevalier et al. conducted two trials to address the safety and efficacy of IL-1-Ra (Anakinra). The first pilot trial reported a significant reduction in pain in 7 of 13 participants and improved ADLs in 10 of 13 individuals. With the exception of one joint effusion that the authors declared was most likely unrelated to the drug, no acute reactions or injection site reactions occurred.32 The second randomized controlled trial (n = 170) had no adverse reactions but the authors were unable to replicate the same clinical benefit observed in the pilot study. The authors concluded that the inability to reach a clinical significant benefit was due to the relatively short half-life of IL-1-Ra. Peak plasma concentrations of IL-1-Ra (t1/2 = 4–6 h) are reached after 3–7 h following a subcutaneous injection but quickly trend down.33 Given the short half-life, repeated injection (weekly) may be beneficial since the IL-1-Ra concentrations reach a suboptimal level after approximately 3 days. The Arthrokinex™ (ACS) protocol institutes a series of injections (6) to maintain supranormal IL-1-Ra levels to competitively inhibit IL-1. Furthermore, the subcutaneous mode of transmission may not be ideal to treat OA. Local IA joint injections are likely to decrease local inflammation of KOA and elicit a greater clinical response when compared to systemic drug delivery. Materials injected directly into the synovium have increased bioavailability, fewer systemic effects, fewer off target cell interactions and lower total drug costs.34 The Arthrokinex™ (ACS) protocol utilizes ultrasound guidance to ensure accuracy of the injection. As many as 50% of blind knee injections performed by experienced physicians do not reach the intended site but end up in extra-articular locations.35, 36 As a local biotherapeutic agent, injections of Arthrokinex™ have an excellent risk to benefit treatment ratio.
The current landscape of available treatment options forces many practitioners to endorse surgery to patients after recommended therapies have inevitably failed. All surgical procedures have inherent risks but many factors compound the risks associated with TKR. New data has revealed an alarming increase in joint infection for individuals that have been administered steroid injections prior to surgery. The infection rate of patients receiving steroid injections was significantly higher (4.4% vs 3.6%) compared to those that were not injected. A significantly higher percent of patients with previous steroid injections had to return to the operating room due to infection (1.5% vs 1.0%). The authors evaluated the duration from injection to TKR and found the increased rate of infection was significant up to seven months. An odds ratio of 1.8 (1.3–2.6, p = 0.0007) was reported in patients receiving the injection the month prior to surgery and 1.4 (p = 0.048) six to seven months prior to surgery.37 Conversely, animal data suggests pre-operative administration of IL-1-Ra to arthritic joints not only halted synovial fibrosis but reversed it.38 The peri-operative role of IL-1-Ra in humans is not fully elucidated but this finding represents an exciting opportunity to improve patient care. Another confounding factor that could complicate surgery is obesity. A well-established body of evidence recognizes obesity as a risk factor for developing KOA.39 One meta-analysis reported an obese or overweight body type conferred a threefold increased risk in developing KOA.40 While not all studies agree,41 data has demonstrated an increased rate of peri-operative complication in obese patients and an increased risk of premature joint failure and revision.42 The majority of patients in our study were classified as obese. Arthokinex™ (ACS) effectively alleviated symptoms in obese patients and could facilitate cartilage repair as well as potentially provide peri-operative benefits if surgery is indicated.
This study has several limitations including a relatively small sample size and lack of a control arm. Despite these limitations, this retrospective chart review serves as a proof of concept for the application of biologic IA injections to reduce joint pain and increase ADLs in people afflicted with KOA. The need for a cost effective medical approach that will provide symptomatic relief as well as alter the course of OA disease progression to delay or prevent surgical intervention is indisputable. Arthrokinex™ (ACS) provides an alternate option and addresses many of the shortcomings faced by current treatment regimens. First, our formulation to induce IL-1-Ra is safe and provides excellent clinical benefits for at least one year. Second, it is cost effective especially when compared to the average cost of a TKR ($22,000), PRP per shot ($1600) and for stem cell treatment ($5500). Third, the protocol is designed to achieve high patient compliance. Patients can schedule a routine office visit, have blood drawn and receive the injection in less than one hour. After the initial blood draw, the IL-1-Ra rich serum is frozen until the patient returns the following week. Finally, serum rich in IL-1-Ra, platelets and growth factors has the potential to repair damaged cartilage to slow or reverse the disease process.
5. Conclusion
Arthrokinex™ (ACS) is a molecular approach that offers a safe, point of care alternative to provide significant clinical benefit. The data from this trial demonstrate the ability of ACS to quickly alleviate pain and significantly improve quality of life in patients with symptomatic KOA. Further research is needed to assess the disease modifying ability of ACS and to compare the clinical effects of ACS to currently recommended therapies.
Conflict of interest
The research being reported in this publication was supported by Memorial Clinical Research, a division of Barreto Healthcare Clinic. Angelique Barreto has equity ownership in, serves as an advisor for, serves on the board of directors of Arthrokinex Joint Health LLC., which is developing a process related to the research being reported. The terms of this arrangement have been reported to the IRB. Timothy Braun is not affiliated with Memorial Clinical Research and reports no conflicts of interest.
References
- 1.Deshpande B.R., Katz J.N., Solomon D.H. The number of persons with symptomatic knee osteoarthritis in the Unisted States: impact of race/ethnicity, age, sex and obesity. Arthritis Care Res. 2016 doi: 10.1002/acr.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HCUPnet . 2005. National statistics on all stays: 2005 outcomes by patient and hospital characteristics for ICD-9-CM principal procedure code 81.54 total knee replacement.http://hcupnetahrqgov/HCUPnetjsp [Google Scholar]
- 3.Healy W.L., Rana A.J., Iorio R. Hospital economics of primary total knee arthroplasty at a teaching hospital. Clin Orthop Relat Res. 2011;469:87–94. doi: 10.1007/s11999-010-1486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello C.A., van der Meer J.W. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25:469–484. doi: 10.1016/j.smim.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seckinger P., Lowenthal J.W., Williamson K., Dayer J.M., MacDonald H.R. A urine inhibitor of interleukin 1 activity that blocks ligand binding. J Immunol. 1987;139:1546–1549. [PubMed] [Google Scholar]
- 6.Hannum C.H., Wilcox C.J., Arend W.P. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg S.P., Evans R.J., Arend W.P. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990;343:341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- 8.Carter D.B., Deibel M.R., Jr., Dunn C.J. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344:633–638. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes J., Tardif G., Martel-Pelletier J. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. Am J Pathol. 1999;154:1159–1169. doi: 10.1016/S0002-9440(10)65368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frisbie D.M., Kawcak C.E., Werpy N.M. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 2007;68:290–296. doi: 10.2460/ajvr.68.3.290. [DOI] [PubMed] [Google Scholar]
- 11.Baltzer A.W., Moser C., Jansen S.A., Krauspe R. Autologous conditioned serum (Orthrokine) is an effective treatment for knee osteoarthritis. Osteoarthr Cartil. 2009;17:152–160. doi: 10.1016/j.joca.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Yang K.G., Raijmakers N.J., van Arkel E.R. Autologous interleukin-1 receptor antagonist improves function and symptoms in OA when compared to placebo in a prospective randomized controlled trial. Osteoarthr Cartil. 2008;16:498–505. doi: 10.1016/j.joca.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Kraus V.B., Birmingham J., Stabler T.V. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: a randomized controlled pilot trial ( NCT00332254) Osteoarthr Cartil. 2012;20:271–278. doi: 10.1016/j.joca.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Darabos N., Haspl M., Moser C. Intra-articular application of autologous conditioned serum (ACS) reduces bone tunnel widening after ACL reconstructive surgery in a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2011;19(suppl 1):36–46. doi: 10.1007/s00167-011-1458-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W., Jones A., Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? A meta-analysis of randomised controlled trials. Ann Rheum Dis. 2004;63:901–907. doi: 10.1136/ard.2003.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan K.M., Arden N.K., Doherty M. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2003;62:1145–1155. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandt K.D., Smith G.N., Jr., Simon L.S. Intraarticular injection of hyaluronan as treatment for knee osteoarthritis: what is the evidence? Arthritis Rheum. 2000;43:1192e203. doi: 10.1002/1529-0131(200006)43:6<1192::AID-ANR2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.GlobalData. Platelet Rich Plasma: A Market Snapshot. http://www.docstoc.com/docs/47503668/Platelet-Rich-Plasma-A-Market-Snapshot.
- 19.Sheth U., Simunovic N., Klein G. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Jt Surg Am. 2012;94:298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 20.Choudhery M.S., Badowski M., Muise A. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Trans Med. 2014;12 doi: 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jedrzejas M., Skowron K., Czekaj P. Stem cell niche exposes to tobacco smoke. Przegl Lek. 2012;69:1063–1073. [PubMed] [Google Scholar]
- 22.Uth K., Trifonov D. Stem cell application of osteoarthritis in the knee joint: a mini review. World J Stem Cells. 2014;6:629–636. doi: 10.4252/wjsc.v6.i5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barreto A., Braun T.R. A method to induce Interleukin-1 Receptor Antagonist Protein from autologous whole blood. Cytokine. 2016;81:137–141. doi: 10.1016/j.cyto.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Chevalier X., Mugnier B., Bouvenot G. Targeting anti-cytokine therapies for osteoarthritis. Bull Acad Natl Med. 2006;190:1411–1420. [PubMed] [Google Scholar]
- 25.Ulrich-Vinther M., Maloney M.D., Schwarz E.M. Articular cartilage biology. J Am Acad Orthop Surg. 2003;11:421–430. doi: 10.5435/00124635-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Wehling P., Moser C., Frisbie D. Autologous conditioned serum in the treatment of orthopedic diseases: the orthokine therapy. BioDrugs. 2007;21:323–332. doi: 10.2165/00063030-200721050-00004. [DOI] [PubMed] [Google Scholar]
- 27.Frizziero A., Giannotti E., Oliva F., Masiero S., Maffulli N. Autologous conditioned serum for the treatment of osteoarthritis and other possible applications in musculoskeletal disorders. Br Med Bull. 2013;105:169–184. doi: 10.1093/bmb/lds016. [DOI] [PubMed] [Google Scholar]
- 28.Bos P.K., Verhaar J.A., van Osch G.J. Age-related differences in articular cartilage wound healing: a potential role for transforming growth factor beta1 in adult cartilage repair. Adv Exp Med Biol. 2006;585:297–309. doi: 10.1007/978-0-387-34133-0_20. [DOI] [PubMed] [Google Scholar]
- 29.Mello M.A., Tuan R.S. Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res. 2006;24:2095–2105. doi: 10.1002/jor.20233. [DOI] [PubMed] [Google Scholar]
- 30.Wollmerstedt N., Kirschner S., Faller H., König A. Reliability, validity and responsiveness of the German Short Musculoskeletal Function Assessment Questionnaire in patients undergoing surgical or conservative inpatient treatment. Qual Life Res. 2006;15:1233–1241. doi: 10.1007/s11136-006-0066-0. [DOI] [PubMed] [Google Scholar]
- 31.Ehrich E.W., Davies G.M., Watson D.J., Bolognese J.A., Seidenberg B.C., Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27 [PubMed] [Google Scholar]
- 32.Chevalier X., Giraudeau B., Conrozier T., Marliere J., Kiefer P., Goupille P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005;32 [PubMed] [Google Scholar]
- 33.Chevalier X., Goupille P., Beaulieu A. Results from a double blind, placebo controlled, multicenter trial of a single intra-articular injection of Anakinra (Kineret) in patients with osteoarthritis of the knee. Arthritis Rheum. 2005;52 [Google Scholar]
- 34.Evans C.H., Kraus V.B., Setton L.A. Progress in intra-articular therapy. Nat Rev Rhematol. 2014;10:11–22. doi: 10.1038/nrrheum.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones A., Regan M., Ledingham J., Pattrick M., Manhire A., Doherty M. Importance of placement of intra-articular steroid injections. BMJ. 1993;307:1329–1330. doi: 10.1136/bmj.307.6915.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson D.W., Evans N.A., Thomas B.M. Accuracy of needle placement into the intra-articular space of the knee. J Bone Jt Surg Am. 2002;84-A:1522–1527. doi: 10.2106/00004623-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Bedard N., Callaghan J.J., Pugely A.J. Do injections increase the risk of infection following total knee arthroplasty? Am Acad Orthop Surg. 2016 [Google Scholar]
- 38.Lewthwaite J., Blake S., Thompson R.C., Hardingham T.E., Henderson B. Antifibrotic action of interleukin-1 receptor antagonist in lapine monoarticular arthritis. Ann Rheum Dis. 1995;54:591e6. doi: 10.1136/ard.54.7.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felson D.T., Anderson J.J., Naimark A., Walker A.M., Meenan R.F. Obesity and knee osteoarthritis. The Framingham study. Ann Intern Med. 1988;5:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 40.Blagojevic M., Jinks C., Jeffery A., Jordan K.P. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthr Cartil. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Bordini B., Stea S., Cremonini S., Viceconti M., De Palma R., Toni A. Relationship between obesity and early failure of total knee prostheses. BMC Musculoskelet Disord. 2009;10:29. doi: 10.1186/1471-2474-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salih S., Sutton P. Obesity, knee osteoarthritis and knee arthroplasty: a review. BMC Sports Sci Med Rehabil. 2013;3:25. doi: 10.1186/2052-1847-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]