Ovarian cancer is generally detected at an advanced stage and is associated with a 5-year survival rate of about 30%. However, survival rates of greater than 90% have been reported with stage I disease, thus fuelling efforts to determine the role of population screening for the detection of early disease. Recent research has focused on two screening strategies: one using ultrasound alone, the other using the serum tumour marker CA125 for primary screening followed by ultrasound as a second-line test (multimodal screening).
Transvaginal or pelvic ultrasonography is used to visualize the adnexae. Benign and malignant tumours are distinguished from one another on the basis of morphology. Complex ovarian cysts with wall abnormalities or solid areas are associated with significant risk for malignant disease,1,2 whereas unilocular ovarian cysts are associated with a less than 1% risk for ovarian cancer in asymptomatic premenopausal women.3,4 The low specificity of ultrasonography for malignant ovarian lesions, combined with recent findings of a high prevalence of benign ovarian lesions in older asymptomatic women,5 results in many women requiring further investigations and undergoing potentially unnecessary surgery when ultrasonography alone is used for screening.
Serum tumour markers for ovarian cancer exist, of which CA125 has been the most extensively studied. It is expressed by about 80% of epithelial cancers (the most common type of malignant tumour) but has limited specificity when used alone, in that it may also be increased in the presence of other cancers (pancreatic, breast, bladder, liver, lung) as well as benign disease (diverticulitis, leiomyoma, endometriosis, benign ovarian cyst, tubo-ovarian abscess, renal disease) and physiologic conditions (pregnancy and menstruation). Specificity of CA125 screening is improved by the addition of pelvic ultrasonography as a second-line test to assess ovarian lesions (multimodal strategy). The sensitivity of CA125 testing has been further refined by using sophisticated computerized calculations to aid in the interpretation of serial serum levels.6
Overall, the data from prospective studies of screening for ovarian cancer in the general population7 suggest that sequential multimodal screening has superior specificity and positive predictive value compared with strategies based on transvaginal ultrasonography alone. However, ultrasonography as a first-line test may offer greater sensitivity for early stage disease.
Perhaps the best evidence to date on the use of screening for ovarian cancer comes from a randomized controlled trial of ovarian cancer screening using the multimodal strategy. Although the authors did not find a difference in the number of deaths from ovarian or fallopian cancer in the group that was screened (relative risk 2.0 [95% confidence interval 0.78–5.13]), they did find a significant difference in the median rate of survival in the screened group compared with the control group (72.9 months v. 41.8 months, p = 0.011).8 Other data from prospective single-arm screening trials have also been encouraging. In Japan, the rates of detection of stage I disease increased from 29.7% to 58.8% after ultrasound screening was introduced.9 In a US study involving nearly 15 000 women, patients who had epithelial ovarian cancer detected by ultrasound screening had a 5-year survival rate of 83.6% (standard deviation 10.8%).10 However, the lack of a control group in the latter study raises the possibility of a “healthy-volunteer effect” (that is, women who volunteer may be healthier and have a better chance of survival than the average woman). It is also important to note that some of the “ovarian cancers” detected by screening in these trials were not primary invasive epithelial cancers but lesions with a lower propensity for malignant disease. Mortality rates are unlikely to be reduced significantly by screen detection of tumours that have a relatively good prognosis.
An important aspect of any screening program is defining the target population. Two distinct populations are at increased risk for ovarian cancer. The first group, women with hereditary risk factors for disease, comprise 10% of all cases. The second, much larger group includes postmenopausal women who are over 50 years of age, in whom 90% of ovarian cancer occurs sporadically. Currently, further risk stratification is not possible within this group, but it is hoped that ongoing research will help us identify individual characteristics, including genetic profiles, associated with increased risk.
The future
Randomized controlled trials are now under way in the general population to examine many of these issues and assess the impact of screening on ovarian cancer mortality. The United Kingdom Collaborative Trial of Ovarian Cancer Screening has recruited 150 000 postmenopausal women from 13 centres in the UK (www.ukctocs.org.uk). The aim is to randomly assign 200 000 postmenopausal women to ultrasound screening, multimodal screening or no screening (1:1:2). The primary end point of the study is death from ovarian cancer; cost of screening, morbidity, compliance and acceptability of screening will also be examined. In the United States, the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial has finished enrolling 74 000 women and will compare a control group with a screened group undergoing primary screening with both CA125 testing and transvaginal ultrasonography for 3 years and then with CA125 testing alone for a further 2 years. Follow-up will continue for at least 13 years to assess health status and cause of death.11 Results of these trials, which are expected in 2012, will form the basis for an informed decision about the implementation of general population screening for ovarian cancer.
Single-arm screening trials involving high-risk women over 35 years of age are also under way in the United States and Britain. High-risk women (those at risk of familial ovarian cancer because of a history of more than 1 first-degree relative with ovarian cancer alone or a combination of early breast and ovarian or colon cancer, or those with confirmed BRCA1 or BRCA2 gene mutations) are being investigated with the goal of developing an optimal screening strategy. It is of note that these women are mainly premenopausal and may have a variety of both physiologic and benign conditions that can give rise to false-positive abnormalities on ultrasonography and CA125 testing. It is imperative that high-risk women who consider screening are told that currently we do not know whether screening can save lives and that they are counselled about the alternative option of having their tubes and ovaries removed (risk-reducing salpingo-oophorectomy). Screening is probably best done in the context of research trials.
The technology used for screening is advancing. In particular, new tumour markers are being discovered that, it is hoped, will improve the accuracy of cancer detection. The large banks of patient serum being developed as part of the screening trials will allow novel markers to be assessed rapidly. Also, as imaging techniques are refined, 3-dimensional views of the complex adnexal masses and quantitative or qualitative differences in blood flow within complex masses may aid in the discrimination of benign from malignant tumours.
Many aspects of ovarian cancer screening, including the natural history of disease and the accuracy of current screening tools, remain poorly understood. What should clinicians do now? They should ensure that women are informed that it is not known whether screening can save lives and that there are concerns that the risks of unnecessary surgery may outweigh benefits.12 Screening is not currently recommended for the general population. It is heartening to know that large trials are under way that will help answer many of the questions around the role of screening for ovarian cancer in the general population, even though these results may be years away. In women at risk for familial ovarian cancer, screening is an option. However, clinicians should ensure that these women are counselled about the alternative of having primary salpingo-oophorectomy.
Usha Menon Senior Lecturer / Consultant Gynaecology Cancer Research Centre Department of Gynaecological Oncology Institute of Women's Health University College London London, UK
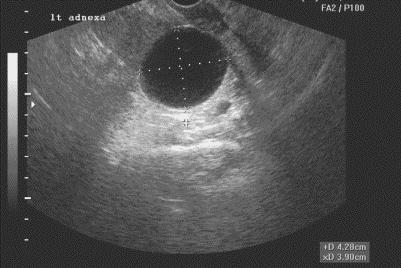
Figure. Simple ovarian cyst.
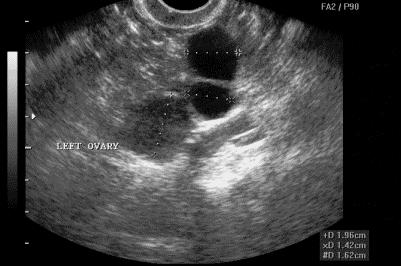
Figure. Complex ovarian lesion suspicious for malignant disease.
References
- 1.Valentin L. Use of morphology to characterize and manage common adnexal masses. Best Pract Res Clin Obstet Gynaecol 2004;18(1):71-89. [DOI] [PubMed]
- 2.Timmerman D. The use of mathematical models to evaluate pelvic masses: Can they beat an expert operator? Best Pract Res Clin Obstet Gynaecol 2004;18(1):91-104. [DOI] [PubMed]
- 3.Bailey CL, Ueland FR, Land GL, DePriest PD, Gallion HH, Kryscio RJ, et al. The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol Oncol 1998; 69(1):3-7. [DOI] [PubMed]
- 4.Roman LD. Small cystic pelvic masses in older women: Is surgical removal necessary? Gynecol Oncol 1998; 69(1):1-2. [DOI] [PubMed]
- 5.Valentin L, Skoog L, Epstein E. Frequency and type of adnexal lesions in autopsy material from postmenopausal women: ultrasound study with histological correlation. Ultrasound Obstet Gynecol 2003;22(3):284-9. [DOI] [PubMed]
- 6.Skates SJ, Menon U, MacDonald N, Rosenthal AN, Oram DH, Knapp RC, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol 2003;21(10 Suppl):206-10. [DOI] [PubMed]
- 7.Lewis S, Menon U. Screening for ovarian cancer. Expert Rev Anticancer Ther 2003;3(1):55-62. [DOI] [PubMed]
- 8.Jacobs IJ, Skates SJ, MacDonald N, Menon U, Rosenthal AN, Davies AP, et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet 1999;353(9160):1207-10. [DOI] [PubMed]
- 9.Sato S, Yokoyama Y, Sakamoto T, Futagami M, Saito Y. Usefulness of mass screening for ovarian carcinoma using transvaginal ultrasonography. Cancer 2000;89(3):582-8. [DOI] [PubMed]
- 10.Van Nagell JR Jr, DePriest PD, Reedy MB, Gallion HH, Ueland FR, Pavlik EJ, et al. The efficacy of transvaginal sonographic screening in asymptomatic women at risk for ovarian cancer. Gynecol Oncol 2000; 77 (3): 350-6. [DOI] [PubMed]
- 11.Hasson MA, Fagerstrom RM, Kahane DC, Walsh JH, Myers MH, Caughman C, et al; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team. Design and evolution of the data management systems in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000; 21(6 Suppl):329S-348S. [DOI] [PubMed]
- 12.Screening for ovarian cancer: recommendation statement. Ann Fam Med 2004;2(3):260-2. [DOI] [PMC free article] [PubMed]


