Abstract
2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and bis(2-ethylhexyl)tetrabromophthalate (BEH-TEBP) are novel brominated flame retardants used in consumer products. A parallelogram approach was used to predict human dermal absorption and flux for EH-TBB and BEH-TEBP. [14C]-EH-TBB or [14C]-BEH-TEBP was applied to human or rat skin at 100 nmol/cm2 using a flow-through system. Intact rats received analogous dermal doses. Treated skin was washed and tape-stripped to remove “unabsorbed” [14C]-radioactivity after continuous exposure (24h). “Absorbed” was quantified using dermally retained [14C]-radioactivity; “penetrated” was calculated based on [14C]-radioactivity in media (in vitro) or excreta+tissues (in vivo). Human skin absorbed EH-TBB (24±1%) while 0.2±0.1% penetrated skin. Rat skin absorbed more (51±10%) and was more permeable (2±0.5%) to EH-TBB in vitro; maximal EH-TBB flux was 11±7 and 102±24 pmol-eq/cm2/h for human and rat skin, respectively. In vivo, 27±5% was absorbed and 13% reached systemic circulation after 24 h (maximum flux was 464±65 pmol-eq/cm2/h). BEH-TEBP in vitro penetrance was minimal (<0.01%) for rat or human skin. BEH-TEBP absorption was 12±11% for human skin and 41±3% for rat skin. In vivo, total absorption was 27±9%; 1.2% reached systemic circulation. In vitro maximal BEH-TEBP flux was 0.3±0.2 and 1±0.3 pmol-eq/cm2/h for human and rat skin; in vivo maximum flux for rat skin was 16±7 pmol-eq/cm2/h. EH-TBB was metabolized in rat and human skin to tetrabromobenzoic acid. BEH-TEBP-derived [14C]-radioactivity in the perfusion media could not be characterized. Less than 1% of the dose of EH-TBB and BEH-TEHP is estimated to reach the systemic circulation following human dermal exposure under the conditions tested.
Keywords: dermal bioavailability; brominated flame retardant; 2-ethylhexyl 2-3,4,5-tetrabromobenzoate; bis(2-ethylhexyl) tetrabromophthalate; parallelogram method; persistent organic pollutant
Introduction
Flame retardant (FR) chemicals are added to consumer products and building materials to decrease the risk of fire (3). However, FRs are also environmental pollutants, especially when incorporated into products as additive agents (4-7). After decades of consumer use it was concluded that pentabrominated diphenyl ether mixtures (pentaBDE), primarily used as FRs in polyurethane foams, bioaccumulate and have undesirable toxicity profiles with evidence for thyroid, liver, neurological, and reproductive toxicities, and cancer endpoints (7-14). As such, pentaBDE (and octaBDE) formulations were voluntarily withdrawn from the US marketplace by their manufacturers at the end of 2004 while decaBDE formulations were withdrawn in 2013 (15). This restriction on the use of pentaBDE has resulted in the utilization of novel brominated FRs as replacements. Penta- and octaBDE congeners are included under the United Nations Environmental Programme (UNEP) Persistent Organic Pollutants (POPs) list (16). As a result, polyurethane foam for soft furnishings produced after 2004 contains a mixture of brominated and chlorinated FRs, including tris(1,3-dichloro-2-propyl) phosphate (TDCPP; “chlorinated tris”), 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (EH-TBB), and bis(2-ethylhexyl) tetrabromophthalate (BEH-TEBP), among others (3, 17). EH-TBB and BEH-TEBP are used in couch foam and baby products (mattresses and high-chair foam). In addition, BEH-TEBP is used as a FR or plasticizer in polyurethane foams, flexible polyvinyl chloride, adhesives, carpet backing, fabric coating, film and sheeting, wire and cable insulation, and wall coverings while the only known application for EH-TBB is in polyurethane foam.
EH-TBB and BEH-TEBP have been found in dust collected in the US, Europe, Oceania, and Asia, indicative of the global distribution of FR foams in consumer products (18-24). In addition to household and office dust, EH-TBB and BEH-TEBP are found worldwide in outdoor dust, sediment, and wildlife (3, 4, 18, 19, 25-28). In studies of the Great Lakes atmosphere, both chemicals appear to be increasing with calculated doubling times of 3-6 years (29). Both EH-TBB and BEH-TEBP are slated to undergo a full risk assessment under the Toxic Substances Control Act (TSCA) Work Plan and Action Plan (30). US national production volume for BEH-TEBP in 2012 was 1,000,000 - 10,000,000 lb/yr. Neither EH-TBB production and import volumes to the US, nor international production volumes are publically available (31). However, EH-TBB is not listed in the US EPA High Production Volume Information System, indicating its US production and import volumes are less than the threshold of “1 million pounds or more per year”. Exact global production volumes for EH-TBB and BEH-TEBP are unavailable; conservative estimates for total novel BFR production is 100,000 tons/year (32, 33). Both EH-TBB and BEH-TEBP have low vapor pressures, high lipophilicity (estimated log P of 7.73-8.75 and 9.48-11.95, respectively (1, 2)), as well as high persistence and bioaccumulation characteristics (19, 29). Toxicity profiles for both chemicals are poorly described (33, 34).
Several studies have detected EH-TBB, BEH-TEBP, or their metabolites in human samples (35, 36). Precise routes of exposure are unclear but ingestion and inhalation of FRs in dust has been well documented (37-40). In addition, dermal contact with FRs has been associated with systemic exposures (4). Unfortunately, few studies have investigated the role of dermal uptake despite repeated demonstration of strong positive correlations between FR levels in the indoor environment (e.g., dust), on human skin (hand wipe collections), and in the bodies of adults and children (serum concentrations) (4, 27, 41). Dermal bioavailability of legacy brominated flame retardants (i.e., BDEs) in humans has been investigated (42-44) but very little is known about the dermal disposition of novel brominated flame retardants (44).
Previous disposition studies investigating EH-TBB and/or BEH-TEBP alone or in commercial preparations (Firemaster 550, Firemaster BZ-54, Uniplex FRP-45), in mammals (45-47) or fish (48), found EH-TBB was more readily absorbed from the gut and excreted as metabolite(s) while BEH-TEBP was less likely to be absorbed but was more likely to bioaccumulate in liver and other organs after repeated administration. Disposition of newer formulations that contain EH-TBB and BEH-TEBP (e.g., Firemaster 600 (49)) have not been tested.
Here, in vivo studies were conducted using female Sprague Dawley (SD) rats and in vitro studies were conducted using split-thickness skin (i.e.., epidermis and upper portion of the dermis) from human donors and female SD rats exposed to 100 nmol/cm2 radiolabeled EH-TBB or BEH-TEBP. This dose was selected based on expert opinion (50) and the need to apply enough [14C]-radioactivity to detect the chemicals in the receptor fluid or excreta. Following 24 h exposure, the treated skin was washed and tape stripped. For these studies, the term ‘absorbed’ is used to describe the portion of the applied dose found within the skin and tape strips. Tape stripping may not be sufficient to completely remove the human stratum corneum (51), but to provide a conservative estimate for potential bioavailability, chemical recovered in tape strips was included in the ‘absorbed’ fractions calculations. Similarly, although dose retained within skin (‘absorbed’) may ultimately be removed by normal desquamation and never reach the bloodstream, amounts recovered in the ‘absorbed’ fraction were included in the estimations of bioavailability in an effort to provide conservative estimates for uptake. In descriptions of in vitro experiments, ‘penetrated’ is used to describe chemical that has completely diffused through the skin into the underlying fluid (termed ‘receptor fluid or perfusion media’), analogous to the amount reaching systemic circulation following in vivo exposure (52, 53). The sum of excreted and retained [14C]-radioactivity in tissues outside the dosed skin was used to determine the total penetrated fraction in vivo. The values for penetration were used to estimate bioavailability and flux for EH-TBB and BEH-TEBP. Finally, the sum of ‘absorbed’ and ‘penetrated’ and the absorptive flux calculated for each model.
Methods & Materials
Chemicals
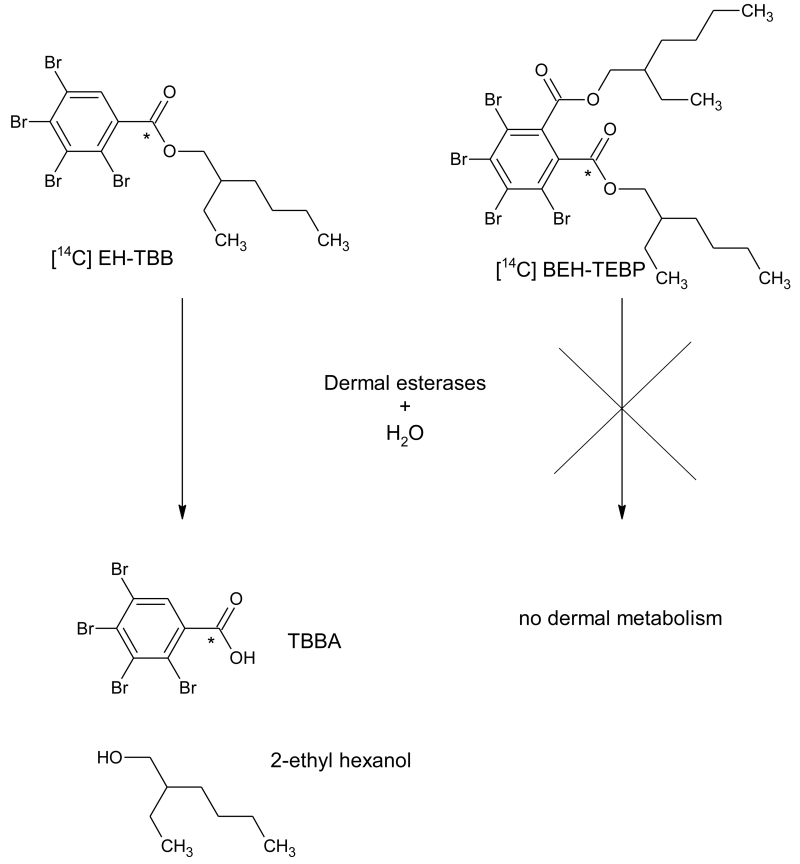
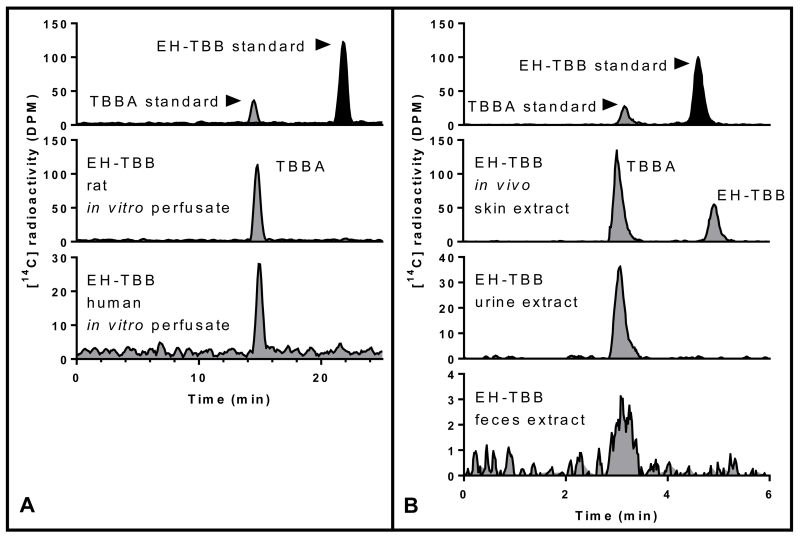
[14C]-labeled EH-TBB and BEH-TEBP were custom synthesized by Moravek Biochemicals (Brea, CA) with the carboxyl carbon radiolabeled (Figure 1). [14C]-EH-TBB (Lot # 256-063-055-A-20130423-DJI) had a radiochemical purity of 99.4% (specific activity = 55 mCi/mmol). [14C]-BEH-TEBP (Lot # 256-061-0605-A-20130419-DJI) had a radiochemical purity of 99.9% (specific activity = 60.5 mCi/mmol). Radiochemical purity was confirmed by radio-HPLC using the methods described below (Figure 3(A) and Figure 4(A), respectively). Both chemicals had a chemical purity of >99%, as compared to their respective reference standard (Accustandard, New Haven, CT). 2,3,4,5-tetrabromobenzoic acid (TBBA; >98% pure) was purchased from the Duke University Small Molecule Synthesis Facility (Durham, NC). Scintillation cocktails were obtained from MP Biomedicals (Ecolume; Santa Ana, CA), Perkin-Elmer (Ultima Gold & PermaFluor E+; Torrance, CA), or Lablogic Inc., (Flow Logic U; Brandon, FL). All other reagents used in these studies were high performance liquid chromatography (HPLC) or analytical grade. Chemical structures were drawn using ACD/Labs Chemsketch (Advanced Chemistry Development, Inc., Toronto, Canada).
Figure 1.
Chemical structure and metabolism scheme for EH-TBB and BEH-TEBP; asterisk indicates the radiolabel location.
Figure 3.
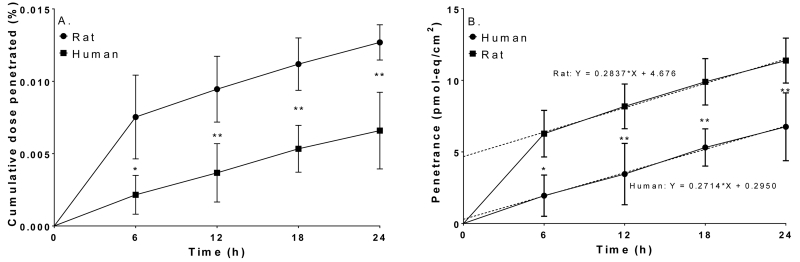
Cumulative recoveries in the receptor fluid after a single application (100 nmol/cm2) of [14C]-BEH-TEBP to rat (●) or human (■) skin. A: Cumulative dose penetrated (%), B: Penetrance (pmol-eq/cm2). Dashed lines show the linear regression of the penetrance data. Data represents mean ± S.D.; N=4 rat; N=3 human. *: p<0.05; **: p<0.01; ****: p<0.0001.
Figure 4.
Characterization of [14C]-radioactivity in EH-TBB study samples from (A) in vitro media and B: extracts from in vivo samples (skin, urine, feces).
Flux calculation
Maximal flux (Jss) was calculated for both in vitro and in vivo studies using the method described by Hughes et al. (54) and derived from Fick’s first law of diffusion (55). Mass was calculated from the amounts of chemical recovered in media (in vitro) or in excreta (in vivo). Briefly, the maximal flux (pmol-eq per square centimeter per hour) was derived from the slopes of the penetrated mass across each barrier plotted versus sampling time period (Equation 1). The experimental duration was expected to be insufficient to produce significant depletion of the applied chemical, i.e., flux was not dose-limited.
Parallelogram calculation
The principles of the parallelogram approach to the dermal exposure assessments were used to estimate bioavailability following in vivo human systemic exposures to a relevant dose of dermally-applied chemical (Equation 2). Because of inconsistent differences in percutaneous absorption between rat and human skin, it is not possible to derive a general adjustment factor for estimation of human percutaneous absorption. However, when these data are available for rat in vivo and for rat and human skin in vitro, the in vivo dermal absorption through human skin can be estimated from the relationship outlined by the parallelogram method (56-59). Briefly, in vivo human exposure is estimated as a function of in vitro human exposure multiplied by a normalization factor based on the same dose applied to rat skin in vivo and in vitro. The parallelogram approach is based on the assumption that the ratio of in vivo to in vitro dermal penetrance of a chemical through the animal model’s skin (here, female SD rat) is the same as the ratio of in vivo to in vitro dermal penetrance in humans.
In vitro experiments
In vitro studies were conducted as described previously by Knudsen et al (53). Briefly, full-thickness human skin was obtained from the National Disease Research Interchange (Philadelphia, PA, USA) from 4 Caucasian individuals aged 78-87 years old (2 male and 2 female, dorsal/scapular skin, excised ≤ 12 h post-mortem, shipped at −80 °C). Two of the human skin samples were of sufficient size to be sampled in both studies; skin from three separate individuals was used in studies for each individual chemical. The skin was shipped and stored frozen (−80°C) until use. Full-thickness female SD rat skin (N=4/chemical, 10-11 weeks old) was obtained from Harlan Bioproducts for Science (Indianapolis, IN, USA). Twenty-four hours prior to excision, hair on the dorsal surface was clipped; the day of shipment, the rats were humanely euthanized by CO2 inhalation and skin excised. The skin was shipped on dry ice and stored at −80°C until use. In vitro dermal absorption tests were conducted according to the OECD Test Guideline 428 (60).
A flow-through diffusion cell system (0.64 cm2 diffusional area; Crown Bio Scientific, Inc., Somerville, NJ, USA) using methodology as described by Bronaugh and Maibach (61) and Bronaugh and Stewart (62) was employed. Experiments for each species/chemical combination were run on separate days. On the day of the experiment, human or rat skin was thawed, direction of hair growth assessed and dermatomed to approximately 300 μm thicknesses using a Padgett dermatome (Kansas City, MO, USA) before placement in receptor fluid. Average sample thickness is shown in Table 1. The integrity of each human skin sample was tested using tritiated water. Samples with scintillation cocktail alone were used as background and the instrument was calibrated with [3H] and [14C] standards prior to each use. Penetrance of <0.05% of applied [3H]-radioactivity was indicative of an intact barrier, analogous to healthy skin and samples that passed this test were used in these studies.
Table 1.
Skin sample thickness (μm) used in dermal studies, mean ± S.D.
| EH-TBB | BEH-TEBP | ||
|---|---|---|---|
| Rat | Human | Rat | Human |
| 335 ± 50 | 414 ± 40 | 321 ± 32 | 433 ± 27 |
[14C]-EH-TBB or [14C]-BEH-TEBP in toluene (100 nmol/cm2, ~1 μCi, 5 uL dose volume) were applied using a blunt tip Hamilton syringe (Franklin, MA, USA) to human and rat skin discs. The specific activity precluded testing at lower doses. Toluene was used as a vehicle due to limited solubility of the test chemicals; both EH-TBB and BEH-TEBP were observed to have limited solubility in more traditional solvents such as ethanol or acetone. The small dose volume combined with the rapid volatilization of the solvent (the flow-through cells were open to the air and the whole system was placed a fume hood) minimized physiological effects (e.g., skin delipidation by toluene). After 24 h, the epidermal surface (with the cell top in place to recover chemical on the surface of the skin sample) was washed six times with 0.5 ml of a mixture of Joy® liquid soap:water (1:1) using a 1 mL pipette to remove unabsorbed chemical. The skin wash fractions were pooled into two vials and mixed with scintillation cocktail. The cell top and cell body were individually washed three times with 0.5 ml ethanol. Skin samples were dried overnight in a hood. The following day, each skin disk was tape stripped up to 10 times with clear tape to remove the stratum corneum; when the stratum corneum visibly separated, tape stripping ceased. Tape stripping was anticipated to remove much of the stratum corneum. Although the stratum corneum is a minimally viable layer that is continuously lost, to be risk conservative, dose fractions recovered in tape strips were assumed to represent absorbed chemical after 24 h. Washed and stripped skin was then chemically solubilized in 1 ml of Soluene 350 (Perkin Elmer) overnight in a water bath set at 37°C. Hionic Fluor (Perkin Elmer) was added to the dissolved skin solution, and “absorbed” [14C]-radioactivity was quantified.
In vivo experiments
In order to link data characterizing the fate of orally-administered EH-TBB (46) and BEH-TEBP (63) in the female rat and the in vitro skin studies described above, 100 nmol/cm2 was applied to the dorsal surface of female SD rats (N = 4 rats/chemical, 11 weeks old, approximately 200 g, Harlan Laboratories, Indianapolis, IA). One day prior to dosing, animals were lightly anesthetized by isoflurane inhalation and an electric clipper was used to remove hair from the dorsal scapular region. Clipped areas were visually inspected for any nicks or cuts; animals were not used if nicks or cuts were found. Animals were returned to polycarbonate shoebox cages for recovery from anesthesia. Immediately prior to dosing, animals were again lightly anaesthetized by isoflurane inhalation, the dosing area visually inspected for nicks and a 1 cm2 area marked. Dosing solution (as described for in vitro samples) was applied inside the marked area using a 25 μL negative displacement pipette with a flexible tip. The vehicle was dried with gentle fanning and the dosing site was then covered with a non-occlusive steel mesh cap attached with polyacrylate glue to prevent ingestion of the test article. After dosing, animals were placed in plastic Nalgene metabolism cages for collection of feces & urine. Animals were provided rat feed (NIH 31; Ziegler, Gardners, PA, USA) and tap water for ad libitum consumption.
Feces, urine, and cage rinses were collected and analyzed as described previously by Knudsen et al. (64) at 4, 8, 12, and 24 h. Animals were euthanized by CO2 inhalation after 24 h. Following euthanasia, blood was collected by cardiac puncture, treated skin was excised, and complete necropsies were performed as described previously (64). Skin from the application area was treated in accordance with the OECD 427 method for in vivo testing of chemicals (65). Briefly, the skin was swabbed 6 times using a 10% Joy® liquid soap solution, 3 times using distilled water, and air dried overnight. Dried skin was tape stripped until the stratum corneum was visibly removed (approximately 10 times) using clear tape. Feces were air-dried and ground to a powder using a mortar and pestle. Tissues (including dosed skin) and feces were sampled in triplicate (approximately 25 mg/sample), combusted in a Packard (Waltham, MA, USA) 307 Biological Sample Oxidizer, and [14C]-radioactivity content was quantified by liquid scintillation counting (LSC) analysis. Skin swabs and tape strips were analyzed by direct LSC as were triplicate samples of urine and cage washes. Data of the combusted tissues and feces, urine, cage washes, skin swabs and tape strips were used to compute an arithmetic sum of residual [14C]-radioactivity. Tape stripping may not be sufficient to completely remove the human stratum corneum (51), and to be risk conservative, radioactivity recovered in tape strips as well as compound in skin that was tape stripped was considered “absorbed”. [14C]-radioactivity in cage washes and urine were combined in the summary urinary recoveries.
Samples of air-dried feces (~500 mg) were extracted with 2 × 2 mL prechilled 1:1 methanol/distilled water. Supernatants from the methanol/water were pooled into glass vials, concentrated to near dryness under vacuum, and reconstituted in 500 μL of HPLC-grade water. Remaining samples were sequentially extracted with 2 × 800 μL of pre-chilled 3:1 dichloromethane/methanol, 1 × 1-2 mL acetonitrile, 1 × 1-2 mL hexanes, and 2 × 2 mL toluene. Organic supernatants were pooled into glass vials, concentrated to near dryness, reconstituted in 500-700 μL of 1:1 ethyl acetate/ethanol, and injected onto the HPLC. Samples of each supernatant (10-100 μL) were subjected to LSC to determine if further extraction was necessary; when samples contained less than 3 × background counts, extractions were deemed exhaustive. Remaining sample pellets were air dried, weighed, and un-extracted [14C]-radioactivity remaining in the sample was quantified by combustion in a Packard 307 Biological Sample Oxidizer followed by LSC counting. Average extraction efficiency for BEH-TEBP study feces was 90% while extraction efficiencies for EH-TBB study feces was ~50%, based on [14C]-radioactivity remaining in the sample.
The protocol for chemical extraction from dosed skin was adapted from Want et al. (66). Prior to extraction, skin was washed and tape stripped as described above. Briefly, samples of dosed skin (23 - 200 mg) were transferred to microcentrifuge tubes and soaked in 1 mL of 1:1 methanol/ distilled water at 4°C. Samples were further minced then transferred to bead-beater tubes. Homogenization occurred in a FastPrep-24 5G benchtop homogenizer (MP Biomedicals) at 6500 Hz for two cycles of 40 seconds with a 1 minute pause. Aqueous supernatants were transferred to microcentrifuge tubes. Remaining samples were sequentially extracted with 1.6 mL of prechilled 3:1 dichloromethane/methanol, 1 mL acetonitrile, 1 mL of hexanes, and 1 mL toluene with the above homogenization settings. Organic supernatants were pooled into glass vials, concentrated to near dryness (Savant SPD1010 SpeedVac), reconstituted in 500 μL of acetonitrile, and injected onto the HPLC. Average extraction efficiency was 96%, based on remaining [14C]-radioactivity in samples.
Urine samples (500 μL) were filtered through PVDF centrifugal filters (Merck Millipore), added to 100 μL of ethanol, and spun down via centrifuge. Supernatants were transferred to glass vials and injected onto the HPLC.
HPLC-radiochemical analyses
The HPLC system used for analysis of receptor fluid (from the in vitro studies) and extracts from dosed skin, urine, and feces (from the in vivo studies) was composed of an Agilent (Santa Clara, CA, USA) 1100 HPLC system with an in-line INUS β-RAM3 radiochemical detector. Mobile phases consisted of 0.1% formic acid in water (mobile phase A) and acetonitrile (mobile phase B). EH-TBB study samples were separated using a Restek (State College, PA) Raptor biphenyl column (2.7 μm, 4.6 mm×50 mm). Elution involved a gradient method: initial conditions (99% A) were maintained for 1 min; A was then reduced to 0% over 1 second and held at 0% A for 5 min at a flow rate of 1 mL/min. BEH-TEBP study samples were separated using the same column and mobile phases with a gradient method: initial conditions (60% A) were maintained for 5 min; A was then reduced to 10% over 2 min then to 0% A over 13 min at a flow rate of 1 mL/min. In all cases, the column was returned to initial conditions and allowed to equilibrate for 2 min before re-use. Scintillant flow (Inflow ES, Lablogic Corp.) was maintained at 2 mL/min initially and increased to 4 mL/min around regions of interest. Laura4 (Lablogic Corp.) software was used for instrument control and analysis software.
Statistical analysis
The data were subjected to statistical analysis using two-way ANOVA followed by the Tukey-Kramer test for pairwise comparisons (GraphPad Prism 6, GraphPad Software, Inc., La Jolla CA). Values were considered to be significantly different at p < 0.05.
Results
EH-TBB studies
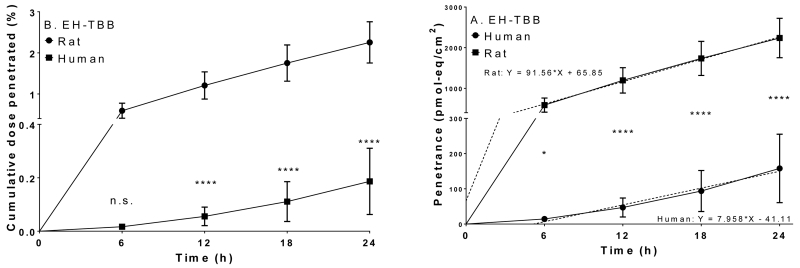
In vivo (rat) and in vitro (rat and human) studies were performed to determine the dermal uptake of a single dose of EH-TBB (~100 nmol/cm2) over 24 h. EH-TBB was well absorbed into and penetrated through skin both in vitro and in vivo. In vitro data showed that penetration over time was significantly lower in human skin (p<0.05) after EH-TBB application (Figure 2). Receptor fluid from human samples dosed with EH-TBB contained 0.2% of the applied [14C]-radioactivity whereas approximately 2% passed through the rat skin in 24 h. The fractional recoveries (expressed as percent of administered dose) for unabsorbed dose (washes), absorbed ([14C]-radioactivity in tape strips and retained in the skin), and penetrated (in vitro receptor fluid or in vivo tissues and excreta) are shown in Table 2. When EH-TBB was administered to the dorsal surface of female SD rats, approximately 10% of the dose was recovered in the skin at the dosing site (absorbed) and 11% was present in tissues or excreta (penetrated). In this same group, 6% of the dose was recovered in urine while 1% of the dose was recovered in feces through 24 h while blood and other tissues contained 4-5% of the administered dose (Table 3, Table S1). As observed in the in vitro studies, most of the administered [14C]-radioactivity was recovered unabsorbed from the in vivo dosing site within 24 h of administration (Table 2). Over the 24 hour exposure period, 18-24% of the EH-TBB dose crossed into the skin and systemic circulation in the rat. From these studies it was determined that maximal EH-TBB flux through rat skin in vitro was 102±24 pmol-eq/cm2/h and occurred between 6 and 12 h post-application. Maximum flux for rat skin in vivo was 464±65 pmol-eq/cm2/h and occurred between 12 and 24 h post dose. Maximal flux for human skin (in vitro) occurred between 18 and 24 h and was 11±7 pmol-eq/cm2/h. HPLC-radiometric analyses of perfusate, extracts, and excreta from EH-TBB studies demonstrated metabolism of EH-TBB to TBBA (Figure 3). Metabolism of EH-TBB was shown to occur in rat skin in the in vitro system. Only TBBA was detected in the receptor fluid. EH-TBB applied dermally to rats was primarily excreted in the urine as TBBA; extractable [14C]-radioactivity from feces resolved as a small peak that also co-eluted with TBBA. Similar results were seen for perfusate collected from human samples. Based on the parallelogram methodology, conservative estimates predict approximately 10±3% of EH-TBB may be absorbed into human skin in vivo, with 0.8±0.6% reaching systemic circulation after 24 h of continuous exposure, likely in the form of TBBA.
Figure 2.
Cumulative recoveries in the receptor fluid after a single application (100 nmol/cm2) of [14C]-EH-TBB to rat (●) or human (■) skin. A: Cumulative dose penetrated (%), B: Penetrance (pmol-eq/cm2). Dashed lines show the linear regression of the penetrance data. Data represents mean ± S.D.; N=4 rat; N=3 human. *: p<0.05; **: p<0.01; ****: p<0.0001.
Table 2.
EH-TBB studies: [14C]-radioactivity recovery in various fractions at 24 h post-dose.
| EH-TBB | ||||
|---|---|---|---|---|
| Species | Human (in vitro) | Rat (in vitro) | Rat (in vivo) | |
| Unabsorbed (%) | Washes | 60 ± 9 | 37 ± 9 | 50 ± 5 |
| Cell | 10 ± 4 | 4 ± 1 | 9 ± 2 | |
| Absorbed (%) | Tape strips | 13 ± 4 | 17 ± 7 | 17 ± 4 |
| Skin | 11 ± 5 | 34 ± 5 | 10 ± 3 | |
| Penetrated (%) | 0.2 ± 0.1 | 2 ± 0.5 | 13 ± 1 | |
| Recovery (%) | 95 ± 7 | 94 ± 7 | 98 ± 1 | |
Table 3.
[14C]-radioactivity recovery in excreta & tissues following in vivo application of EH-TBB or BEH-TEBP (%).
| Feces | Urine | Blood | Non-GI tissues | GI tract | GI contents | |
|---|---|---|---|---|---|---|
| EH-TBB | 1 ± 0.3 | 6 ± 0.7 | 1 ± 0.4 | 3 ± 0.2 | 0.2 ± 0.02 | 1 ± 0.1 |
| BEH-TEBP | 0.3 ± 0.1 | 0.1 ± 0.03 | 0.3 ± 0.3 | 0.4 ± 0.3 | 0.01 ± 0.003 | 0.1 ± 0.1 |
BEH-TEBP studies
Dermal penetration of BEH-TEBP was lower compared to EH-TBB, both in vivo and in vitro (Table 4, Table S2, Table S3). Levels of BEH-TEBP [14C]-radioactivity in perfusion media were approximately 100-fold lower than seen for EH-TBB. Penetration was significantly lower in human skin (p<0.05) at all time points after BEH-TEBP dosing. The nature of the radioactivity in the perfusion media for either rats or humans could not be determined due to limits of detection for the HPLC system. However, HPLC-radiometric analyses of extracts from dosed skin and feces of rats showed all extractable [14C]-radioactivity was recovered as parent (Figure 4). From these studies it was determined that maximal BEH-TEBP flux through rat skin in vitro was 1±0.3 pmol-eq/cm2/h and occurred between 0 and 6 h post-application. In vivo, maximum flux for rat skin was 16±7 pmol-eq/cm2/h and occurred between 12 and 24 h post dose. Maximal flux for human skin (in vitro) was 0.3±0.2 pmol-eq/cm2/h and was reached between 0 and 6 h. Based on the parallelogram methodology, conservative estimates predict approximately 7.8±7.6% of BEH-TEBP may be absorbed into human skin in vivo; about 0.8±0.4% of the parent chemical is expected to reach systemic circulation after 24 h of continuous exposure.
Table 4.
BEH-TEBP studies: [14C]-radioactivity recovery in various fractions at 24 h.
| BEH-TEBP | ||||
|---|---|---|---|---|
| Species | Human (in vitro) | Rat (in vitro) | Rat (in vivo) | |
| Unabsorbed (%) | Washes | 80 ± 20 | 56 ± 6 | 63± 9 |
| Cell | 4 ± 2 | 2 ± 0.4 | 6 ± 2 | |
| Absorbed (%) | Tape strips | 4 ± 3 | 13 ± 5 | 19 ± 6 |
| Skin | 8 ± 8 | 29 ± 2 | 8 ± 3 | |
| Penetrated (%) | 0.007 ± 0.002 | 0.01 ± 0.002 | 1.2 ± 0.4 | |
| Recovery (%) | 95 ± 7 | 98 ± 6 | 98 ± 2 | |
Discussion
The objective of this study was to measure the dermal absorption of EH-TBB and BEH-TEBP in rat and human skin to provide an assessment of bioavailable fractions of both chemicals following in vivo dermal exposures to humans. As expected, based on known physicochemical characteristics, rat skin was more permeable to EH-TBB and BEH-TEBP than human skin in vitro. Further, human skin absorbed and retained much less of either chemical than rat skin. In BEH-TEBP studies, [14C]-radioactivity that was absorbed into the skin was recovered as parent BEH-TEBP, as was [14C]-radioactivity recovered in rat feces, which leads to the conclusion that dermally applied BEH-TEBP likely follows similar pathways as those found previously for oral- or intravenous-administered BEH-TEBP in the rat (63). Analyses of urine and extracts of skin and feces showed EH-TBB is metabolized in skin to TBBA and excreted as metabolite(s) in feces and urine after dermal absorption in the rat, putatively due to carboxylesterase activity. In the current study (as seen in Table 3), and consistent with previous studies conducted by the oral route in rats (46), there was minimal retention of either chemical in tissues after administration. Similar to previous observations for highly brominated polybrominated diphenyl ethers (PBDEs) (42), the skin appeared to act as a lipophilic “trap” for BEH-TEBP. Good hygiene practices may aid in decreasing residence time on the skin, which in turn could limit bioavailability and systemic exposure.
Strong positive correlations exist between FR levels in dust collected from homes schools and businesses, on hand wipes, and serum concentrations in adults and children (4, 27, 41); despite this, very little is known about the dermal disposition of FRs (44). The stratum corneum, a biologically inactive layer of the epidermis, is often the primary barrier to dermal absorption. However, given the highly lipophilic nature of EH-TBB and BEH-TEBP, diffusion into the stratum corneum may be significant; in these cases the viable epidermis and upper layers of the dermis are likely to be the primary barrier. However, given the observations that most of the material was washed off 24 h after application, it is likely that the stratum corneum is still an effective barrier for percutaneous uptake of EH-TBB and BEH-TEBP. Passive diffusion through this layer is governed by the lipophilicity of the agent and varies inversely with molecular weight (the MW of both compounds is > 500 g/mol). Once past the stratum corneum, a chemical may be metabolized in situ or move by diffusion or facilitated transport through the epidermal stratum geminativum, spinosum, and granulosum and into the dermis where it may enter the systemic circulation through capillary beds.
Skin is a metabolically active organ, with phase I (oxidative metabolism), phase II (conjugative metabolism), and phase III (transport) processes occurring (67-69). Organic anion transporting polypeptide (OATP) proteins have been shown to facilitate increased systemic exposure of PBDEs and other lipophilic chemicals in intestine and liver (70-72). At least one OATP transporter (OATP2B1) has been described in human skin (73) and human-derived keratinocytes appear to express functional OATP transporters, with immune-reactive staining apparent in viable regions of the epidermis (68). However, the role of transporters in the dermal absorption of EH-TBB and BEH-TEBP is not known.
A limitation of the study is the large mass of each compound applied to the skin. This was due to the low specific activity of the compounds, and the need to assure there was a sufficient amount of radioactivity applied to be detected in the receptor fluid, excreta, or tissues. Other than potential occupational exposure, it is unlikely that one would be exposed to the mass of either chemical applied in this study. The dose level tested was in excess of that detected in environmental samples (27) but given the possibility that lower surface loads may actually result in higher uptake efficiency (74), fractional recoveries and subsequent predictions for human exposure may actually under-predict the real-world bioavailable fraction experienced by continuously exposed individuals. In addition, residential exposures to these chemicals would be in the form bound to dust. For absorption to occur if the dust contacts the skin, the chemical would have to partition from the dust to the skin. While absorption of neat BFR in this study was observed, dermal absorption of BFR bound to dust would most likely be lower (75).
Another limitation was the choice of vehicle. Toluene is not a common vehicle for dermal absorption studies, but was chosen because it could more easily dissolve the test compounds than other vehicles such as acetone. Being an organic solvent, the toluene could have damaged the skin, primarily by altering the lipids in the skin (76), but we are uncertain of this potential effect. Also, a fraction of the toluene may have penetrated the stratum corneum and could have enhanced overall absorption. However, given the small volume applied (5 uL) and the volatility of the toluene, the skin effects of toluene in this experiment were most likely minimal.
Chronic exposure to BFRs and structurally similar chemicals via the skin is a common occurrence but only a few studies have been conducted to describe their dermal disposition. An in vivo dermal study of 2,2’,4,4’-tetrabromodiphenyl ether (BDE 47) using female C57BL6 mice showed less than 10% of a 1 mg/kg dose eliminated in urine and feces over 24 h where approximately 77% of the dose was systemically available (e.g., recovered at the dose site or within tissues & excreta) over 5 days (77). In vitro studies using BDE-47 applied to human or rat skin found similar levels of penetration as those reported for BEH-TEBP (43). An in vitro dermal assessment of decabromodiphenyl oxide (BDE 209) using mouse skin estimated 2% of a 60 nmol dose was recovered in skin and receptor fluid (78). In vitro assessments of a series of PBDEs found lower levels of bromination corresponded to increased dermal penetration while more highly brominated congeners were more likely to accumulate in skin (42). Previous studies in this laboratory (53) estimated approximately 6% of a 100 nmol/cm2 dose of tetrabromobisphenol A would be dermally bioavailable to humans based on in vitro human data (4%) normalized to rat in vitro and in vivo data (13% and 22%, respectively). A recent study describing dermal disposition of ten different BFRs (including EH-TBB and BEH-TEBP) in full thickness human skin showed similar results when the chemicals were applied in ethanol and allowed to perfuse for 72 h (79).
Dermal absorption in children, especially infants, is different than adults (80). In the very young (i.e., the first few days of life), the keratinocyte layer has not fully formed, which has a substantial impact on dermal absorption (81). However, even after this layer has formed, the child’s skin remains quite different from the adult for the first year of life (82). In adulthood, the barrier function(s) of the skin remain intact and may even increase as the skin ages, when transepithelial water loss decreases and the stratum corneum thickens (83). The relatively thin stratum corneum and small corneocytes found in infant skin has been proposed to result in weaker stratum corneum barrier function compared to that of adults (84). While we were unable to test skin from young individuals, the bioavailability and flux rates for these compounds may be higher in younger individuals, especially pre-term infants and newborns exposed to these chemicals.
Systemic quantification of internal EH-TBB and BEH-TEBP levels after occupational, consumer, and environmental exposures to dust containing these and other FRs likely results from at least two routes, ingestion and dermal contact. To quantify the dermal contact component, we applied the principles of the parallelogram approach to the dermal exposure assessments for both chemicals to estimate a likely level following in vivo human systemic exposures to a relevant dose of dermally-applied FR, as described by Ross et al (59). The hypothesis behind the parallelogram approach has shown data from rat to be within ±3-fold of the values measured in human subjects; however, when studies have been conducted under analogous conditions as part of a matched protocol study, the uncertainty for the predicted value is much lower (85-87). In the present study, the possible fraction remaining at the dosing site may eventually reach the systemic circulation or become removed by desquamation since steady state may not have been achieved during the 24 h study. In addition, these studies were designed to provide a conservative estimate for dermal uptake of these novel BFRs. In addition, the exposure matrix has been shown to significantly affect the bioavailability of flame retardants (88). This study, using neat compound applied directly to skin likely provides an upper bound estimate of possible absorption from soil or dust, or data relevant only to dusts that are fully saturated, an unlikely scenario for environmentally-relevant concentrations. Flux rates derived from this type of dose configuration likely represent an upper bound for dermal penetrance of these chemical and relationships between tested conditions and real-world exposures to EH-TBB and BEH-TEBP in dust should be further investigated. Therefore, these findings represent conservative assumptions, but may be useful in assessing the contribution of dermal exposure to aggregate exposures to susceptible populations. While percutaneous penetration was generally low, both compounds accumulated in the skin to varying degrees and may reach systemic circulation over time even after surface washing and removal, either as parent or metabolite (89).
These data provided herein are expected to aid in risk assessment for dermal exposures to EH-TBB and BEH-TEBP. Over a 24 hour period, the amounts of administered EH-TBB or BEH-TEBP that penetrated the human and rat skin in vitro continually increased for all samples, indicating that duration of exposure increases the risk of toxicity. Human skin is less permeable than rat skin for a variety of chemicals (56, 90, 91) due to differences in anatomical, physiological, and biochemical factors (61, 85). These differences necessitate the use of a normalizing mechanism to account for differences in dermal uptake between species. Despite species differences in uptake, it is clear that EH-TBB can be absorbed by the skin and dermal contact should be expected to be a relevant route of exposure to humans. We anticipate these data will be useful in estimating human exposure risk, especially pregnant women and small children who are exposed to higher levels of household dust (37). This is of particular importance because, coupled with their increased surface area to volume ratio and immature detoxification pathways (92), early-life exposure to endocrine disrupting chemicals like EH-TBB and BEH-TEBP enhances susceptibility to diseases like obesity and other chronic pathologies (93, 94). There is increasing evidence that the combination of EH-TBB and BEH-TEBP is becoming the predominant replacement for pentaBDE in children’s products (95, 96), increasing the likelihood of exposure in homes with small children and adults of child-bearing age.
Equation 1. Estimation of percutaneous flux.
Equation 2. Estimation of human in vivo systemic exposure relative to the ratio of animal to human absorption (penetrated + absorbed) of dermally applied chemicals.
Supplementary Material
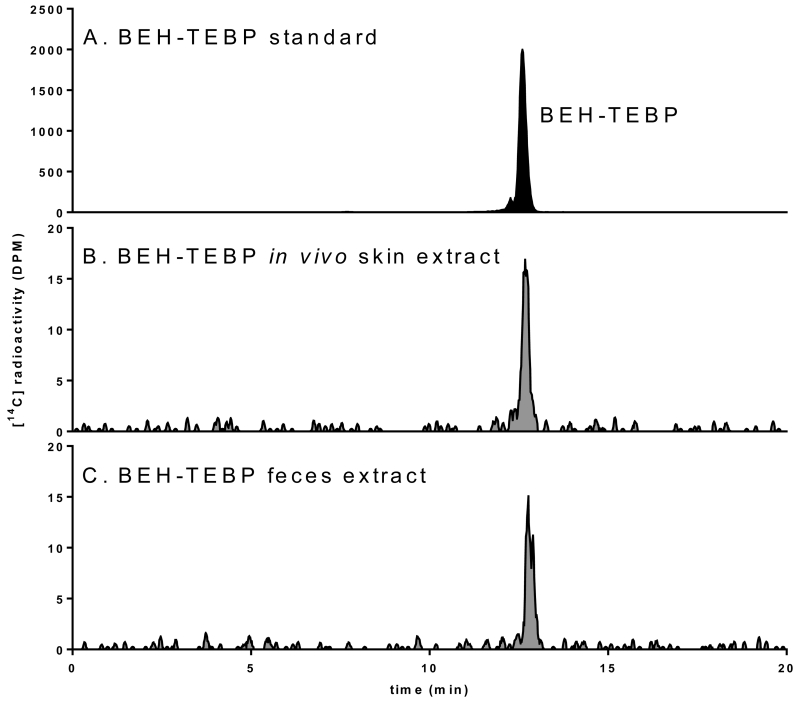
Figure 5.
Representative HPLC-radiochromatograms from BEH-TEBP studies: (A) BEH-TEBP standard, (B) in vivo rat study skin extracts, (C) in vivo rat study feces extracts.
Highlights for review.
Human skin maximal flux was 11±7 (EH-TBB) & 0.3±0.2 (BEH-TEBP) pmol-eq/cm2/h.
Predicted systemic bioavailability was <1% for either chemical after 24h.
Skin retained EH-TBB & BEH-TEBP after 24 h dermal exposure.
EH-TBB was hydrolyzed to tetrabromobenzoic acid; BEH-TEBP was not metabolized.
Skin contact is an important route of human exposure to EH-TBB & BEH-TEBP.
Acknowledgements
The authors would like to thank Ms. Brenda Edwards, Mr. Ethan Hull, Ms. Katelyn McIntosh and Mr. Vivek Miyani, for technical assistance. This article has been reviewed in accordance with the policy of the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. This research was supported in part by the Intramural Research Program of NIH/NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds studied in this article
2-ethylhexyl 2,3,4,5-tetrabromobenzoate (PubChem CID: 71316600; CAS No. 183658-27-7, FW: 549.92 g/mol, logPest: 7.73-8.75 (1, 2)). Other published abbreviations for 2-ethylhexyl-2,3,4,5-tetrabromobenzoate are TBB, EHTeBB, or EHTBB.
bis(2-ethylhexyl) tetrabromophthalate (PubChem CID: 117291; CAS No. 26040-51-7, FW: 706.14 g/mol, logPest: 9.48-11.95 (1, 2)). Other published abbreviations for bis(2-ethylhexyl)tetrabromophthalate are TeBrDEPH, TBPH, or BEHTBP.
References
- 1.Bergman A, Ryden A, Law RJ, de Boer J, Covaci A, Alaee M, Birnbaum L, Petreas M, Rose M, Sakai S, Van den Eede N, van der Veen I. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environment international. 2012;49:57–82. doi: 10.1016/j.envint.2012.08.003. Epub 2012/09/18. doi: 10.1016/j.envint.2012.08.003. PubMed PMID: 22982223; PubMed Central PMCID: PMC3483428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.USEPA Estimation Programs Interface Suite™ for Microsoft® Windows. v 4.11 Available from, as of 25 March 2016: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface2016.
- 3.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environmental science & technology. 2012;46(24):13432–9. doi: 10.1021/es303471d. doi: 10.1021/es303471d. PubMed PMID: 23186002; PubMed Central PMCID: PMC3525014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carignan CC, Heiger-Bernays W, McClean MD, Roberts SC, Stapleton HM, Sjodin A, Webster TF. Flame retardant exposure among collegiate United States gymnasts. Environmental science & technology. 2013;47(23):13848–56. doi: 10.1021/es4037868. Epub 2013/11/08. doi: 10.1021/es4037868. PubMed PMID: 24195753; PubMed Central PMCID: PMC3885979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environmental health perspectives. 2004;112(1):9–17. doi: 10.1289/ehp.6559. Epub 2003/12/31. PubMed PMID: 14698924; PubMed Central PMCID: PMC1241790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orta-Garcia S, Perez-Vazquez F, Gonzalez-Vega C, Varela-Silva JA, Hernandez-Gonzalez L, Perez-Maldonado I. Concentrations of persistent organic pollutants (POPs) in human blood samples from Mexico City, Mexico. The Science of the total environment. 2014;472:496–501. doi: 10.1016/j.scitotenv.2013.11.059. Epub 2013/12/05. doi: 10.1016/j.scitotenv.2013.11.059. PubMed PMID: 24300460. [DOI] [PubMed] [Google Scholar]
- 7.Klosterhaus SL, Stapleton HM, La Guardia MJ, Greig DJ. Brominated and chlorinated flame retardants in San Francisco Bay sediments and wildlife. Environment international. 2012;47:56–65. doi: 10.1016/j.envint.2012.06.005. doi: 10.1016/j.envint.2012.06.005. PubMed PMID: 22766500. [DOI] [PubMed] [Google Scholar]
- 8.La Guardia MJ, Hale RC, Harvey E, Mainor TM, Ciparis S. In situ accumulation of HBCD, PBDEs, and several alternative flame-retardants in the bivalve (Corbicula fluminea) and gastropod (Elimia proxima) Environmental science & technology. 2012;46(11):5798–805. doi: 10.1021/es3004238. Epub 2012/05/11. doi: 10.1021/es3004238. PubMed PMID: 22571713. [DOI] [PubMed] [Google Scholar]
- 9.Vorkamp K, Riget FF. A review of new and current-use contaminants in the Arctic environment: evidence of long-range transport and indications of bioaccumulation. Chemosphere. 2014;111:379–95. doi: 10.1016/j.chemosphere.2014.04.019. Epub 2014/07/07. doi: 10.1016/j.chemosphere.2014.04.019. PubMed PMID: 24997943. [DOI] [PubMed] [Google Scholar]
- 10.Chen SJ, Ma YJ, Wang J, Chen D, Luo XJ, Mai BX. Brominated flame retardants in children’s toys: concentration, composition, and children’s exposure and risk assessment. Environmental science & technology. 2009;43(11):4200–6. doi: 10.1021/es9004834. Epub 2009/07/03. PubMed PMID: 19569352. [DOI] [PubMed] [Google Scholar]
- 11.de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46(5):583–624. doi: 10.1016/s0045-6535(01)00225-9. Epub 2002/05/10. PubMed PMID: 11999784. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PI, Stapleton HM, Mukherjee B, Hauser R, Meeker JD. Associations between brominated flame retardants in house dust and hormone levels in men. The Science of the total environment. 2013;445-446:177–84. doi: 10.1016/j.scitotenv.2012.12.017. Epub 2013/01/22. doi: 10.1016/j.scitotenv.2012.12.017. PubMed PMID: 23333513; PubMed Central PMCID: PMC3572297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scanlan LD, Loguinov AV, Teng Q, Antczak P, Dailey KP, Nowinski DT, Kornbluh J, Lin XX, Lachenauer E, Arai A, Douglas NK, Falciani F, Stapleton HM, Vulpe CD. Gene Transcription, Metabolite and Lipid Profiling in Eco-Indicator Daphnia magna Indicate Diverse Mechanisms of Toxicity by Legacy and Emerging Flame-Retardants. Environmental science & technology. 2015 doi: 10.1021/acs.est.5b00977. Epub 2015/05/20. doi: 10.1021/acs.est.5b00977. PubMed PMID: 25985095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NTP Draft NTP Technical Report on the toxicology of a pentabromodiphenyl ether mixture [DE-71 (technical grade)] (CAS No. 32534-81-9) in F344/N rats and B6C3F1/N mice and toxicology and carcinogenesis studies of a pentabromodiphenyl ether mixture [DE-71 (technical grade)] in Wistar Han [Crl:WI)Han)] rats and B6C3F1/N mice (gavage studies) https://ntp.niehs.nih.gov/results/pubs/longterm/reports/longterm/index.html2015 [cited 2015 11/5/2015] Available from: http://ntp.niehs.nih.gov/ntp/about_ntp/trpanel/2015/june/tr589_peerdraft.pdf.
- 15.USEPA . In: An exposure assessment of polybrominated diphenyl ethers. Assessment NCfE, editor. Washington, DC: 2010. [Google Scholar]
- 16.UNEP Listing of POPs in the Stockholm Convention. 2008 [updated 5/24/2016]. Available from: http://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx.
- 17.Ma Y, Venier M, Hites RA. 2-Ethylhexyl tetrabromobenzoate and bis(2-ethylhexyl) tetrabromophthalate flame retardants in the Great Lakes atmosphere. Environmental science & technology. 2012;46(1):204–8. doi: 10.1021/es203251f. Epub 2011/12/02. doi: 10.1021/es203251f. PubMed PMID: 22128844. [DOI] [PubMed] [Google Scholar]
- 18.Ali N, Dirtu AC, Van den Eede N, Goosey E, Harrad S, Neels H, t Mannetje A, Coakley J, Douwes J, Covaci A. Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere. 2012;88(11):1276–82. doi: 10.1016/j.chemosphere.2012.03.100. Epub 2012/05/04. doi: 10.1016/j.chemosphere.2012.03.100. PubMed PMID: 22551874. [DOI] [PubMed] [Google Scholar]
- 19.Ali N, Harrad S, Goosey E, Neels H, Covaci A. “Novel” brominated flame retardants in Belgian and UK indoor dust: implications for human exposure. Chemosphere. 2011;83(10):1360–5. doi: 10.1016/j.chemosphere.2011.02.078. Epub 2011/04/05. doi: 10.1016/j.chemosphere.2011.02.078. PubMed PMID: 21458020. [DOI] [PubMed] [Google Scholar]
- 20.Ali N, Harrad S, Muenhor D, Neels H, Covaci A. Analytical characteristics and determination of major novel brominated flame retardants (NBFRs) in indoor dust. Analytical and bioanalytical chemistry. 2011;400(9):3073–83. doi: 10.1007/s00216-011-4966-7. Epub 2011/04/12. doi: 10.1007/s00216-011-4966-7. PubMed PMID: 21479791. [DOI] [PubMed] [Google Scholar]
- 21.Fromme H, Hilger B, Kopp E, Miserok M, Volkel W. Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and “novel” brominated flame retardants in house dust in Germany. Environment international. 2014;64:61–8. doi: 10.1016/j.envint.2013.11.017. Epub 2013/12/26. doi: 10.1016/j.envint.2013.11.017. PubMed PMID: 24368294. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman K, Fang M, Horman B, Patisaul HB, Garantziotis S, Birnbaum LS, Stapleton HM. Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster(R) 550. Environmental health perspectives. 2014;122(9):963–9. doi: 10.1289/ehp.1308028. Epub 2014/05/16. doi: 10.1289/ehp.1308028. PubMed PMID: 24823833; PubMed Central PMCID: PMC4154220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahlstrom L, Sellstrom U, de Wit CA. Clean-up method for determination of established and emerging brominated flame retardants in dust. Analytical and bioanalytical chemistry. 2012;404(2):459–66. doi: 10.1007/s00216-012-6160-y. Epub 2012/06/14. doi: 10.1007/s00216-012-6160-y. PubMed PMID: 22692590. [DOI] [PubMed] [Google Scholar]
- 24.Qi H, Li WL, Liu LY, Zhang ZF, Zhu NZ, Song WW, Ma WL, Li YF. Levels, distribution and human exposure of new non-BDE brominated flame retardants in the indoor dust of China. Environmental pollution (Barking, Essex: 1987) 2014;195C:1–8. doi: 10.1016/j.envpol.2014.08.008. Epub 2014/08/30. doi: 10.1016/j.envpol.2014.08.008. PubMed PMID: 25170815. [DOI] [PubMed] [Google Scholar]
- 25.Abdallah MA, Harrad S. Tetrabromobisphenol-A, hexabromocyclododecane and its degradation products in UK human milk: relationship to external exposure. Environment international. 2011;37(2):443–8. doi: 10.1016/j.envint.2010.11.008. Epub 2010/12/21. doi: 10.1016/j.envint.2010.11.008. PubMed PMID: 21167604. [DOI] [PubMed] [Google Scholar]
- 26.Davis EF, Klosterhaus SL, Stapleton HM. Measurement of flame retardants and triclosan in municipal sewage sludge and biosolids. Environment international. 2012;40:1–7. doi: 10.1016/j.envint.2011.11.008. Epub 2012/01/28. doi: 10.1016/j.envint.2011.11.008. PubMed PMID: 22280921. [DOI] [PubMed] [Google Scholar]
- 27.Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere. 2014;116:54–60. doi: 10.1016/j.chemosphere.2013.12.100. Epub 2014/02/04. doi: 10.1016/j.chemosphere.2013.12.100. PubMed PMID: 24485814; PubMed Central PMCID: PMC4116470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu B, Lai NL, Wai TC, Chan LL, Lam JC, Lam PK. Changes of accumulation profiles from PBDEs to brominated and chlorinated alternatives in marine mammals from the South China Sea. Environment international. 2014;66C:65–70. doi: 10.1016/j.envint.2014.01.023. Epub 2014/02/18. doi: 10.1016/j.envint.2014.01.023. PubMed PMID: 24530800. [DOI] [PubMed] [Google Scholar]
- 29.Liu LY, Salamova A, Venier M, Hites RA. Trends in the levels of halogenated flame retardants in the Great Lakes atmosphere over the period 2005-2013. Environment international. 2016;92-93:442–9. doi: 10.1016/j.envint.2016.04.025. Epub 2016/05/11. doi: 10.1016/j.envint.2016.04.025. PubMed PMID: 27160856. [DOI] [PubMed] [Google Scholar]
- 30.USEPA . In: Design for the Environment Program Alternatives Assessment Criteria for Hazard Evaluation, Version 2.0. Design for the Environment Program OoPPaT, editor. Washington, DC: 2011. [Google Scholar]
- 31.USEPA Chemical Data Access Tool (CDAT) Search Query: CAS No. 26040-51-7 http://java.epa.gov/oppt_chemical_search/2015 [updated 9/24/2015; cited 2015 10/5/2015]
- 32.Harju M, Heimstad ES, Herzke D, Sandanger T, Posner S, Wania F. In: Emerging “new” brominated flame retardants in flame retarded products and the environment. Authority NPC, editor. Oslo, Norway: 2009. p. 113. [Google Scholar]
- 33.Covaci A, Harrad S, Abdallah MA, Ali N, Law RJ, Herzke D, de Wit CA. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environment international. 2011;37(2):532–56. doi: 10.1016/j.envint.2010.11.007. Epub 2010/12/21. doi: 10.1016/j.envint.2010.11.007. PubMed PMID: 21168217. [DOI] [PubMed] [Google Scholar]
- 34.EFSA EFSA Panel on Contaminants in the Food Chain (CONTAM): Scientific Opinion on Emerging and Novel Brominated Flame Retardants (BFRs) in Food. EFSA Journal. 2012;10(10):133. doi: 10.2903/j.efsa.2012.2908. [Google Scholar]
- 35.Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environmental science & technology. 2014;48(17):10432–8. doi: 10.1021/es5025299. Epub 2014/08/05. doi: 10.1021/es5025299. PubMed PMID: 25090580. [DOI] [PubMed] [Google Scholar]
- 36.Butt CM, Miranda ML, Stapleton HM. Development of an analytical method to quantify PBDEs, OH-BDEs, HBCDs, 2,4,6-TBP, EH-TBB, and BEH-TEBP in human serum. Analytical and bioanalytical chemistry. 2016;408(10):2449–59. doi: 10.1007/s00216-016-9340-3. Epub 2016/02/13. doi: 10.1007/s00216-016-9340-3. PubMed PMID: 26864867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environmental science & technology. 2008;42(18):6910–6. doi: 10.1021/es801070p. Epub 2008/10/16. PubMed PMID: 18853808. [DOI] [PubMed] [Google Scholar]
- 38.Boyce CP, Sonja N, Dodge DG, Pollock MC, Goodman JE. Human exposure to decabromodiphenyl ether, tetrabromobisphenol A, and decabromodiphenyl ethane in indoor dust. JEPS. 2009;3:75–96. [Google Scholar]
- 39.Hays SM, Pyatt DW. Risk assessment for children exposed to decabromodiphenyl (oxide) ether (Deca) in the United States. Integrated environmental assessment and management. 2006;2(1):2–12. Epub 2006/04/28. PubMed PMID: 16640311. [PubMed] [Google Scholar]
- 40.Gomes G, Ward P, Lorenzo A, Hoffman K, Stapleton HM. Characterizing Flame Retardant Applications and Potential Human Exposure in Backpacking Tents. Environmental science & technology. 2016;50(10):5338–45. doi: 10.1021/acs.est.6b00923. Epub 2016/04/16. doi: 10.1021/acs.est.6b00923. PubMed PMID: 27082445. [DOI] [PubMed] [Google Scholar]
- 41.Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Webster TF. Associations between PBDEs in office air, dust, and surface wipes. Environment international. 2013;59:124–32. doi: 10.1016/j.envint.2013.06.001. Epub 2013/06/26. doi: 10.1016/j.envint.2013.06.001. PubMed PMID: 23797055; PubMed Central PMCID: PMCPmc3759556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdallah MA, Pawar G, Harrad S. Effect of Bromine Substitution on Human Dermal Absorption of Polybrominated Diphenyl Ethers. Environmental science & technology. 2015;49(18):10976–83. doi: 10.1021/acs.est.5b03904. Epub 2015/08/25. doi: 10.1021/acs.est.5b03904. PubMed PMID: 26301594. [DOI] [PubMed] [Google Scholar]
- 43.Roper CS, Simpson AG, Madden S, Serex TL, Biesemeier JA. Absorption of [14C]-tetrabromodiphenyl ether (TeBDE) through human and rat skin in vitro. Drug and chemical toxicology. 2006;29(3):289–301. doi: 10.1080/01480540600652954. Epub 2006/06/17. doi: 10.1080/01480540600652954. PubMed PMID: 16777707. [DOI] [PubMed] [Google Scholar]
- 44.Abdallah MA, Pawar G, Harrad S. Evaluation of in vitro vs. in vivo methods for assessment of dermal absorption of organic flame retardants: a review. Environment international. 2015;74:13–22. doi: 10.1016/j.envint.2014.09.012. Epub 2014/10/14. doi: 10.1016/j.envint.2014.09.012. PubMed PMID: 25310507. [DOI] [PubMed] [Google Scholar]
- 45.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster(R) 550 in rats: an exploratory assessment. Journal of biochemical and molecular toxicology. 2013;27(2):124–36. doi: 10.1002/jbt.21439. Epub 2012/11/10. doi: 10.1002/jbt.21439. PubMed PMID: 23139171; PubMed Central PMCID: PMC3788594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knudsen GA, Sanders JM, Birnbaum LS. Disposition of the emerging brominated flame retardant, 2-Ethylhexyl 2,3,4,5-Tetrabromobenzoate, in female SD rats and male B6C3F1 mice: effects of dose, route, and repeated administration. Toxicol Sci. 2016 doi: 10.1093/toxsci/kfw176. Epub 2016/09/11. doi: 10.1093/toxsci/kfw176. PubMed PMID: 27613714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva MJ, Hilton D, Furr J, Gray LE, Preau JL, Calafat AM, Ye X. Quantification of tetrabromo benzoic acid and tetrabromo phthalic acid in rats exposed to the flame retardant Uniplex FPR-45. Archives of toxicology. 2016;90(3):551–7. doi: 10.1007/s00204-015-1489-6. Epub 2015/03/26. doi: 10.1007/s00204-015-1489-6. PubMed PMID: 25804200; PubMed Central PMCID: PMC4583349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bearr JS, Stapleton HM, Mitchelmore CL. Accumulation and DNA damage in fathead minnows (Pimephales promelas) exposed to 2 brominated flame-retardant mixtures, Firemaster 550 and Firemaster BZ-54. Environmental toxicology and chemistry / SETAC. 2010;29(3):722–9. doi: 10.1002/etc.94. Epub 2010/09/08. doi: 10.1002/etc.94. PubMed PMID: 20821500; PubMed Central PMCID: PMC4332595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chemtura . FIREMASTER® 600 Material Safety Data Sheet. 2016. updated 4/7/2016; cited 2016 6/6/2016. [Google Scholar]
- 50.Maibach HI. In: Personal Communication: Mass loading recommendations for novel and legacy brominated flame retardants. Knudsen GA, editor. 2012. [Google Scholar]
- 51.Escobar-Chavez JJ, Merino-Sanjuan V, Lopez-Cervantes M, Urban-Morlan Z, Pinon-Segundo E, Quintanar-Guerrero D, Ganem-Quintanar A. The tape-stripping technique as a method for drug quantification in skin. Journal of pharmacy & pharmaceutical sciences: a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2008;11(1):104–30. doi: 10.18433/j3201z. Epub 2008/05/01. PubMed PMID: 18445368. [DOI] [PubMed] [Google Scholar]
- 52.Demierre AL, Peter R, Oberli A, Bourqui-Pittet M. Dermal penetration of bisphenol A in human skin contributes marginally to total exposure. Toxicology letters. 2012;213(3):305–8. doi: 10.1016/j.toxlet.2012.07.001. Epub 2012/07/17. doi: 10.1016/j.toxlet.2012.07.001. PubMed PMID: 22796587. [DOI] [PubMed] [Google Scholar]
- 53.Knudsen GA, Hughes MF, McIntosh KL, Sanders JM, Birnbaum LS. Estimation of tetrabromobisphenol A (TBBPA) percutaneous uptake in humans using the parallelogram method. Toxicology and applied pharmacology. 2015 doi: 10.1016/j.taap.2015.09.012. Epub 2015/09/22. doi: 10.1016/j.taap.2015.09.012. PubMed PMID: 26387765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes MF, Edwards BC. In vitro dermal absorption of pyrethroid pesticides in human and rat skin. Toxicology and applied pharmacology. 2010;246(1-2):29–37. doi: 10.1016/j.taap.2010.04.003. Epub 2010/04/20. doi: 10.1016/j.taap.2010.04.003. PubMed PMID: 20398685. [DOI] [PubMed] [Google Scholar]
- 55.Niedorf F, Schmidt E, Kietzmann M. The automated, accurate and reproducible determination of steady-state permeation parameters from percutaneous permeation data. Alternatives to laboratory animals: ATLA. 2008;36(2):201–13. doi: 10.1177/026119290803600209. Epub 2008/06/05. PubMed PMID: 18522486. [DOI] [PubMed] [Google Scholar]
- 56.van Ravenzwaay B, Leibold E. A comparison between in vitro rat and human and in vivo rat skin absorption studies. Human & experimental toxicology. 2004;23(9):421–30. doi: 10.1191/0960327104ht471oa. Epub 2004/10/23. PubMed PMID: 15497817. [DOI] [PubMed] [Google Scholar]
- 57.van Ravenzwaay B, Leibold E. The significance of in vitro rat skin absorption studies to human risk assessment. Toxicology in vitro: an international journal published in association with BIBRA. 2004;18(2):219–25. doi: 10.1016/j.tiv.2003.08.002. Epub 2004/02/06. PubMed PMID: 14757113. [DOI] [PubMed] [Google Scholar]
- 58.Jakasa I, Kezic S. Evaluation of in-vivo animal and in-vitro models for prediction of dermal absorption in man. Human & experimental toxicology. 2008;27(4):281–8. doi: 10.1177/0960327107085826. Epub 2008/08/08. doi: 10.1177/0960327107085826. PubMed PMID: 18684798. [DOI] [PubMed] [Google Scholar]
- 59.Ross JH, Reifenrath WG, Driver JH. Estimation of the percutaneous absorption of permethrin in humans using the parallelogram method. Journal of toxicology and environmental health Part A. 2011;74(6):351–63. doi: 10.1080/15287394.2011.534425. Epub 2011/01/29. doi: 10.1080/15287394.2011.534425. PubMed PMID: 21271436. [DOI] [PubMed] [Google Scholar]
- 60.OECD . Test No. 428: Skin Absorption: In Vitro Method. OECD Publishing; 2004. [Google Scholar]
- 61.Bronaugh RL, Maibach HI. In vitro Percutaneous Absorption: Principles, Fundamentals, and Applications. CRC Press; Boca Raton, FL: 1991. [Google Scholar]
- 62.Bronaugh RL, Stewart RF. Methods for in vitro percutaneous absorption studies IV: The flow-through diffusion cell. Journal of pharmaceutical sciences. 1985;74(1):64–7. doi: 10.1002/jps.2600740117. Epub 1985/01/01. PubMed PMID: 3981421. [DOI] [PubMed] [Google Scholar]
- 63.Knudsen GA, Sanders JM, Birnbaum LS. Disposition of the emerging brominated flame retardant, bis(2-ethylhexyl) tetrabromophthalate, in female Sprague Dawley rats: effects of dose, route and repeated administration. Xenobiotica; the fate of foreign compounds in biological systems. 2016:1–10. doi: 10.1080/00498254.2016.1174793. doi: 10.1080/00498254.2016.1174793. PubMed PMID: 27098498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knudsen GA, Sanders JM, Sadik AM, Birnbaum LS. Disposition and kinetics of Tetrabromobisphenol A in female Wistar Han rats. Toxicol Rep. 2014;1(0):214–23. doi: 10.1016/j.toxrep.2014.03.005. doi: 10.1016/j.toxrep.2014.03.005. PubMed PMID: 24977115; PubMed Central PMCID: PMC4071299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.OECD . Test No. 427: Skin Absorption: In Vivo Method. OECD Publishing; 2004. [Google Scholar]
- 66.Want EJ, Masson P, Michopoulos F, Wilson ID, Theodoridis G, Plumb RS, Shockcor J, Loftus N, Holmes E, Nicholson JK. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat Protocols. 2013;8(1):17–32. doi: 10.1038/nprot.2012.135. [DOI] [PubMed] [Google Scholar]
- 67.Imai T, Ariyoshi S, Ohura K, Sawada T, Nakada Y. Expression of Carboxylesterase Isozymes and Their Role in the Behaviour of a Fexofenadine Prodrug in Rat Skin. Journal of pharmaceutical sciences. 2015 doi: 10.1002/jps.24648. Epub 2015/10/08. doi: 10.1002/jps.24648. PubMed PMID: 26444870. [DOI] [PubMed] [Google Scholar]
- 68.Schiffer R, Neis M, Holler D, Rodriguez F, Geier A, Gartung C, Lammert F, Dreuw A, Zwadlo-Klarwasser G, Merk H, Jugert F, Baron JM. Active influx transport is mediated by members of the organic anion transporting polypeptide family in human epidermal keratinocytes. The Journal of investigative dermatology. 2003;120(2):285–91. doi: 10.1046/j.1523-1747.2003.12031.x. Epub 2003/01/25. doi: 10.1046/j.1523-1747.2003.12031.x. PubMed PMID: 12542534. [DOI] [PubMed] [Google Scholar]
- 69.Eilstein J, Lereaux G, Arbey E, Daronnat E, Wilkinson S, Duche D. Xenobiotic metabolizing enzymes in human skin and SkinEthic reconstructed human skin models. Experimental dermatology. 2015;24(7):547–9. doi: 10.1111/exd.12694. Epub 2015/03/27. doi: 10.1111/exd.12694. PubMed PMID: 25808006. [DOI] [PubMed] [Google Scholar]
- 70.Pacyniak E, Roth M, Hagenbuch B, Guo GL. Mechanism of polybrominated diphenyl ether uptake into the liver: PBDE congeners are substrates of human hepatic OATP transporters. Toxicol Sci. 2010;115(2):344–53. doi: 10.1093/toxsci/kfq059. Epub 2010/02/24. doi: 10.1093/toxsci/kfq059. PubMed PMID: 20176623; PubMed Central PMCID: PMCPmc2871754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, Artursson P, Tsuji A. PREDOMINANT CONTRIBUTION OF ORGANIC ANION TRANSPORTING POLYPEPTIDE OATP-B (OATP2B1) TO APICAL UPTAKE OF ESTRONE-3-SULFATE BY HUMAN INTESTINAL CACO-2 CELLS. Drug Metabolism and Disposition. 2006;34(8):1423–31. doi: 10.1124/dmd.106.009530. doi: 10.1124/dmd.106.009530. [DOI] [PubMed] [Google Scholar]
- 72.Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120(2):525–33. doi: 10.1053/gast.2001.21176. Epub 2001/02/13. PubMed PMID: 11159893. [DOI] [PubMed] [Google Scholar]
- 73.Fujiwara R, Takenaka S, Hashimoto M, Narawa T, Itoh T. Expression of human solute carrier family transporters in skin: possible contributor to drug-induced skin disorders. Scientific reports. 2014;4:5251. doi: 10.1038/srep05251. Epub 2014/06/12. doi: 10.1038/srep05251. PubMed PMID: 24918694; PubMed Central PMCID: PMCPmc4052716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kissel JC. The mismeasure of dermal absorption. Journal of exposure science & environmental epidemiology. 2011;21(3):302–9. doi: 10.1038/jes.2010.22. Epub 2010/04/29. doi: 10.1038/jes.2010.22. PubMed PMID: 20424648. [DOI] [PubMed] [Google Scholar]
- 75.Fang M, Stapleton HM. Evaluating the bioaccessibility of flame retardants in house dust using an in vitro Tenax bead-assisted sorptive physiologically based method. Environmental science & technology. 2014;48(22):13323–30. doi: 10.1021/es503918m. Epub 2014/10/21. doi: 10.1021/es503918m. PubMed PMID: 25330458; PubMed Central PMCID: PMCPmc4238594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ranasinghe AW, Wertz PW, Downing DT, Mackenzie IC. Lipid composition of cohesive and desquamated corneocytes from mouse ear skin. The Journal of investigative dermatology. 1986;86(2):187–90. doi: 10.1111/1523-1747.ep12284246. Epub 1986/02/01. PubMed PMID: 3745944. [DOI] [PubMed] [Google Scholar]
- 77.Staskal DF, Diliberto JJ, DeVito MJ, Birnbaum LS. Toxicokinetics of BDE 47 in female mice: effect of dose, route of exposure, and time. Toxicol Sci. 2005;83(2):215–23. doi: 10.1093/toxsci/kfi018. Epub 2004/10/29. doi: 10.1093/toxsci/kfi018. PubMed PMID: 15509665. [DOI] [PubMed] [Google Scholar]
- 78.Hughes MF, Edwards BC, Mitchell CT, Bhooshan B. In vitro dermal absorption of flame retardant chemicals. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2001;39(12):1263–70. doi: 10.1016/s0278-6915(01)00074-6. Epub 2001/11/07. PubMed PMID: 11696400. [DOI] [PubMed] [Google Scholar]
- 79.Frederiksen M, Vorkamp K, Jensen NM, Sorensen JA, Knudsen LE, Sorensen LS, Webster TF, Nielsen JB. Dermal uptake and percutaneous penetration of ten flame retardants in a human skin ex vivo model. Chemosphere. 2016;162:308–14. doi: 10.1016/j.chemosphere.2016.07.100. Epub 2016/08/12. doi: 10.1016/j.chemosphere.2016.07.100. PubMed PMID: 27513551. [DOI] [PubMed] [Google Scholar]
- 80.Fluhr JW, Darlenski R, Lachmann N, Baudouin C, Msika P, De Belilovsky C, Hachem JP. Infant epidermal skin physiology: adaptation after birth. The British journal of dermatology. 2012;166(3):483–90. doi: 10.1111/j.1365-2133.2011.10659.x. Epub 2011/10/05. doi: 10.1111/j.1365-2133.2011.10659.x. PubMed PMID: 21967466. [DOI] [PubMed] [Google Scholar]
- 81.Stamatas GN, Nikolovski J, Luedtke MA, Kollias N, Wiegand BC. Infant Skin Microstructure Assessed In Vivo Differs from Adult Skin in Organization and at the Cellular Level. Pediatric dermatology. 2010;27(2):125–31. doi: 10.1111/j.1525-1470.2009.00973.x. doi: 10.1111/j.1525-1470.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- 82.King A, Balaji S, Keswani SG. Biology and function of fetal and pediatric skin. Facial plastic surgery clinics of North America. 2013;21(1):1–6. doi: 10.1016/j.fsc.2012.10.001. Epub 2013/02/02. doi: 10.1016/j.fsc.2012.10.001. PubMed PMID: 23369584; PubMed Central PMCID: PMCPmc3654382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boireau-Adamezyk E, Baillet-Guffroy A, Stamatas GN. Age-dependent changes in stratum corneum barrier function. Skin research and technology: official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI) 2014;20(4):409–15. doi: 10.1111/srt.12132. Epub 2014/02/13. doi: 10.1111/srt.12132. PubMed PMID: 24517174. [DOI] [PubMed] [Google Scholar]
- 84.Stamatas GN, Nikolovski J, Mack MC, Kollias N. Infant skin physiology and development during the first years of life: a review of recent findings based on in vivo studies. International Journal of Cosmetic Science. 2011;33(1):17–24. doi: 10.1111/j.1468-2494.2010.00611.x. doi: 10.1111/j.1468-2494.2010.00611.x. [DOI] [PubMed] [Google Scholar]
- 85.Jung EC, Maibach HI. Animal models for percutaneous absorption. Journal of Applied Toxicology. 2015;35(1):1–10. doi: 10.1002/jat.3004. doi: 10.1002/jat.3004. [DOI] [PubMed] [Google Scholar]
- 86.Franz TJ, Lehman PA, Raney SG. Use of excised human skin to assess the bioequivalence of topical products. Skin pharmacology and physiology. 2009;22(5):276–86. doi: 10.1159/000235828. Epub 2009/08/27. doi: 10.1159/000235828. PubMed PMID: 19707043; PubMed Central PMCID: PMCPmc2790798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lehman PA, Raney SG, Franz TJ. Percutaneous absorption in man: in vitro-in vivo correlation. Skin pharmacology and physiology. 2011;24(4):224–30. doi: 10.1159/000324884. Epub 2011/04/02. doi: 10.1159/000324884. PubMed PMID: 21455015. [DOI] [PubMed] [Google Scholar]
- 88.Pawar G, Abdallah MA, de Saa EV, Harrad S. Dermal bioaccessibility of flame retardants from indoor dust and the influence of topically applied cosmetics. Journal of exposure science & environmental epidemiology. 2016 doi: 10.1038/jes.2015.84. Epub 2016/01/07. doi: 10.1038/jes.2015.84. PubMed PMID: 26732374. [DOI] [PubMed] [Google Scholar]
- 89.Frasch HF, Dotson GS, Bunge AL, Chen CP, Cherrie JW, Kasting GB, Kissel JC, Sahmel J, Semple S, Wilkinson S. Analysis of finite dose dermal absorption data: Implications for dermal exposure assessment. J Expo Sci Environ Epidemiol. 2014;24(1):65–73. doi: 10.1038/jes.2013.23. doi: 10.1038/jes.2013.23. PubMed PMID: WOS:000328604900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Api AM, Ritacco G, Hawkins DR. The fate of dermally applied [14C]d-limonene in rats and humans. International journal of toxicology. 2013;32(2):130–5. doi: 10.1177/1091581813479979. Epub 2013/03/16. doi: 10.1177/1091581813479979. PubMed PMID: 23493903. [DOI] [PubMed] [Google Scholar]
- 91.Moody RP, Nadeau B, Chu I. In vivo and in vitro dermal absorption of benzo[a]pyrene in rat, guinea pig, human and tissue-cultured skin. Journal of dermatological science. 1995;9(1):48–58. doi: 10.1016/0923-1811(94)00356-j. Epub 1995/01/01. PubMed PMID: 7727354. [DOI] [PubMed] [Google Scholar]
- 92.USEPA . In: Dermal Exposure Assessment: Principles and Applications. Exposure Assessment Group OoHaEA, editor. Washington, DC: 1992. [Google Scholar]
- 93.Stel J, Legler J. The Role of Epigenetics in the Latent Effects of Early Life Exposure to Obesogenic Endocrine Disrupting Chemicals. Endocrinology. 2015;156(10):3466–72. doi: 10.1210/en.2015-1434. Epub 2015/08/05. doi: 10.1210/en.2015-1434. PubMed PMID: 26241072; PubMed Central PMCID: PMCPmc4588824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vaiserman A. Early-life Exposure to Endocrine Disrupting Chemicals and Later-life Health Outcomes: An Epigenetic Bridge? Aging and disease. 2014;5(6):419–29. doi: 10.14336/AD.2014.0500419. Epub 2014/12/10. doi: 10.14336/ad.2014.0500419. PubMed PMID: 25489493; PubMed Central PMCID: PMCPmc4249811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environmental science & technology. 2011;45(12):5323–31. doi: 10.1021/es2007462. Epub 2011/05/20. doi: 10.1021/es2007462. PubMed PMID: 21591615; PubMed Central PMCID: PMC3113369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schreder ED, La Guardia MJ. Flame retardant transfers from U.S. households (dust and laundry wastewater) to the aquatic environment. Environmental science & technology. 2014;48(19):11575–83. doi: 10.1021/es502227h. Epub 2014/10/08. doi: 10.1021/es502227h. PubMed PMID: 25288150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.