Abstract
Thrombosis and its complications are the leading cause of death in patients with diabetes. Metformin, a first-line therapy for type 2 diabetes, is the only drug demonstrated to reduce cardiovascular complications in diabetic patients. However, whether metformin can effectively prevent thrombosis and its potential mechanism of action is unknown. Here we show, metformin prevents both venous and arterial thrombosis with no significant prolonged bleeding time by inhibiting platelet activation and extracellular mitochondrial DNA (mtDNA) release. Specifically, metformin inhibits mitochondrial complex I and thereby protects mitochondrial function, reduces activated platelet-induced mitochondrial hyperpolarization, reactive oxygen species overload and associated membrane damage. In mitochondrial function assays designed to detect amounts of extracellular mtDNA, we found that metformin prevents mtDNA release. This study also demonstrated that mtDNA induces platelet activation through a DC-SIGN dependent pathway. Metformin exemplifies a promising new class of antiplatelet agents that are highly effective at inhibiting platelet activation by decreasing the release of free mtDNA, which induces platelet activation in a DC-SIGN-dependent manner. This study has established a novel therapeutic strategy and molecular target for thrombotic diseases, especially for thrombotic complications of diabetes mellitus.
Worldwide, 415 million people, or 1 in 11 adults, are estimated to have diabetes mellitus (DM). Approximately 5.0 million people died from DM in 2015, which is equivalent to one death every six seconds1,2,3. Thrombosis is the leading cause of morbidity and mortality in patients with DM, with a reported 65% of diabetic patients eventually dying from thrombotic diseases4,5,6. However, an increased understanding of the mechanisms of thrombogenesis has led to a new era in the discovery of antithrombotic agents that target many of the key steps in blood coagulation and platelet activation7. Metformin is widely used as the first-line therapy for type-2 diabetic patients. It has been reported that metformin reduced mortality and diabetes-associated thrombotic complications8,9,10,11,12. However, its mechanism of action is largely unknown with respect to thrombosis prevention, and further investigation is merited.
In 2000, two independent publications showed that metformin protects mitochondrial function by inhibiting complex I in the mitochondrial respiratory chain13,14. In 2010, it was reported that metformin use may decrease mortality among patients with diabetes and atherothrombosis9. Numerous studies have reported that mitochondrial function is associated with platelet activation and thrombosis15,16,17,18. For example, Brownlee and colleagues demonstrated that hyperglycaemia induces mitochondrial hyperpolarization in normal platelets, resulting in the augmentation of reactive oxygen species (ROS) generation and subsequent activation16. Cardenes et al. found that mitochondrial alterations cause platelet activation in vitro and in vivo17, while Boudreau et al. reported that activated platelets can release mitochondria18. However, it is not known whether metformin is associated with platelet activation. We hypothesized that metformin can inhibit platelet activation and platelet-activated blood coagulation by preventing platelet mitochondrial dysfunction and release.
Results
Metformin suppresses mtDNA release and mitochondrial dysfunction of activated platelets
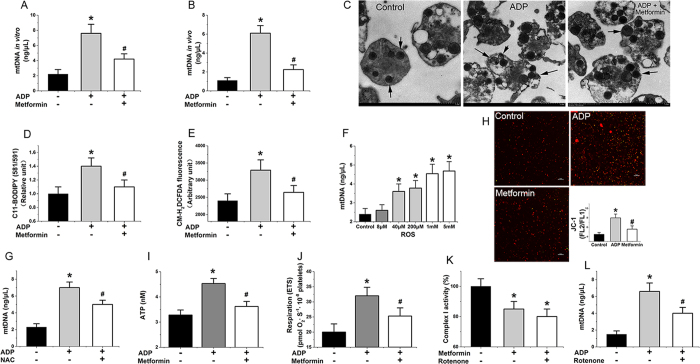
We first determined the amounts of extracellular mtDNA detected from ADP-activated platelets in vitro (Fig. 1A, metformin: 1 mM, 6 h, 37 °C) and in vivo (Fig. 1B, metformin: 400 mg/kg/d, 7 d) in the absence and presence of metformin with qPCR assay. As expected, metformin inhibited mtDNA release from activated platelets (Fig. 1A,B). Furthermore metformin also inhibited mtDNA release from arachidonic acid (AA)- and thrombin-activated platelets (Supplementary Fig. 1A). However, the mechanism behind these inhibitory effects of metformin is poorly recognized. As shown in Supplementary Fig. 1D, metformin has no effects on apoptosis in platelets. This study eliminated the possibility that the changes of extracellular mtDNA level are because of the changes of apoptotic bodies. Our results suggest that metformin prevented platelet membrane damage in activated platelets (Fig. 1C), Activated platelets treated with metformin also showed lowered lipid peroxidation levels as measured by Lipid Peroxidation Sensor, BODIPY® 581/591 C11 (Fig. 1D). It has been reported that cell membranes are highly susceptible to injury by an over-load of ROS, which then cause enhanced lipid peroxidation leading to damage of the bio-membrane system19,20. In our study, we observed an elevated level of ROS in ADP-activated platelets as detected by CM-H2DCFDA fluorescence (Fig. 1E), but a lower level of ROS production when metformin was added (Fig. 1E). We found that metformin also reduced level of platelet mitochondrial ROS production (Supplementary Fig. 1F). Furthermore, we identified a hydrogen peroxide-induced increase in free mtDNA from activated platelets (Fig. 1F), while N-acetylcysteine (NAC), an ROS inhibitor, also inhibited mtDNA release (Fig. 1G). We further determined the hyperpolarization level of the mitochondrial membrane potential (Fig. 1H), intracellular ATP level (Fig. 1I) and mitochondrial routine respiration (Fig. 1J), and found that these three key indexes for evaluating mitochondrial function were improved from mitochondrial dysfunction with metformin (1 mM, 6 h) treatment in activated platelets. The mitochondrial electron transport chain (ETC) is an important target that regulates mitochondrial function, including mitochondrial membrane potential, ROS, and ATP levels19. Our study found that metformin inhibited complex I activity of the platelet mitochondrial respiratory chain (Fig. 1K). In addition, other complexes of the mitochondrial respiratory chain were also evaluated. However, we did not observe any statistical differences in complex activity between metformin treatment and non-treatment. The low concentrations of rotenone, a known mitochondrial complex I inhibitor, had similar effects in this assay (Fig. 1K,L) and, as expected, also inhibited mitochondrial release (Fig. 1L). The effect of rotenone on platelet mtDNA release should be looking at the impact of rotenone dose response, high concentrations of rotenone cause platelet mitochondrial dysfunction, apoptosis and mtDNA release. As shown in Supplementary Fig. 1B, high concentrations of metformin also increase platelet mtDNA release. Thus, our results indicated that metformin lowered mtDNA release from activated platelets and suppressed mitochondrial dysfunction via inhibition of complex I in the ETC.
Figure 1. Metformin suppresses the release of platelet mtDNA via inhibition of complex I.
(A,B) Metformin influences mtDNA escape from activated platelets in vitro (metformin: 1 mM, 6 h) and in vivo (metformin: 400 mg/kg/d, 7 d). (C) Transmission electronic microscopy imaging of platelets and mitochondria (black arrows) treated with metformin or without. (D) C11-BODIPY 581/591 (lipid peroxidation sensor), (E) ROS(CM-H2DCFDA fluorescence), (I) ATP, (H) hyperpolarization (JC-1 fluorescence ratio, FL2/FL1) ΔΨ, (J) mitochondrial routine respiration, (K) complex I values in activated platelets treated with metformin (metformin: 1 mM, 6 h). (F) ROS (hydrogen peroxide, 6 h) induces mtDNA escape from platelets. (G) NAC reduce mtDNA release from platelets (NAC: 10 mM, 6 h). (L) Low concentrations of rotenone reduce mtDNA release from platelets (rotenone: 12.5 nM, 6 h). Data are expressed as mean ± SD. n = 8. *P < 0.05 vs control, #P < 0.05 vs ADP.
mtDNA-induced platelet activation may through DC-SIGN-dependent pathway
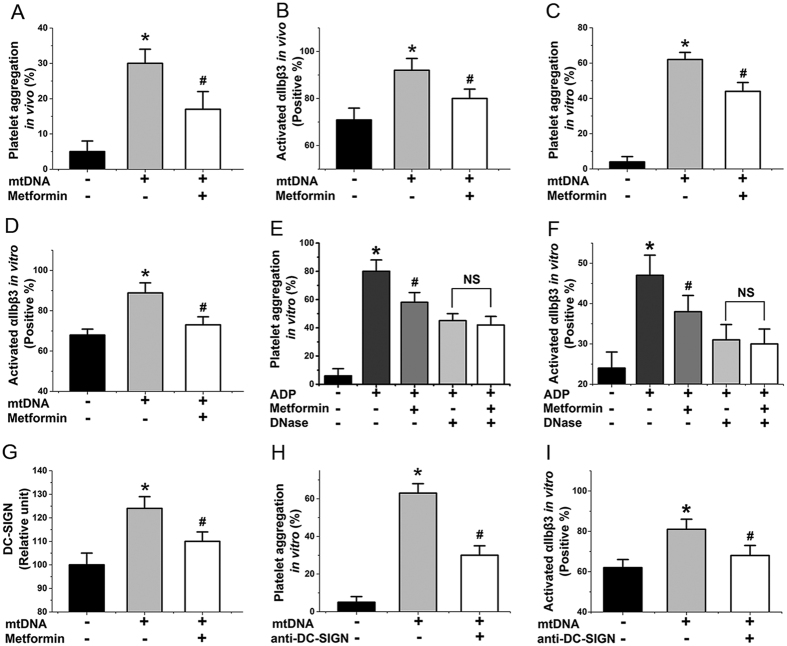
Overall, our above studies showed that metformin protected against mitochondrial dysfunction by inhibiting complex I, hyperpolarization, ROS production, and ROS-induced membrane damage, and subsequently prevented mtDNA release from activated platelets. However, because it is unclear whether mtDNA induces platelet activation, we also performed additional related experiments. We found that mtDNA caused increased levels of platelet aggregation (Fig. 2A,C) and αIIbβ3 expression (Fig. 2B,D) in vivo (mtDNA: 50 μg/kg/d, 7 d) and in vitro (mtDNA: 40 ng/μL, 6 h, 37 °C), while the addition of metformin reduced these levels (Fig. 2A–D). To further confirm the importance of mtDNA in metformin inhibited platelet activation it would been useful to demonstrate that elimination of DNA using a DNase eliminated the signal. As expected, DNase prevents inhibition of metformin on platelet activation (Fig. 2E,F). Furthermore, we found that the level of a certain protein, with a comparable weight to that of DC-SIGN, increased when we incubated platelets with mtDNA (Fig. 2G). By using a co-immunoprecipitation assay with anti-DC-SIGN antibodies, we confirmed that this protein was DC-SIGN21. Platelets express this surface molecule, which acts as a critical receptor in further platelet activation21,22,23, but had not been linked previously to mtDNA-induced platelet activation. Less DC-SIGN was expressed in the presence of metformin (Fig. 2G). Anti-DC-SIGN antibody (neutralizing antibodies against DC-SIGN, 25 μg/mL, 0.5 h) decreased mtDNA-induced platelet aggregation and αIIbβ3 expression (Fig. 2H,I). Taken together, our study has been the first to reveal that mtDNA released from activated platelets can act as a cellular damage factor and subsequently induce platelet activation may in a DC-SIGN-dependent manner. Next, we considered to confirm that mtDNA induces platelet activation through DC-SIGN with knockout approaches.
Figure 2. mtDNA induced platelet activation may through DC–SIGN dependent pathway.
(A,C) mtDNA affects aggregation of platelets with or without metformin treatment in vivo (mtDNA: 50 μg/kg/d, 7 d) and in vitro (mtDNA: 40 ng/μL, 6 h). (B,D) mtDNA influences αIIbβ3 level with or without metformin treatment in vivo and in vitro. (E,F) DNase decreases inhibition of metformin on platelet aggregation and αIIbβ3 level induced by ADP in vitro (Pre-incubated for 0.5 h at 20 μg/mL DNase I). (G) mtDNA increases DC-SIGN expression in vitro. (H,I) Anti-DC-SIGN (Pre-incubated for 0.5 h at 25 μg/mL) agent decreases platelet aggregation and αIIbβ3 level induced by mtDNA in vitro. Data are expressed as mean ± SD. n = 8. *P < 0.05 vs control, #P < 0.05 vs mtDNA.
Metformin inhibits platelet activation in vivo and in vitro
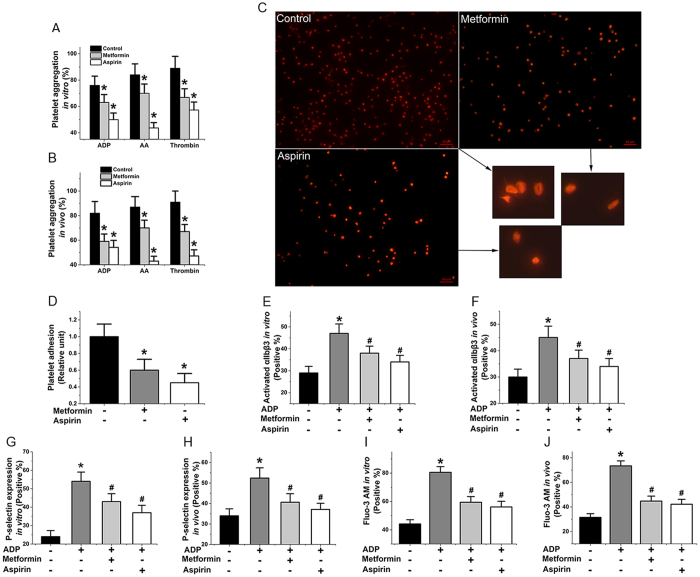
To further assess the effect of metformin on platelet activation, we assessed the drug’s effect on aggregation of platelets induced by ADP, AA, and thrombin in vitro (Fig. 3A, metformin: 1 mM, 6 h, 37 °C) and in vivo (Fig. 3B, metformin: 400 mg/kg/d, 7 d). The results showed that metformin significantly suppressed platelet aggregation in all assays (Fig. 3A,B). We also found that three key indicators of platelet activation, αIIbβ3 expression (Fig. 3E,F), P-selectin (Fig. 3G,H), and cytosolic calcium (Fig. 3I,J), were decreased in vitro and in vivo in ADP-activated platelets in the presence of metformin. The effects were comparable with those of aspirin used as a positive control (Fig. 3D–J). The αIIbβ3 expression that key indicator of platelet activation also were decreased in AA, and thrombin-activated platelets in the presence of metformin (Supplementary Fig. 2A). Furthermore, because thrombosis is known to be mediated by adhesion, activation, and aggregation of platelets24, we evaluated the effects of metformin on platelet adhesion and activation on collagen-coated surfaces. The data showed that metformin effectively inhibited these processes (Fig. 3C,D).
Figure 3. Metformin inhibits platelet activation in vivo and in vitro.
(A,B) Influence of metformin on aggregation of platelets induced by ADP, AA, and thrombin in vitro (metformin: 1 mM, 6 h) and in vivo (metformin: 400 mg/kg/d, 7 d). (C,D) Effect of metformin on platelet adhesion to collagen-coated surfaces (phalloidin-labelled platelets). (E,F) αIIbβ3 expression in ADP-activated platelets with metformin in vitro and in vivo. (G,H) P-selectin expression in ADP-activated platelets with treatment in vitro and in vivo. (I,J) Influence of metformin on the elevation of cytosolic calcium levels in ADP-stimulated platelets in vitro and in vivo. Data are expressed as mean ± SD. n = 9–10. *P < 0.05 vs control, #P < 0.05 vs ADP.
Metformin inhibits the formation of arterial and venous thrombosis in animals
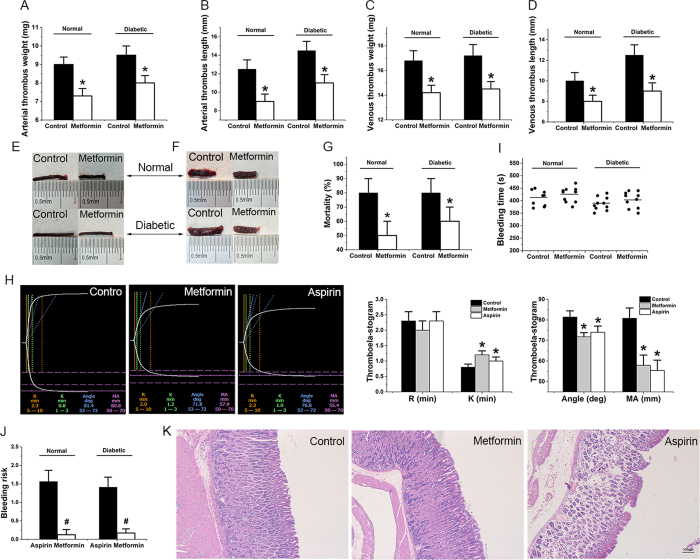
In addition, we evaluated the effect of metformin on thrombus (metformin: 200 mg/kg twice a day for 7 days)25,26, particularly on diabetic rat thrombus, since metformin is an antidiabetic drug6. Metformin showed similar effects on preventing arterial (Fig. 4A,B,E) and venous (Fig. 4C,D,F, Supplementary Fig. 3A,B) thrombosis in normal and diabetic rat models. The metformin treatment significantly decreased the incidence of pulmonary embolism (Fig. 4G), reduced the weight and length of arterial and venous thrombus (Fig. 4A–F), prolonged the average time to occlusive thrombosis in FeCl3-induced inferior vena cava and carotid arteries injury (Supplementary Fig. 3C,D), and improved blood viscosity (Supplementary Table 1). These results suggest that metformin may have great potential as an anti-thrombotic agent and are consistent with published reports stating that metformin decreases cardiovascular events and mortality in diabetic patients27,28,29. Our studies also demonstrate that metformin reduces the amount of plasma mtDNA and platelet DC-SIGN expression in FeCl3-induced carotid arterial thrombosis and partial inferior vena cava ligation induced venous thrombosis model (Supplementary Fig. 3E–H). From analysis of a thromboelastogram (TEG)30, we found that metformin treatment significantly improved indexes for viscoelastic properties of the developing clot, thrombogenesis, and platelet activation (Fig. 4H). However, metformin treatment appeared not to have a significant influence on prothrombin time (PT), activated partial thrombin time (APTT), fibrinogen (FIB), thrombin time (TT), coagulation factors II, V, VII–XII, clot weight, and platelet counts (Supplementary Fig. 2B–D, Supplementary Table 2). Furthermore, the metformin treatment did not significantly prolong bleeding time in either normal or diabetic rats (Fig. 4I). These findings are notable, because a known major limitation of current antithrombotic therapies is their inability to separate thrombotic events from bleeding occurrences31. And the mice treated with metformin did not show any spontaneous bleeding and had far less bleeding risk than those treated with aspirin (Fig. 4J), the most commonly used oral antithrombotic drug. Our results in mice are consistent with the fact that bleeding side effects do not generally occur with clinical metformin treatment12. Our results also showed that, compared with aspirin32, metformin treatment (metformin: 200 mg/kg twice a day for 60 days) led to decreased incidence of gastric ulcer (Fig. 4K). In our studies, gastric ulcers developed in two mice (2/10) in the aspirin-treated group (aspirin: 15 mg/kg twice a day for 60 days), but none appeared in mice (0/10) in the metformin-treated group. These data suggest that metformin can prevent both venous and arterial thrombosis by inhibiting platelet activation, notably without a significant risk of bleeding.
Figure 4. Metformin inhibits formation of FeCl3-induced carotid arterial thrombosis and partial inferior vena cava ligation induced venous thrombosis in animals.
Decreases weight (A,E) and length (B,E) of arterial thrombus in diabetic and normal rats (pre-treated with 400 mg/kg/d metformin for 7 d). Decreases weight (C,F) and length (D,F) of venous thrombus in normal and diabetic rats. (G) Reduces mortality from pulmonary thromboembolism in diabetic and normal mice (metformin: 400 mg/kg/d, 7 d). (H) Thromboelastogram of whole blood from rats treated with or without metformin. (I) Does not significantly prolong bleeding times in normal and diabetic C57/BL6 mice (metformin: 400 mg/kg/d, 7 d). (J) Reduces bleeding risk (increased bleeding time %/inhibited thrombosis %) in diabetic and normal rats compared with the use of aspirin. (K) Metformin decreases the incidence of gastric ulcer in normal rats compared with the use of aspirin (metformin: 400 mg/kg/d, 60 d. Aspirin: 30 mg/kg/d, 60 d). Data are expressed as mean ± SD. n = 9–10. *P < 0.05 vs control, #P < 0.05 vs aspirin.
Discussion
Metformin, an insulin-sensitizer, may improve vascular function and several physiologic abnormalities related to insulin resistance with fewer reported side-effects in patients with type 2 DM10. Literature reports have connected metformin with reduced mortality and diabetes-associated cardiovascular and cerebrovascular events in diabetic patients8,9,10,11. However, it is not known whether metformin is effective against thrombosis in both diabetic and normal individuals, and further investigation is merited to determine its precise mechanism of action with respect to thrombosis prevention. One advantage of normal animal thrombotic models, as opposed to diabetes-associated studies, is that the contribution of metformin can be assessed independently of other cardiovascular risk factors, such as hypercholesterolemia, hyperglycemia, obesity, and hypertension33. By using models of carotid artery and inferior vena cava thrombosis, we found that thrombus formation was strongly impaired in metformin-treated diabetic and normal rats. We also demonstrated that metformin-treated mice were more resistant to pulmonary thromboembolism than control mice. Normal activity of blood coagulation requires platelets as a critical factor. Studies have shown that platelets are involved in many biological clotting processes, including detection of vascular lesions, coagulation cascade startup, and activation of other platelets15. Additional studies presented here provided evidence that, in the presence of metformin, platelet prothrombinase activity was markedly impaired. This finding is consistent with the observed decrease in αIIbβ3 expression, P-selectin, and cytosolic calcium of platelets preincubated with metformin. Our results also indicated that the platelet adhesive function was reduced after administration of metformin. However, metformin treatment appeared to have no significant influence on coagulation factors and clot weight.
Antiplatelet therapy has been effective in reducing the mortality and morbidity of cardiovascular disease, the most common cause of death in developed countries. However, current antiplatelet agents approved by the US Food and Drug Administration have serious side effects, including bleeding episodes, gastrointestinal toxicity, neutropenia and thrombocytopenia. As stated previously, a recognized limitation of current antiplatelet therapies is their inability to separate thrombotic events from bleeding occurrences31,33,34. The impact of metformin on hemostatic function is best exemplified by our tail bleeding time studies and chronic-type gastric ulcer evaluation. Intriguingly, metformin-treated mice did not show any spontaneous bleeding, abnormal tail bleeding times, or gastrointestinal toxicity (without gastric ulcer occurrences). Our mouse studies also demonstrated that metformin does not have deleterious effects on platelet properties and, therefore, suggested that long-term medication would not lead to the negative effects of induced platelet death or altered platelet counts. In this study, we have demonstrated that metformin uniquely prevents both venous and arterial thrombosis with no significant prolonged bleeding time by inhibiting platelet activation. Our work presented herein suggests that further investigation on metformin could lead to a novel antiplatelet therapy with fewer side-effects. Increased understanding of metformin’s antithrombotic activity should lead to the development of new antiplatelet drugs that are potentially more effective with minimal side effects. Such new therapies targeting the underlying mechanism for platelet dysfunction are urgently warranted.
Some indications as to the role mitochondria play in platelet function have been elucidated15,16,17. Early reports primarily examined the role that mitochondria play in meeting the energy demands required for platelet aggregation and granule release16. For example, Barile et al. pointed out that inhibition of mitochondrial respiration in platelets could suppress platelet-stimulated blood coagulation15. Interestingly, previous work by Boudreau and colleagues demonstrated that activated platelets can release mitochondria18. Previously, various studies indicated that metformin has variable effects on hepatic mitochondrial function by inhibiting complex I in the mitochondrial respiratory chain13,14. While it was not determined whether metformin can protect against activated platelet-induced mitochondrial dysfunction or prevent mitochondria release from activated platelets, we hypothesized that metformin can inhibit platelet activation and platelet-activated blood coagulation in this manner. Consistent with our hypothesis, decreased extracellular mtDNA was detected with quantitative real-time PCR in metformin-treated platelets. As shown in Supplementary Fig. 1D, metformin had no significant effect on apoptosis of platelets. Therefore it was conclude that the lowered level of extracellular mtDNA may not be caused by the variation of platelets apoptotic bodies. Our observations also indicated that metformin inhibited mtDNA release by preventing membrane damage, and mitochondrial dysfunction in activated platelets. However, the mechanism behind these inhibitory effects of metformin is poorly recognized and deserves further investigation. Membranes are redox-sensitive, and an increased generation of ROS is one mechanism for membrane damage19. Our results showed that lower levels of ROS were produced when platelets were preincubated with metformin. We subsequently explored several possible mechanisms for metformin-induced decreased accumulation of ROS in activated platelets. We first considered decreased NADPH oxidase in platelets as a potential mechanism, because decreased NADPH oxidase has been associated with platelet activation33. However, we did not observe any differences in platelet NADPH oxidase levels between metformin treatment and untreated control (Supplementary Fig. 1G). Glutathione peroxidase-1 (Gpx1) was also evaluated, as decreased erythrocyte Gpx1 activity is associated with increased cardiovascular risk in patients with coronary artery disease; however, no change was seen (Supplementary Fig. 1H). We next considered the potential role of superoxide dismutase as an enzymatic source of elevated ROS in platelets. Because ROS can be generated from superoxide by superoxide dismutase33, metformin-associated decreased generation of ROS in platelets could result from decreased conversion of superoxide to H2O2 by superoxide dismutase. However, we consider this possibility to be unlikely, because we also did not detect any significant change of superoxide dismutase (Sod1) in platelets from metformin treatment (Supplementary Fig. 1I).
ETC is an important target that regulates ROS levels and mitochondrial function, including mitochondrial membrane potential, respiration and ATP levels19. As expected, our findings that complex I in the ETC was inhibited in metformin-treated platelet were consistent with the observations of Piel et al. in human platelets35. In this study, we also found that intracellular ATP level, mitochondrial routine respiration, and hyperpolarization level of the mitochondrial membrane potential, three key indexes for evaluating mitochondrial function, were improved with metformin treatment in ADP-stimulated platelets. These findings suggest that the decrease in platelet ROS is caused by decreased activity of mitochondrial complex I. In agreement with this hypothesis, we found that preincubation of platelets with low concentrations of rotenone, a mitochondrial complex I inhibitor, resulted in a significant decrease in ADP-induced activation of αIIbβ3 (data not shown). We also observed that low concentrations of rotenone decreased mtDNA release from ADP-induced activated platelets. Meanwhile, high concentrations of rotenone caused platelet mitochondrial dysfunction, apoptosis, and mtDNA release. Similarly to rotenone, the effect of metformin on platelet mtDNA release should be looking at the impact of metformin dose response, high concentrations of metformin increased mtDNA release by increasing platelet apoptosis, low concentrations of metformin inhibited mtDNA release by protecting mitochondrial function. Taken together, our results indicated that metformin lowers mtDNA release from activated platelets and suppresses mitochondrial dysfunction via inhibition of complex I in the ETC.
Increasing evidence demonstrates that mtDNA plays key roles in immune and inflammatory responses and their related diseases27,36,37,38,39, and our results indicated that metformin inhibits mtDNA release as well as platelet activation. However, because it is unclear whether mtDNA induces platelet activation, additional related experiments were performed. We examined the effect of mtDNA on platelet pro-coagulant activity, and found that mtDNA-treated platelets can support efficient platelet activation in vitro and in vivo. However, the nature of the downstream pathways for mtDNA-mediated platelet formation remains largely unknown. DC-SIGN, a type II membrane protein and C-type lectin receptor that binds high-mannose-containing glycoproteins, was originally cloned from a placental library via its ability to bind the glycan-rich HIV-1 envelope without the assistance of the classic CD4 virus receptor22. Although previous studies have shown that DC-SIGN interacts with platelet activation21, they were not then linked to mtDNA-induced platelet activation. When we incubated platelets with mtDNA, the level of a certain protein was increased. Next, we confirmed that this protein was DC-SIGN by using a co-immunoprecipitation assay with anti-DC-SIGN antibodies. In addition, we found that anti-DC-SIGN (neutralizing antibodies against DC-SIGN) inhibition abolished the potentiating effects of mtDNA on platelet activation, further confirming the critical role of DC-SIGN in mtDNA-mediated platelet activation. We next studied the role of metformin in mtDNA-mediated platelet activation. We found that platelet activation was dramatically decreased following metformin preincubation. Taken together, our study is the first to reveal that the mtDNA released from activated platelets can act as a cellular damage factor and subsequently induce platelet activation may via a DC-SIGN-dependent pathway. We next considered to confirm and assure mtDNA induces platelet activation through DC-SIGN with gene knockout approaches. Our work presented here is consistent with other recent findings and suggests possible mechanism(s) for metformin’s role in reduced mortality and diabetes-associated thrombotic complications, related to inhibition of platelet activation, prevention of mitochondrial dysfunction, and reduction in the release of free mtDNA.
As of 2014, DM was diagnosed in more than 20 million adults in the United States and likely remained undiagnosed in millions more. Thrombotic cardiovascular events, in which platelets have essential functions, will be a likely cause of death in an estimated 65% of diabetic patients. Although aspirin is most commonly used to prevent and treat heart attacks and strokes in clinical practice, previous work has shown that 10% to 40% of diabetic patients are biochemically resistant to this drug. Due to this important concern, a better understanding of and new therapies to target the underlying mechanism for platelet dysfunction must be found1,2,3,4. Our findings suggest that metformin could be a promising lead as a new class of antiplatelet agents that are highly effective at inhibiting platelet activation in vitro and in vivo.
In conclusion, the research presented here has established a novel therapeutic strategy for platelet abnormalities in DM and a general strategy to prevent cardiovascular complications. Future studies will be required to develop new antiplatelet drugs that are potentially more effective without bleeding risk and as possible treatments for metabolic syndrome. Furthermore, our team will evaluate whether or not metformin also inhibits anionic phospholipid exposure, microvesicle release, and other damage-associated molecular pattern molecules (DAMPs) such as high-mobility group box 1 (HMGB1) in the future. We will use other pharmacological and knockout approaches to confirm and assure mtDNA induces platelet activation through DC-SIGN, and research DC-SIGN downstream signaling events, and consider other receptors that might be implicated in mtDNA dependent platelet activation e.g. TLR9. On the basis of our studies, we now propose that drugs targeting platelet mtDNA may be effective against platelet dysfunction and thrombosis. These mechanistic studies provide new insights into platelet abnormalities to inhibit thrombosis. Beyond the well-recognized pivotal role in thrombosis and hemostasis, platelets also play a critical role in inflammatory and infectious diseases, and increasing evidence indicates that bacterial infection predisposes for atherosclerosis and thrombotic events26. Several previous studies provided support that mtDNA is also involved in immune and inflammatory responses and their related diseases36,37,38,39. In this study, we reported that mtDNA is released from activated platelets and acts as an ‘agonist’ to induce platelet activation and thrombosis. To our knowledge, this study is the first to report that mtDNA may play a connecting role in thrombosis and inflammation/immunity during bacterial infection and, thus, is an important link to study in elucidating the underlying mechanisms that thrombotic events play in inflammation, infection, cancer, DM, etc.
Materials and Methods
Animal models and diets
Male Type 2 diabetic rats (type 2 DM), Sprague-Dawlecy (SD) rats and C57/BL6 mice were obtained from the West China Hospital Experiment Animal Center (Chengdu, China). The rats were maintained in an animal house with a constant temperature of 25 °C and a 12/12-hour of light/dark cycle. Food and water were provided ad libitum. All animals were treated according to the experimental protocols approved by the Bioethics Committee of West China Center of Medical Sciences. Diabetic rats were induced by combination of high-fat diet-fed and low-dose streptozotocin (STZ, 45 mg/kg, ip) injection (Final fasting blood glucose level: Control 4.8 ± 0.8 mmol/L; Diabetic 16.4 ± 2.2 mmol/L)40. All animal studies included 9–10 rats or mice per group, aged 9–12 weeks at the time of study. Animals were randomly allocated to control groups, model groups, and treatment groups. Metformin was administrated orally to animals at 200 mg/kg twice daily for seven days. Aspirin was administrated at 15 mg/kg in the same manner. Ferric chloride induced carotid arterial injury and venous thrombosis experiments were conducted according to the previously described methods with a minor modification25. Briefly, a piece of filter paper (2 mm × 10 mm) pre-saturated with 3 μL of 1 mM FeCl3 solution (arterial) or 10 mm × 10 mm paper pre-saturated with 20 μL of 2 mM FeCl3 solution (venous) was placed on the arterial or venous cava. Blood flow was monitored continuously for 90 minutes or until stable occlusion occurred, at which time the experiment was terminated. Stable occlusion was defined as the time at which blood flow remained absent for ≥10 minutes. Finally, weight and length were measured. Inferior vena cava ligation experiments were performed as described under aseptic conditions41. Briefly, rats were anesthetized with 5% chloral hydrate (0.7 mL/100 g) and placed in a supine position. A midline laparotomy was performed, and then intestines were exteriorized and sterile saline was applied to prevent drying during the whole procedure. The inferior vena cava (IVC) was ligated by a 7.0 polypropylene suture below the renal veins for obtain complete blood stasis. After surgery, peritoneum and skin were closed and the animal was allowed to recover. After 48 hours, the IVC was harvested and weighed for analysis. Pulmonary thromboembolism was induced by a method as described42. The number of dead or paralyzed mice was recorded and the percentage of protection was calculated. The bleeding time was measured by a previously described method25,42.
Chronic-type gastric ulcer
Male, C57/BL6 mice (20 ± 3 g) were administrated orally metformin 200 mg/kg twice a day for 60 days. Aspirin was administrated 15 mg/kg in the same way, and mice were sacrificed on the 61st day. The stomach was examined by an observer unaware of the treatments and any macroscopically visible ulcers were measured with calipers. An ulcer area (in mm2) was calculated for each stomach. Samples of macroscopically normal and damaged tissue were excised, fixed in neutral buffered formalin, and processed by routine techniques for subsequent histological evaluation. The small intestine was examined for signs of injury32.
Platelet preparation
Washed platelets were prepared by differential centrifugation as described previously33,41,43. Briefly, venous blood samples were collected in citrate by standard venous puncture from rats, and the first 2 mL of blood was discarded to avoid artificial activation. Whole blood was centrifuged (160 g for 10 min) in the presence of PGI2 (1 mM) to obtain platelet-rich plasma (PRP). Platelets were subsequently pelleted by centrifugation (1000 g for 5 min). The platelet pellet was then suspended in a washing buffer containing Tyrode’s buffer, 10% of acid-citrate-dextrose solution, 2 mM EDTA and 1 mM PGI2 and was centrifuged again. Final samples were resuspended in modified Tyrode buffer. Platelet purity was confirmed by flow cytometric measurement of CD41a expression.
Platelet adhesion under flow conditions assay
Platelets were incubated at 37 °C for 6 h in the absence or presence of metformin (1 mM), and stained with phalloidin (200 nM) (Sigma-Aldrich) for 30 min at 37 °C as described. A collagen-coated coverslip (Neuvitro) was mounted on a custom-made flow chamber (Chamlide CF; Live Cell Instruments). The fluorescently labeled platelets were then perfused over a matrix of collagen at 150 s−1 using a syringe pump (Harvard Apparatus). Non-adherent platelets in the chamber were washed with PBS. Adherent platelets were fixed with cold 4% paraformaldehyde for 15 min and then washed with PBS. The perfusion was live-monitored with a fluorescence microscope (Nikon TE-2000S; Nikon, Melville, NY, USA) equipped with a Nikon DS-2MBWc-U1 CCD camera (Nikon)34,43,44.
P-selectin and integrin αIIbβ3 assay
After treatment (in vitro: metformin: 1 mM, 6 h, 37 °C; aspirin: 0.1 mM, 6 h, 37 °C; mtDNA: 40 ng/μL, 6 h, 37 °C. In vivo: metformin: 200 mg/kg twice a day for 7 days; aspirin: 15 mg/kg twice a day for 7 days; mtDNA: 25 μg/kg twice a day for 7 days), platelets were incubated with PE-CD62P (Sigma, USA) or PAC-1-FITC (Sigma, USA) for 30 min in the dark, and reaction was stopped by adding ice-cold PBS. The P-selectin and integrin-αIIbβ3 expression on the platelets was measured at 585 nm (FL2) and 530 nm (FL1) by using flow cytometer (Becton - Dickinson, San Jose, CA, USA). In selected experiments platelets were pre-incubated for 0.5 h at 37 °C with neutralizing antibodies against DC-SIGN (120507; R&D Systems, USA, 25 μg/mL) or DNase I (Genentech, USA, 20 μg/mL)33,45.
Lipid peroxidation, ROS, Ca2+ and ATP assay
Platelets were incubated with metformin (1 mM) for 6 h at 37 °C. Subsequently, platelets were loaded with CM-H2DCFDA (General Oxidative Stress Indicator, Thermo Fisher Scientific, USA) or MitoSOX™ Red Mitochondrial Superoxide Indicator (Thermo Fisher Scientific, USA) at room temperature in the dark and measured by a fluorescence microplate reader (Aynergy Mx, BioTek, USA). Lipid peroxidation of platelets was assayed using a commercial kit (BODIPY® 581/591 C11, Thermo Fisher Scientific, USA). The levels of cytosolic calcium were analyzed by flow cytometer using Fluo-3-AM (Sigma, USA) as a probe, and ATP levels were determined using a commercial kit (Sigma, USA)15,35,46,47.
Complex I assay
Platelets were incubated with metformin (1 mM) for 6 h at 37 °C. Complex I (NADH-ubiquinone 1 reductase) levels were assessed with MitoCheck® Complex I Activity Assay Kit (Item No 700930, Cayman Chemical, USA) (Abcam, USA)35,46.
Mitochondrial membrane potential assay
Treated platelets implanted in a 48-well plate were incubated with JC-1 solution (10 μg/mL) at 37 °C for 15 min. Then, the platelets were harvested and resuspended in 0.2 mL of Tyrode’s buffer. The Δψ of labeled platelets was measured by a confocal microscope (Nikon ECLIPSE Ti, Japan)35,46.
Mitochondrial routine respiration assay
The effect of metformin on mitochondrial respiration in platelets was assay as described previously35. Briefly, after stabilization of routine respiration in MiR05 medium, 1 mM metformin, 12.5 nM rotenone, or either vehicle was added. Oligomycin (1 μg/mL), ATP-synthase inhibitor was added to assess LEAK respiration. The maximal capacity of electron respiratory chain using endogenous substrates (ETS) was measured by application of FCCP. Respiration was sequentially blocked by rotenone (the complex I inhibitor, 2 μM), antimycin (the complex III inhibitor, 1 μg/mL) and sodium azide (the complex IV inhibitor, 10 mM) to assess residual oxygen consumption.
Transmission electronic microscopy imaging of platelets and mitochondria assay
Washed platelets were prepared treated with and without metformin (1 mM, 6 h) and activated or not by ADP, then were fixed with 2.5% glutaraldehyde for at least 24 h and processed for standard dehydration. Briefly, samples were first washed (3 × 10 min) with Tyrode’s buffer then fixed with osmium tetroxide (1% in sodium cacodylate buffer) for 90 min. Samples were washed again (3 × 10 min) with Tyrode’s buffer and subsequently processed for alcohol dehydration steps (50, 70, 95 and 100% EtOH, 10 min each steps). Samples were then dipped in 100% EtOH for 40 and 10 min respectively, and air-dried overnight. Samples were then coated with palladium and observed with a JEOL 6360LV transmission electron microscope (Tokyo, Japan)18,27,48.
In vitro blood clot dissolution assay
Dissolution of blood clot assay in vitro was measured as described with slight modification. In brief, venous blood of rats was gathered into a dry Petri dish and clotted at room temperature. The blood clot was cut into 100 ± 20 mg weight slices after 24 h. Subsequently, these clot slices were put into a 12-well plate (one slice per well) and incubated with or without metformin (metformin: 1 mM, 6 h, 37 °C). The dissolution rate of blood clot was calculated and expressed as: Dissolution rate (%) = [(W1–W2)/W1]*100; where W1 represents clot weigh, and W2 indicates the residual clot weight49.
Hematological parameters
The whole blood was collected by cardiac puncture and treated by different anticoagulants: 3.8% trisodium citrate (9:1, v/v), 10% EDTA (9:1, v/v), and heparin. Platelet-rich plasma (PRP) was obtained by centrifugation at 100 g for 10 min. Platelet-poor plasma (PPP) was prepared by centrifugation at 1000 g for 10 min. Thrombelastogram was measured by thrombelastography (Haemoscope, USA). Blood coagulation factor was measured by a kaolin-activated one-stage partial thromboplastin time method using mice Factor plasma (George King Biomedical). An automated hematology analyzer was used to evaluate the viscosity of those samples. Platelet counts (PLT), mean platelet volume (MPV), immature platelet fraction (IPF), plateletcrit (PCT) and platelet distribution width (PDW) of blood samples were measured by automatic blood cell analyzer (SYSMEX SF-3000, Japan). The platelet aggregation assay was performed at 37 °C using a Lumi-Aggregometer (Chrono-log). Aggregation was initiated by the addition of ADP solution (final concentration, 20 μM), thrombin (0.1 U/mL) and arachidonic acid (AA: 900 μM) in vivo (pre-treated with 400 mg/kg/d metformin, 50 μg/kg/d mtDNA or control buffer for 7 d) and in vitro (pre-incubated with 1 mM metformin, 40 ng/μL or control buffer for 6 h at 37 °C), and recorded for 5 min. In selected experiments platelets were pre-incubated for 0.5 h at 37 °C with neutralizing antibodies against DC-SIGN (120507; R&D Systems, USA, 25 μg/mL)41,33,49,50,51.
mtDNA preparation
The mtDNA was isolated from platelets using a commercial kit (Mitochondria DNA Isolation Kit, Biovision), using western blotting confirm the absence of non-mitochondrial proteins to demonstrate the purity of the mtDNA preparations, and using consistent approaches in this study. Quantification of mtDNA was determined by qPCR assay using a PRISM 7300 sequence detection system (Applied Biosystems)18.
mtDNA quantification in vitro
Platelets were isolated from the blood of rats and purity was confirmed by flow cytometric measurement of CD41a expression. Then platelets treated with 1 mM metformin for 6 h at 37 °C. After centrifugation at 700 g for 10 min to remove the pellet (platelet), extracellular mtDNA was isolated from the supernatant using a Mitochondrial DNA Isolation Kit (Catalog K280-50, Biovision, USA), using western blotting confirm the absence of non-mitochondrial proteins to demonstrate the purity of the mtDNA. The concentration of mtDNA was determined by a spectrophotometer (Nanodrop2000, Thermo) or qPCR assay using a PRISM 7300 sequence detection system (Applied Biosystems)18,36,50,52.
mtDNA quantification in vivo
Seven days after treatment with 400 mg/kg/d metformin, rats were sacrificed and blood was collected, and mtDNA was extracted with Mitochondrial DNA Isolation Kit (Catalog K280-50, Biovision, USA) for analysis, and confirm the purity of the mtDNA. mtDNA level was measured by qPCR assay using a PRISM 7300 sequence detection system (Applied Biosystems) as previously described18,36,50,52. The primer sequences were rat NADH dehydrogenase 1 gene (mtDNA): forward CGCCTGACCAATAGCCATAA, reverse ATTCGACGTTAAAGCCTGAGA. All samples were measured with standards at the same time.
DC-SIGN expression assay
Platelets were resuspended in Tyrode’s buffer, then treatment with or without mtDNA (mtDNA: 40 ng/μL, 6 h). Immunoprecipitation of protein assemblies was performed by incubation of these pools with purified and identified monoclonal antibodies against DC-SIGN (MR-1, Santa Cruz Biotech, USA), and then capture of the immune complexes on Protein G superparamagnetic Microbeads (Miltenyi Biotec, Germany) as described previously22. Immunoprecipitates were subjected to Western blot using polyclonal antibodies against DC-SIGN. Immunoprecipitated with Streptavidin-agarose (Sigma Aldrich, USA), and then immunoprecipitated material was analyzed by SDS-PAGE, Western blot with antibodies specific for DC-SIGN.
mRNA Levels of Nox2, Gpx1, Sod1 assay
Levels of mRNA for Nox2, Gpx1, Sod1, and 18S were measured by quantitative real-time PCR as described previously33. First, isolating total RNA from washed platelets with Trizol reagent (Invitrogen, Carlsbad, CA). Reverse-transcribed cDNA was incubated with PCR primers, 6-carboxy fluorescein-labeled probes (Applied Biosystems) and TaqMan Universal PCR mix at 50 °C for 2 minutes and then at 95 °C for 10 minutes. After that, 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. Using the comparative threshold cycle (ΔΔCT) method to quantification with values normalized of 18S and expressed relative to levels in platelet without metformin preincubation. Platelet purity was confirmed by flow cytometric measurement of CD41a expression33.
Western blot analysis
Washed platelets were incubated with metformin for 6 h. The platelet were harvested and lysed using lysis buffer, after that the solution was centrifuged. After protein concentrations were determined, individual platelets lysates were separated with sodium dodecyl sulfate polyacrylamide gel electrophoresis (10% gel, SDS–PAGE), subsequently, platelets were transferred onto nitrocellulose membranes. The target proteins in the membranes was blocked with 5% nonfat milk, and probed with anti-caspase 3 (Cell Signaling Technology, Danvers, USA), overnight. The membranes were incubated with peroxidase-conjugated secondary antibody for 2 h. and then visualized with ECL Western Blotting Detection Reagents (Amersham Pharmacia Biotech)53.
Statistical analysis
The data are expressed as mean ± SD. Multiple comparisons were made by appropriate analysis of variance, whereas individual group samples were compared by Student’s t-test. In all cases P values were two tailed and P values < 0.05 were considered to indicate significance.
Additional Information
How to cite this article: Xin, G. et al. Metformin Uniquely Prevents Thrombosis by Inhibiting Platelet Activation and mtDNA Release. Sci. Rep. 6, 36222; doi: 10.1038/srep36222 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No: 81673710) awarded to Wen Huang and NIH grant CA177584 from the National Cancer Institute awarded to Kuo-hsiung Lee. We thank Jie Zhang and Ling Bai for using confocal microscope, Fangfang Zhang for using flow cytometer, Guang Yang for animal care, Jingyi Zhang for assisting using fluorescence microplate reader, Hongwei Zhang for providing diabetic rats.
Footnotes
Author Contributions W.H. and K.-H.L. designed experiments and analysed data, interpreted results and edited the paper; G.X. performed all experiments, analysed data, interpreted results and wrote the manuscript; H.Z. and Z.W. took care of animals, prepared and quantified mtDNA; C.J. designed mitochondrial function protocol; L.M. and R.Z. performed mitochondrial function assay; J.G. established venous and arterial thrombosis rats; K.L. and Y.L. analysed data; S.L.M.-N. wrote the manuscript; C.Q. performed rt-PCR assay; L.W. and Z.X. performed biochemistry assay; J.-L.Y., L.L., Q.X. and Y.C. revised manuscript; H.N. performed statistical analysis.
References
- Cho N. H. et al. The IDF Diabetes Atlas 7th edition (2015).
- Mackman N. Triggers, targets and treatments for thrombosis. Nature 451, 914–918 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H. et al. Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation 129, 1598–1609 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creager M. A., Lüscher T. F., Cosentino F. & Beckman J. A. Diabetes and Vascular Disease Pathophysiology, Clinical Consequences, and Medical Therapy: Part I. Circulation 108, 1527–1532 (2003). [DOI] [PubMed] [Google Scholar]
- Vazzana N., Ranalli P., Cuccurullo C. & Davì G. Diabetes mellitus and thrombosis. Thromb. Res. 129, 371–377 (2012). [DOI] [PubMed] [Google Scholar]
- Randriamboavonjy V. et al. Metformin reduces hyper-reactivity of platelets from patients with polycystic ovary syndrome by improving mitochondrial integrity. Thromb Haemost. 114, 569–578 (2015). [DOI] [PubMed] [Google Scholar]
- Cannegieter S. C. et al. Risk of venous and arterial thrombotic events in patients diagnosed with superficial vein thrombosis: a nationwide cohort study. Blood 125, 229–235 (2015). [DOI] [PubMed] [Google Scholar]
- Inzucchi S. E. Metformin and heart failure. Diabetes Care. 28, 2585–2587 (2005). [DOI] [PubMed] [Google Scholar]
- Roussel R. et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch. Intern. Med. 170, 1892–1899 (2010). [DOI] [PubMed] [Google Scholar]
- Madiraju A. K. et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 150, 542–546 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D. Y. et al. Metformin use in patients with type 2 diabetes mellitus is associated with reduced risk of deep vein thrombosis: a non-randomized, pair-matched cohort study. BMC Cardiovasc Disord. 14, 187 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A. et al. Effects of exenatide (Exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 28, 1092–1100 (2005). [DOI] [PubMed] [Google Scholar]
- El-Mir M. Y. et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 275, 223–228 (2000). [DOI] [PubMed] [Google Scholar]
- Owen M. R., Doran E. & Halestrap A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex I of the mitochondrial respiratory chain. Biochem J. 348, 607–614 (2000). [PMC free article] [PubMed] [Google Scholar]
- Barile C. J. et al. Inhibiting platelet-stimulated blood coagulation by inhibition of mitochondrial respiration. PNAS USA 109, 2539–2543 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S. I., Edelstein D., Du X. L. & Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes 50, 1491–1494 (2001). [DOI] [PubMed] [Google Scholar]
- Cardenes N. et al. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood 123, 2864–2872 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau L. H. et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124, 2173–2183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. P. How mitochondria produce reactive oxygen species. Biochem J. 417, 1–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecien S. et al. Lipid peroxidation, reactive oxygen species and antioxidative factors in the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress-induced gastric injury. J Physiol Pharmacol. 65, 613–622 (2014). [PubMed] [Google Scholar]
- Hottz E. D. et al. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases. J Thromb Haemost. 11, 951–962 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparrós E. et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood 107, 3950–3958 (2006). [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T. B. et al. Identification of DC-SIGN, a novel dendritic cell–specific ICAM-3 receptor that supports primary immune responses. Cell 100, 575–585 (2000). [DOI] [PubMed] [Google Scholar]
- King S. M. et al. Platelet dense-granule secretion plays a critical role in thrombosis and subsequent vascular remodeling in atherosclerotic mice. Circulation 120, 785–791 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Murine model of ferric chloride-induced vena cava thrombosis: evidence for effect of potato carboxypeptidase inhibitor. J Thromb Haemost. 4, 403–410 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang S. et al. Nucleotide-binding oligomerization domain 2 receptor is expressed in platelets and enhances platelet activation and thrombosis. Circulation 131, 1160–1170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger S. W. et al. Hydrogen peroxide and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 86, 960–966 (2000). [DOI] [PubMed] [Google Scholar]
- Inzucchi S. E., Masoudi F. A. & McGuire D. K. Metformin in heart failure. Diabetes Care. 28, 2585–2587 (2007). [DOI] [PubMed] [Google Scholar]
- Grant P. J. Beneficial effects of metformin on haemostasis and vascular function in man. Diabetes Metab. 29, 6S44–6S52 (2003). [DOI] [PubMed] [Google Scholar]
- Jy W. et al. Comparison of pharmacokinetics and hemostatic efficacy of red cell microparticles (RMP) in rabbits using different infusion regimens. Blood 124, 2811–2811 (2014). [Google Scholar]
- Musumeci L. et al. Dual-specificity phosphatase 3 deficiency or inhibition limits platelet activation and arterial thrombosis. Circulation 131, 656–668 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L. et al. A diclofenac derivative without ulcerogenic properties. Eur J Pharmacol. 257, 249–255 (1994). [DOI] [PubMed] [Google Scholar]
- Dayal S. et al. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation 127, 1308–1316 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. et al. Ginsenoside Rg1 inhibits platelet activation and arterial thrombosis. Thromb Res. 133, 57–65 (2014). [DOI] [PubMed] [Google Scholar]
- Piel S., Ehinger J. K., Elmér E. & Hansson M. J. Metformin induces lactate production in peripheral blood mononuclear cells and platelets through specific mitochondrial complex I inhibition. Acta Physiol. 213, 171–180 (2015). [DOI] [PubMed] [Google Scholar]
- Qin C. et al. Variation of perioperative plasma mitochondrial DNA correlate with peak inflammatory cytokines caused by cardiac surgery with cardiopulmonary bypass. J Cardiothorac Surg. 10, 85 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal I., Dittus K. & Holmes C. E. Platelets, coagulation and fibrinolysis in breast cancer progression. Breast Cancer Res. 15, 207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. R., Foster. T. J. & Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 4, 445–457 (2006). [DOI] [PubMed] [Google Scholar]
- Milne J. C. et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450, 712–716 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill A. et al. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood 117, 1400–1407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anil-Kumar K. S. et al. Synthesis and identification of chiral aminomethylpiperidine carboxamides as inhibitor of collagen induced platelet activation. Eur. J Med Chem. 81, 456–472 (2014). [DOI] [PubMed] [Google Scholar]
- Wang S. B. et al. Kaempferol suppresses collagen-induced platelet activation by inhibiting NADPH oxidase and protecting SHP-2 from oxidative inactivation. Free Radic Biol Med. 83, 41–53 (2015). [DOI] [PubMed] [Google Scholar]
- Bae O. N. et al. Trivalent methylated arsenical-induced phosphatidylserine exposure and apoptosis in platelets may lead to increased thrombus formation. Toxicol Appl Pharmacol. 239, 144–153 (2009). [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S. M. et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 118, 1663–1674 (2011). [DOI] [PubMed] [Google Scholar]
- Shirakabe A. et al. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation 133, 1249–1263 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges H. R., Jones A. J., Pollak M. N. & Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 462, 475–487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouseph M. M. et al. Autophagy is induced upon platelet activation and is essential for hemostasis and thrombosis. Blood 126, 1224–1233 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G. H., Qin Y. & Huang W. Anti-thrombosis effect of diosgenin extract from Dioscorea zingiberensis CH Wright in vitro and in vivo. Phytomedicine 18, 458–463 (2011). [DOI] [PubMed] [Google Scholar]
- Priestley E. S. et al. Structure-based design of macrocyclic coagulation Factor VIIa inhibitors. J Med Chem. 58, 6225–6236 (2015). [DOI] [PubMed] [Google Scholar]
- Citirik M., Beyazyildiz E., Simsek M., Beyazyildiz O. & Haznedaroglu I. C. MPV may reflect subcinical platelet activation in diabetic patients with and without diabetic retinopathy. Eye 29, 376–379 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K. et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 10, e1001577 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M. et al. Caspase-7 deficiency protects from endotoxin-induced lymphocyte apoptosis and improves survival. Blood 113, 2742–2745 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.