Abstract
The advantages of organic field-effect transistors (OFETs), such as low cost, flexibility and large-area fabrication, have recently attracted much attention due to their electronic applications. Practical transistors require high mobility, large on/off ratio, low threshold voltage and high stability. Development of new organic semiconductors is key to achieving these parameters. Recently, organic semiconductors have been synthesized showing comparable mobilities to amorphous-silicon-based FETs. These materials make OFETs more attractive and their applications have been attempted. New organic semiconductors resulting in high-performance FET devices are described here and the relationship between transistor characteristics and chemical structure is discussed.
Keywords: organic semiconductors, organic field-effect transistors, electron donors, electron acceptors, pentacene, acenes, thiophene oligomers, tetrathiafulvalenes
Introduction
Organic field-effect transistors (OFETs) have been of great interest for applications, such as display drivers, identification tags and smart cards, because they have advantages of low cost, flexibility and light weight [1–4]. Organic semiconductors can be processed at low temperatures compatible with plastic substrates, whereas higher temperatures are required for alternative Si-based FETs. Using solution techniques such as spin coating, inkjet printing and screen printing, large-area fabrication is possible at low costs. Modification of organic semiconductors can easily tune the transistor characteristics. Organic FETs have great perspectives in electronics.
The OFETs have a simple structure shown in figure 1. It includes source, drain and gate electrodes, and an active layer of organic semiconductor. An insulator layer, such as SiO2, exists between the gate electrode and organic semiconductor. Device characteristics are evaluated mainly by the carrier mobility, on/off current ratio and threshold voltage. Good transistor performance means high mobility, large on/off ratio and low threshold voltage. In addition, high stability in air is essential for applications. To achieve such performance, development of new organic semiconductors as well as the improvement of device structure is important. Recently, organic semiconductors have been developed exhibiting mobilities comparable to those of amorphous silicon. These materials make OFETs more attractive, and applications using them have been attempted. In this review, new organic semiconductors suitable for high-performance FETs are described, and the relationship between the transistor characteristics and chemical structure is discussed.
Figure 1.
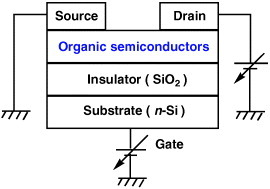
Structure of OFET device.
p-Type semiconductors
Molecules involving π-conjugation have high HOMO levels and exhibit electron-donating properties. Those molecules are good candidates for p-type semiconductors. On the other hand, electron-accepting molecules with low HOMO levels become n-type semiconductors. Many organic semiconductors showing p-type FET behavior have been reported, and some of these materials exhibit higher hole mobilities than amorphous Si (∼1.0 cm2 Vs−1). Typical p-type semiconductors are acenes, such as pentacene, and heterocyclic oligomers, such as oligothiophenes. In this chapter, recent examples are introduced according to structure types, i.e., acenes, oligomers and tetrathiafulvalenes (TTFs). Mobility does depend on the device structure. Therefore, only the highest reported values are mentioned below; ‘high mobility’ refers to values above 0.1 cm2 Vs−1.
Acenes
Pentacene 1 shows the highest hole mobility of 3.0 cm2 Vs−1 in thin-film OFETs [5]. However, pentacene has disadvantages such as instability in air and low solubility in solvents. Good solubility is crucial for device fabrication using solution methods such as inkjet printing. To overcome those disadvantages, various pentacene derivatives and analogues have been developed and the FETs based on them have been fabricated.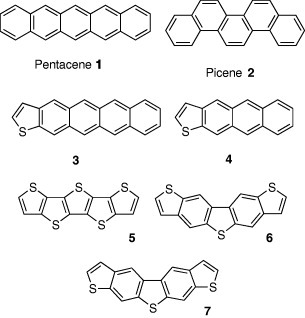
Picene 2, an isomer of pentacene, is stable in air because of the phenanthrene-type structure leading to the lower HOMO level. The mobility in air (1.1 cm2 Vs−1) is higher than in vacuum [6]. Replacement of the benzene rings of pentacene by thiophene rings also enhances the air stability. Thiophene-containing compound 3 exhibits high mobility of 0.31 cm2 Vs−1 and low threshold voltage (7 V) [7]. The air stability is improved compared to pentacene. The mobility of tetracene analogue 4 is 0.1 cm2 Vs−1, indicating that systems with more conjugation show higher mobilities [7]. Compounds 5–7 containing more thiophene rings were developed. Compound 5, composed of only thiophene rings, shows lower mobility (0.045 cm2 Vs−1) [8]. The mobility of 6 (0.011 cm2 Vs−1) is lower than that of its isomer 7 (0.12 cm2 Vs−1) [9]. These facts suggest that skeletal modification strongly affects electronic properties.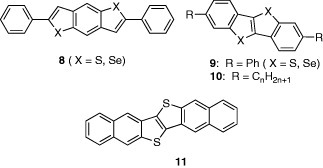
As new semiconductors, Takimiya et al developed diphenyl-substituted tri- or tetra-cyclic systems 8 and 9. In 8, the selenium-containing form showed enhanced mobility of 0.2 cm2 Vs−1 [10], whereas even higher mobility of 2.0 cm2 Vs−1 was measured in sulfur-containing 9 [11]. The device was stable for several months in air.
The stability was discussed based on the molecular orbital calculations [12]. Interestingly, alkyl-substituted derivatives 10 resulted in high-performance FETs fabricated by the solution method. The mobility was dependent on the alkyl chain length and the highest mobility of 2.75 cm2 Vs−1 was achieved in the compound with C13H27 groups [13]. Higher mobility of 2.9 cm2 Vs−1 was reported using 11. The device was fabricated by vacuum deposition and it was stable in air [14]. The mobility is comparable to that of pentacene. The high air stability is ascribed to the lower HOMO level.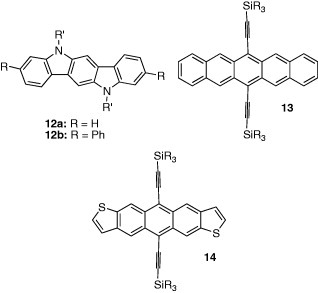
Instead of the thiophene rings, pyrrole rings were introduced to synthesize 12. The advantage of 12 is that long alkyl groups can be attached at the N-position to increase the solubility in solvents. The mobility of 12a was dependent on the alkyl substituents, and the highest mobility of 0.12 cm2 Vs−1 was observed in the octyl derivative [15]. The introduction of phenyl groups in 12b increased the mobility to 0.20 cm2 Vs−1 [16].
Pentacene crystal has a herringbone structure reducing intermolecular electron repulsion in the stack. On the other hand, intermolecular interactions are generally stronger in π-stacking structures than in the herringbone ones. Because carrier mobilities are related to intermolecular interactions, higher mobilities are expected in compounds with π-stacking structures possessing strong intermolecular interactions. Therefore, construction of π-stacking structures in acenes has been attempted to enhance mobilities. One way to produce a π-stacking structure is to use steric interactions by introducing bulky substituents. A pentacene derivative 13 containing acetylene units with a bulky substituent takes a π-stacking columnar structure to avoid steric interactions. The derivative containing iso-propyl groups resulted in an FET with a high mobility of 0.4 cm2 Vs−1 [17]. The advantage of 13 is its good solubility, favoring the solution process for the device fabrication. This method of using bulky acetylene groups was applied to thiophene analogues 14. The ethyl derivative takes a two-dimensional columnar structure and exhibits a high mobility of 1.0 cm2 Vs−1 [18].
Another method for constructing π-stacking structures is to use intermolecular charge-transfer interactions. Pentacene derivative 15 with an electron-accepting quinone unit takes a π-stacking columnar structure, owing to the intermolecular electron donor-acceptor interaction where the pentacene unit works as a donor part [19]. The mobility of 15 is 0.05 cm2 Vs−1, which is much lower than that of pentacene with a herringbone structure. This result can be explained considering a grain-boundary effect—the devices are thin polycrystalline films, where hopping conduction takes place. The grain-boundary effect is generally smaller in herringbone structures than in π-stacking ones because the former has higher dimensionality than the latter. This is why pentacene with a herringbone structure exhibits the highest mobility among thin film devices. On the other hand, in single-crystal devices, compounds with π-stacking structures show higher mobilities. For example, rubrene 16, having π-stacking structure, shows mobility of 15 cm2 Vs−1 [20]. A tetracene derivative 17, showing mobility of 1.6 cm2 Vs−1 in a single-crystal device, also assumes a π-stacking structure [21].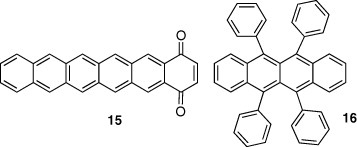
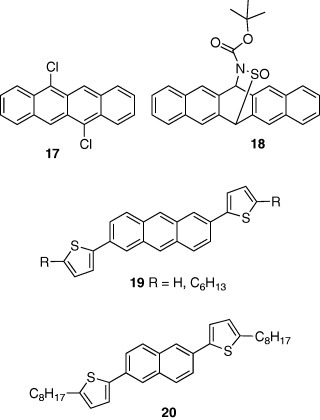
Solution methods are more suitable than vacuum deposition for large-area low-cost fabrication. Although pentacene devices cannot be prepared by the solution method from pentacene itself due to its low solubility in solvents, they could be obtained by a solution method using a precursor 18 which affords pentacene by heating [22]. The device showed a high mobility of 1.0 cm2 Vs−1 after annealing.
Oligomers
Anthracene did not show FET characteristics because of its small size of π-electronic system. However, the bisthienyl derivative 19 showed good p-type behavior [23], where the introduction of hexyl group greatly increases the mobility to 0.50 cm2 Vs−1 [24]. Even compound 20 containing a naphthalene core with alkylthienyl groups demonstrated a high mobility of 0.14 cm2 Vs−1 [25]. Phenylvinyl derivative 21 with a more π-extended system showed a higher mobility of 1.3 cm2 Vs−1 [26]. On the other hand, the dimer 22 and trimer 23 exhibited FET performance, and the hexyl derivatives showed higher mobilities (22; 0.13 cm2 Vs−1, 23; 0.17 cm2 Vs−1) [27]. Hexyl group is effective for arranging molecules perpendicularly to the substrates. On the other hand, the FET of the trimer of phenanthrene 24 showed a lower mobility of 0.11 cm2 Vs−1 indicating that acenes are superior to phenanthrene systems on mobilities [28]. However, the co-oligomer 25 with a bithiophene unit showed a high mobility of 0.12 cm2 Vs−1 and good stability in air.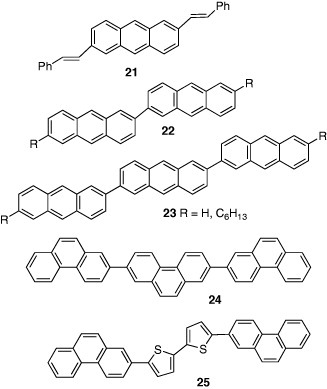
Thiophene oligomers 26 have been extensively studied, and the effects of ring numbers and alkyl chain lengths on FET characteristics have been investigated. Four to six thiophene rings and 2–6 carbon numbers of alkyl chains are necessary for high mobilities [29]. Thiophene-phenylene co-oligomers have higher air stability than thiophene oligomers owing to the lower HOMO levels. In co-oligomers 27, an odd–even effect of the thiophene ring numbers on the FET performance was found, where oligomers with even number of thiophene rings showed higher mobilities than oligomers with odd number of rings [30]. This observation is explained considering that the molecular symmetry is higher in the former oligomers, which leads to better-ordered molecular arrangement. Compounds 28 and 29 were also developed containing fluorene groups, where more planar molecular structures are expected. Phenylene systems 29 showed higher mobilities than 28, and the highest mobility of 0.32 cm2 Vs−1 was observed in the compound with a biphenyl core [31].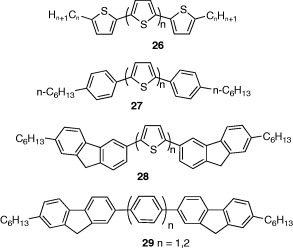
Soluble polythiophenes have attracted much attention from application viewpoints because uniform films can be formed using solution methods. High-performance polymer-based FETs require crystalline rather than amorphous structure. To prepare crystalline polythiophenes with good solubility, long alkyl chains should be introduced at suitable positions of the thiophene rings. Polythiophene 30 (R=C12H25) showed liquid–crystal characteristics, and the ordered structure was confirmed by x-ray analysis [32]. The mobility was 0.02–0.05 cm2 Vs−1 and improved to 0.07–0.12 cm2 Vs−1 after annealing. Polythiophene 31 is also crystalline and showed a mobility of 0.015–0.022 cm2 Vs−1 [33]. Introduction of condensed heterocyclic units is considered to increase intermolecular interactions leading to high mobilities. Polymer 32 was designed according to this idea and its mobility was 0.012 cm2 Vs−1[34]. Thienothiophene-containing polymer 33 ( R= C14
H29) showed a mobility of 0.6 cm2 Vs−1, which is the highest among polymer FETs [35]. On the other hand, to enhance air stability, the introduction of electron-acceptor units is effective to lower the HOMO levels. Polymer 34, containing an electron-accepting silole part, was found stable in air [36]. Furthermore, thiazolothiazole-containing polymer 35 showed high air stability as well as high mobility of 0.14 cm2 Vs−1 [37].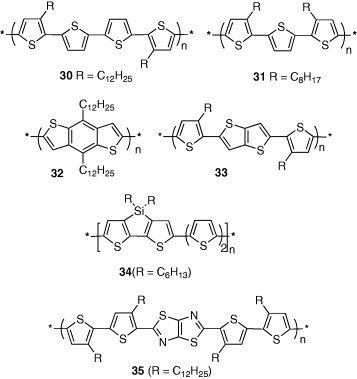
Tetrathiafulvalenes
Tetrathiafulvalenes (TTFs) are famous for electron donors resulting in organic conductors and superconductors. They are also promising semiconductors for high-performance OFETs because of self-assembling properties. However, the electron-donating properties of TTFs are so strong that their thin films easily oxidize leading to poor FET performance. Therefore, to use TTFs as semiconductors, the electron-donating properties should be reduced. For this purpose, we have used a TTF analogue 36 containing electron-accepting thiadiazole rings, which resulted in high hole mobility of 0.2 cm2 Vs−1 and high on/off ratio of 108 [38]. Introduction of fused aromatic rings to the TTF is also useful to decrease the electron-donating property. Benzene and thiophene-fused derivatives 37 and 38 exhibited high mobilities of 1.0 [39] and 1.4 cm2 Vs−1 [40], respectively, in the single-crystal devices. Although the mobility of 37 was only 0.06 cm2 Vs−1 in thin film device, the mobility of naphthalene-fused derivative 39 increased to 0.42 cm2 Vs−1 [41]. This increase is attributed to the extended π-conjugation resulting in stronger intermolecular interactions. However, the FET characteristics of 39 could not be observed in air owing to the still high HOMO level. In contrast, TTF derivative 40 containing electron-accepting quinoxaline rings showed enhanced stability to oxygen, although the mobility decreased to 0.2 cm2 Vs−1. Compound 40 has electron donor-acceptor parts leading to a π-stacking structure by an intermolecular charge-transfer interaction in the crystal, whereas TTFs 37 and 39 assume herringbone structures. Following a similar idea, electron-accepting diimide groups were introduced to dibenzoTTF to yield high hole mobility of 0.40 cm2 Vs−1 in 41 [42]. Pyrrole-fused derivatives 42 have an advantage of good solubility, facilitating solution processing [43]. The FET fabricated by spin coating showed a mobility of 0.013 cm2 Vs−1.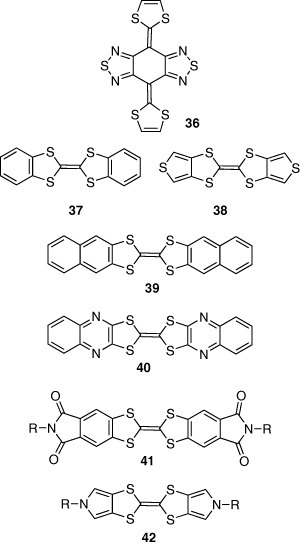
n-Type organic semiconductors
Compared to p-type organic semiconductors, n-type semiconductors are not fully developed, and their FET performance is not satisfactory yet. Perylenediimides 43 are famous as typical n-type semiconductors. The octyl derivative showed a high electron mobility of 0.6 cm2 Vs−1 [44], although the device was not stable in air. In the n-type organic semiconductors, radical anions are produced by electron injection. They are labile to oxygen and the stability is related to the electron-accepting properties of semiconductors. Therefore, cyano groups were introduced to give 44 which has high electron affinity. As expected, 44 showed better air stability as well as a high electron mobility of 0.64 cm2 Vs−1 [45]. Tetrachloro derivative 45 also showed a high mobility of 0.18 cm2 Vs−1 and good air stability [46]. Similarly, naphthalenediimide 46 [47] and anthracenediimide 47 [48] containing cyano groups were developed. Their reduction potentials were + 0.08 and - 0.33 V versus saturated calomel reference electrode, respectively, suggesting that the anion radicals are stable in air. Actually, their FETs showed air stability and the mobilities were 0.15 and 0.03 cm2 Vs−1, respectively. Tetracyanoquinodimethane (TCNQ) and its analogues are known as strong electron acceptors. Among them, terthiophene analogues resulted in good FETs. Compound 48 has good solubility, and the spin-coated thin film showed a high mobility of 0.16 cm2 Vs−1 after annealing at 150 ∘
C [49].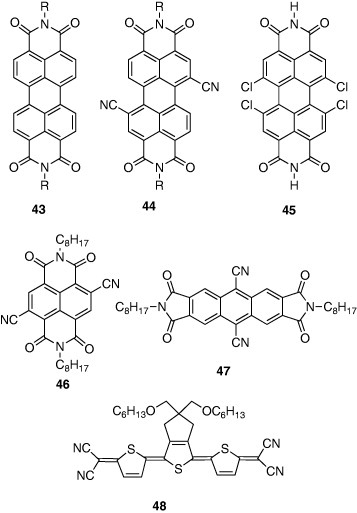
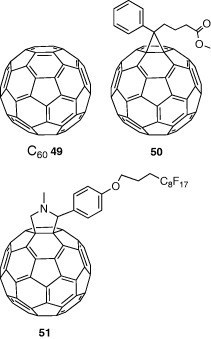
Fullerene C60 49 has a high electron affinity and yields high-performance FETs. A device fabricated by molecular-beam deposition showed mobility of 0.56 cm2 Vs−1 in vacuum [50]. Fullerene derivatives were developed to increase the solubility in solvents. The derivative 50 yielded ambipolar FETs by a solution method, where the electron and hole mobilities were 0.01 and 0.008 cm2 Vs−1, respectively [51]. A device fabricated from derivative 51 by solution method showed a high electron mobility of 0.15 cm2 Vs−1 [52].
Novel n-type organic semiconductors can be prepared by introducing electron acceptor groups to p-type semiconductors. Perfluoronated pentacene 52 showed a high electron mobility of 0.11 cm2 Vs−1 under high vacuum conditions [53]. Tetrafluoro derivative 53 of the thiophene analogue with acetylene groups showed ambipolar behavior in vacuum [54]. The highest electron and hole mobilities were 0.2 and 0.06 cm2 Vs−1, respectively. In air, the n-type behavior disappeared and the hole mobility increased to 0.1 cm2 Vs−1. The changes were attributed to oxygen.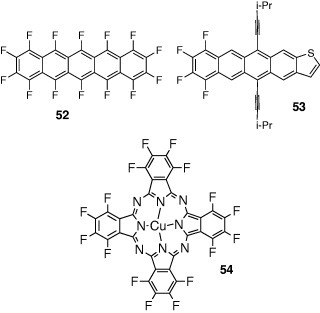
On the other hand, perfluorophthalocyanine derivative 54 was stable in air, but showed low electron mobility (0.03 cm2 Vs−1) [55]. Thiophene oligomers 55 with perfluorohexyl groups also exhibited n-type characteristics. The highest mobility of 0.048 cm2 Vs−1 was observed in the oligomer with a quarterthiophene core [56]. The oligomer 56 with pentafluorophenyl groups showed an electron mobility of 0.08 cm2 Vs−1 [57]. These findings could be rationalized in terms of HOMO–LUMO levels—introduction of electron-withdrawing groups decreased the LUMO levels leading to the n-type behavior. However, compound 57, having similar HOMO and LUMO energies as 56, showed p-type behavior [58]. This result reveals the effect of the end groups on the FET polarity; those groups might play an important role in accumulating carriers at the insulator-semiconductor interface. Introduction of acyl groups to the thiophene-oligomer core also induces n-type characteristics. The electron mobilities of hexyl derivative 58a and perfluorohexyl derivative 58b were 0.1 and 0.6 cm2 Vs−1, respectively [58]. Those compounds are ambipolar, and the hole mobility is better in the hexyl derivative 58a. On the other hand, a benzoyl derivative 58c revealed only p-type behavior (0.043 cm2 Vs−1), while a perfluoro derivative 58d showed only n-type behavior (0.45 cm2 Vs−1) [59].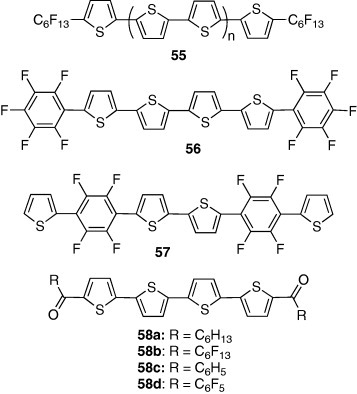
High electron mobilities were achieved using a trifluoromethylphenyl group as end substituent. Bithiophene derivative 59 with the substituents showed mobility of 0.18 cm2 Vs−1, which is higher than that of the corresponding perfluorohexyl derivative [60]. The weak electron affinity of 59 results in large barrier for electron injection from the electrode, resulting in a high threshold voltage of 70 V. Therefore, thiazolothiazole-containing compound 60 was designed to increase the electron affinity [59]. This bicyclic heterocycle is a rigid polarized ring which is expected to induce strong intermolecular π–π interactions. Actually, 60 has a π–π stacking structure and short heteroatom contacts between columns in the crystal. The FET based on 60 showed a high mobility of 0.30 cm2 Vs−1 with a threshold voltage of 60 V. Optimization of the device structure improved the mobility to 1.2 cm2 Vs−1 [61]. Furthermore, higher mobilities were achieved using bisthiazole derivative 62 [62]. The mobility was 1.83 cm2 Vs−1 when an octadecyltrichlorosilane-modified SiO2 substrate was used. Although high mobilities were also achieved in oligomers with trifluoromethylphenyl groups, as described above, the threshold voltages were high (above 60 V). To decrease these values, the LUMO levels of semiconductors should be lowered for easy electron injection. Replacement of the thiophene rings of 60 by thiazole rings to give 61 increases the electron affinity [63]. The FET based on compound 61 exhibited a lower threshold voltage of 24 V with a high mobility of 0.64 cm2 Vs−1.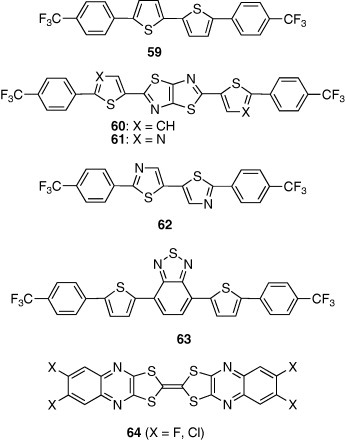
As an electron-accepting heterocycle, benzothiadiazole with a quinoid structure was also used for n-type semiconductors. Compound 63 containing this unit resulted in high-performance n-type FETs, where a high mobility of 0.19 cm2 Vs−1 and a low threshold voltage of 3 V were observed [64]. In addition, an FET made of compound 63 showed light emission with intensity increasing with increasing gate voltage. This effect is attributed to the small HOMO–LUMO gap leading to direct hole injection from the electrode.
Although quinoxaline-fused TTF derivatives 40 showed p-type behavior, the halogen-substituted derivatives 64 showed n-type behavior. The mobilities were 0.1 cm2 Vs−1 in both derivatives and they are the first examples of n-type FETs based on TTF derivatives [65]. The effect of halogen substituents was rationalized by the lower LUMO levels. This result confirms that the FET polarity can be determined by the frontier orbital energies of semiconductors, and the end-substituents can control the polarity.
Summary
As described here, a large number of novel organic semiconductors have recently been reported, which make great progress in OFETs. The FET carrier mobilities are summarized in tables 1 and 2. Some semiconductors show higher mobilities than amorphous silicon (1.0 cm2 Vs−1). These findings make OFETs attractive for applications. However, some problems have to be solved, such as stability. For solution processing, present FET performance is not satisfactory. New semiconductors will hopefully be developed to overcome these problems and bring about practical appreciation of OFETs.
Table 1.
Mobilities of the p-type FETs.
| Compounda | Mobility (cm2 Vs−1)b | Reference |
|---|---|---|
| Pentacene 1 | 3.0 | [5] |
| Picene 2 | 1.1 | [6] |
| 3 | 0.31 | [7] |
| 4 | 0.1 | [7] |
| 5 | 0.045 | [8] |
| 6 | 0.011 | [9] |
| 7 | 0.12 | [9] |
| 8 | 0.2 | [10] |
| 9 | 2.0 | [11] |
| 10 | 2.75 | [13] |
| 11 | 2.9 | [14] |
| 12a | 0.12 | [15] |
| 12b | 0.20 | [16] |
| 13 | 0.4 | [17] |
| 14 | 1.0 | [18] |
| 15 | 0.05 | [19] |
| 16 | 15c | [20] |
| 17 | 1.6c | [21] |
| 19 | 0.50 | [24] |
| 20 | 0.14 | [25] |
| 21 | 1.3 | [26] |
| 22 | 0.13 | [27] |
| 23 | 0.17 | [27] |
| 24 | 0.011 | [28] |
| 25 | 0.12 | [28] |
| 26 | 1.1 | [29] |
| 27 | 0.09 | [30] |
| 28 | 0.11 | [31] |
| 29 | 0.32 | [31] |
| 30 | 0.12 | [32] |
| 31 | 0.022 | [33] |
| 32 | 0.012 | [34] |
| 33 | 0.6 | [35] |
| 34 | 0.06 | [36] |
| 35 | 0.14 | [37] |
| 36 | 0.2 | [38] |
| 37 | 1.0c | [39] |
| 38 | 1.4c | [40] |
| 39 | 0.42 | [41] |
| 40 | 0.2 | [41] |
| 41 | 0.40 | [42] |
| 42 | 0.013 | [43] |
aCompounds 1–17 are acene-type, 19–35 are oligomer-type, and 36–42 are TTF-type.
bHighest values reported in the reference.
cSingle-crystal device.
Table 2.
Mobilities of the n-type FETs.
| Compound | Mobility (cm2 Vs−1)a | Reference |
|---|---|---|
| 43 | 0.6 | [44] |
| 44 | 0.64 | [45] |
| 45 | 0.18 | [46] |
| 46 | 0.15 | [47] |
| 47 | 0.03 | [48] |
| 48 | 0.16 | [49] |
| 49 | 0.56 | [50] |
| 50b | 0.01 | [51] |
| 51 | 0.15 | [52] |
| 52 | 0.11 | [53] |
| 53b | 0.2 | [54] |
| 54 | 0.03 | [55] |
| 55 | 0.048 | [56] |
| 56 | 0.08 | [57] |
| 58ab | 0.1 | [58] |
| 58bb | 0.6 | [58] |
| 58d | 0.45 | [59] |
| 59 | 0.18 | [60] |
| 60 | 1.2 | [61] |
| 61 | 0.64 | [63] |
| 62 | 1.83 | [62] |
| 63 | 0.19 | [64] |
| 64 | 0.1 | [65] |
aHighest values reported in the reference.
bAmbipolar behavior.
Acknowledgment
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (no. 15073212) from the Ministry of Education, Culture, Sports and Technology, Japan.
References
- Sun Y, Liu Y. and Zhu D. J. Mater. Chem. 2005;15:53. doi: 10.1039/b411245h. [DOI] [Google Scholar]
- Murphy A R. and Fréchet J M J. Chem. Rev. 2007;107:1066. doi: 10.1021/cr0501386. [DOI] [PubMed] [Google Scholar]
- Anthony J E. Angew. Chem., Int. Ed. Engl. 2008;47:452. doi: 10.1002/anie.200604045. [DOI] [PubMed] [Google Scholar]
- Allard S, Forster M, Souharce B, Thiem H. and Scherf U. Angew. Chem., Int. Ed. Engl. 2008;47:4070. doi: 10.1002/anie.200701920. [DOI] [PubMed] [Google Scholar]
- Klauk H, Halik M, Zschieschang U, Eder F, Schmid G. and Dehm C. Appl. Phys. Lett. 2003;82:4175. doi: 10.1063/1.1579870. [DOI] [Google Scholar]
- Okamoto H, Kawasaki N, Kaji Y, Kubozono Y. and Yamaji M. J. Am. Chem. Soc. 2008;130:10470. doi: 10.1021/ja803291a. [DOI] [PubMed] [Google Scholar]
- Tang M L, Okamoto T. and Bao Z. J. Am. Chem. Soc. 2006;128:16002. doi: 10.1021/ja066824j. [DOI] [PubMed] [Google Scholar]
- Xioa K.et al2005J. Am. Chem. Soc. 12713281. 10.1021/ja052816b [DOI] [PubMed] [Google Scholar]
- Wex B, Kaafarani B R, Schroeder R, Majewski L A, Burckel P, Grell M. and Neckers D C. J. Mater. Chem. 2006;16:1121. doi: 10.1039/b512191d. [DOI] [Google Scholar]
- Takimiya K, Kunugi Y, Konda Y, Niihara N. and Otsubo T. J. Am. Chem. Soc. 2004;126:5084. doi: 10.1021/ja0496930. [DOI] [PubMed] [Google Scholar]
- Takimiya K, Ebata H, Sakamoto K, Izawa T, Otsubo T. and Kunugi Y. J. Am. Chem. Soc. 2007;128:12604. doi: 10.1021/ja064052l. [DOI] [PubMed] [Google Scholar]
- Takimiya K, Yamamoto T, Ebata H. and Izawa T. Sci. Technol. Adv. Mater. 2007;8:273. doi: 10.1016/j.stam.2007.02.010. [DOI] [Google Scholar]
- Ebata H, Izawa T, Miyazaki E, Takimiya K, Ikeda M, Kuwabara H. and Yui T. J. Am. Chem. Soc. 2007;129:15732. doi: 10.1021/ja074841i. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. and Takimiya K. J. Am. Chem. Soc. 2007;129:2224. doi: 10.1021/ja068429z. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li Y, Gardner S. and Ong B S. J. Am. Chem. Soc. 2005;127:614. doi: 10.1021/ja0456149. [DOI] [PubMed] [Google Scholar]
- Boudreault P-L, Wakim S, Blouin N, Simard M, Tessier C, Tao Y. and Leclerc M. J. Am. Chem. Soc. 2007;129:9129. doi: 10.1021/ja071923y. [DOI] [PubMed] [Google Scholar]
- Sheraw C D, Jackson T N, Eaton D L. and Anthony J E. Adv. Mater. 2003;15:2009. doi: 10.1002/adma.200305393. [DOI] [Google Scholar]
- Payne M P, Parkin S R, Anthony J E, Kuo C-C. and Jackson T N. J. Am. Chem. Soc. 2005;127:4986. doi: 10.1021/ja042353u. [DOI] [PubMed] [Google Scholar]
- Miao Q, Lefenfeld M, Nguyen T-Q, Siegrist T, Kloc C. and Nuckolls C. Adv. Mater. 2005;17:407. doi: 10.1002/adma.200401251. [DOI] [Google Scholar]
- Sundar V C, Zaumseil J, Podzorov V, Menard E, Willett R L, Someya T, Gershenson M E. and Rogers J A. Science. 2004;303:1644. doi: 10.1126/science.1094196. [DOI] [PubMed] [Google Scholar]
- Moon H, Zeis R, Borkent E-J, Besnard C, Lovinger A J, Siegrist T, Kloc C. and Bao Z. J. Am. Chem. Soc. 2004;126:15322. doi: 10.1021/ja045208p. [DOI] [PubMed] [Google Scholar]
- Weidkamp K P, Afzali A, Tromp R M. and Hamers R J. J. Am. Chem. Soc. 2004;126:12740. doi: 10.1021/ja045228r. [DOI] [PubMed] [Google Scholar]
- Ando S, Nishida J, Fujiwara E, Tada H, Inoue Y, Tokito S. and Yamashita Y. Chem. Mater. 2005;17:1261. doi: 10.1021/cm0478632. [DOI] [Google Scholar]
- Meng H, Sun F, Goldfinger M B, Jaycox G D, Li Z, Marshall W J. and Blackman G S. J. Am. Chem. Soc. 2005;127:2406. doi: 10.1021/ja043189d. [DOI] [PubMed] [Google Scholar]
- Oikawa K, Monobe H, Nakayama K, Kimoto T, Tsuchiya K, Heinrich B, Guillon D, Shimizu Y. and Yokoyama M. Adv. Mater. 2007;19:1864. doi: 10.1002/adma.200602608. [DOI] [Google Scholar]
- Klauk H, Zschieschang U, Weitz R T, Meng H, Sun F, Nunes G, Keys D E, Fincher C R. and Xiang Z. Adv. Mater. 2007;19:3882. doi: 10.1002/adma.200701431. [DOI] [Google Scholar]
- Ito K, Suzuki T, Sakamoto Y, Kubota D, Inoue Y, Sato F. and Tokito S. Angew. Chem., Int. Ed. Engl. 2003;42:1159. doi: 10.1002/anie.200390305. [DOI] [PubMed] [Google Scholar]
- Tian H, Shi J, Dong S, Yan D, Wang L, Geng Y. and Wang F. Chem. Commun. 2006;33:3498. doi: 10.1039/b606759j. [DOI] [PubMed] [Google Scholar]
- Halik M, Klauk H, Zschieschang U, Schmid G, Ponomarenko S, Kirchmeyer S. and Weber W. Adv. Mater. 2003;15:917. doi: 10.1002/adma.200304654. [DOI] [Google Scholar]
- Mushrush M, Facchetti A, Lefenfeld M, Katz H E. and Marks T J. J. Am. Chem. Soc. 2003;125:9414. doi: 10.1021/ja035143a. [DOI] [PubMed] [Google Scholar]
- Meng H, Zheng J, Lovinger A J, Wang B-C, Van Patten P G. and Bao Z. Chem. Mater. 2003;15:1778. doi: 10.1021/cm020866z. [DOI] [Google Scholar]
- Ong B S, Wu Y, Liu P. and Gardner S. J. Am. Chem. Soc. 2004;126:3378. doi: 10.1021/ja039772w. [DOI] [PubMed] [Google Scholar]
- Wu Y, Liu P, Gardner S. and Ong B S. Chem. Mater. 2005;17:221. doi: 10.1021/cm048678r. [DOI] [Google Scholar]
- Pan H, Li Y, Wu Y, Liu P, Ong B S, Zhu S. and Xu G. Chem. Mater. 2006;18:3237. doi: 10.1021/cm0602592. [DOI] [Google Scholar]
- Mcculloch I.et al2006Nat. Mater. 5328. 10.1038/nmat1612 [DOI] [PubMed] [Google Scholar]
- Usta H, Lu G, Facchetti A. and Marks T J. J. Am. Chem. Soc. 2006;128:9034. doi: 10.1021/ja062908g. [DOI] [PubMed] [Google Scholar]
- Osaka I, Sauvé G, Zhang R, Kowalewski T. and McCullough R D. Adv. Mater. 2007;19:4160. doi: 10.1002/adma.200701058. [DOI] [Google Scholar]
- Takada M, Graaf H, Yamashita Y. and Tada H. Japan. J. Appl. Phys. 2002;41:L4. doi: 10.1143/JJAP.41.L4. [DOI] [Google Scholar]
- Mas-Torrent M, Hadley P, Bromley S T, Crivillers N, Veciana J. and Rovira C. Appl. Phys. Lett. 2005;86:012110. doi: 10.1063/1.1848179. [DOI] [Google Scholar]
- Mas-Torrent M, Durkut M, Hadley P, Ribas X. and Rovira C. J. Am. Chem. Soc. 2004;126:984. doi: 10.1021/ja0393933. [DOI] [PubMed] [Google Scholar]
- Naraso, Nishida J, Tada H, Inoue Y, Tokito S. and Yamashita Y. J. Am. Chem. Soc. 2005;127:10142. doi: 10.1021/ja051755e. [DOI] [PubMed] [Google Scholar]
- Gao X.et al2007Adv. Mater. 193037. 10.1002/adma.200700007 [DOI] [Google Scholar]
- Doi I, Miyazaki E, Takimiya K. and Kunugi Y. Chem. Mater. 2007;19:5230. doi: 10.1021/cm070956+. [DOI] [PubMed] [Google Scholar]
- Malenfant P R L, Dimitrakopoulos C D, Gelorme J D, Kosbar L L, Graham T O, Curioni A. and Andreoni W. Appl. Phys. Lett. 2002;80:2517. doi: 10.1063/1.1467706. [DOI] [Google Scholar]
- Jones B A, Ahrens M J, Yoon M-H, Facchetti A, Marks T J. and Wasielewski M R. Angew. Chem., Int. Ed. Engl. 2004;43:6363. doi: 10.1002/anie.200461324. [DOI] [PubMed] [Google Scholar]
- Ling M-M, Erk P, Gomez M, Koenemann M, Locklin J. and Bao Z. Adv. Mater. 2007;19:1123. doi: 10.1002/adma.200601705. [DOI] [Google Scholar]
- Jones B A, Facchetti A, Marks T J. and Wasielewski M R. Chem. Mater. 2007;19:2703. doi: 10.1021/cm0704579. [DOI] [Google Scholar]
- Wang Z, Kim C, Facchetti A. and Marks T J. J. Am. Chem. Soc. 2007;129:13362. doi: 10.1021/ja073306f. [DOI] [PubMed] [Google Scholar]
- Handa S, Miyazaki E, Takimiya K. and Kunugi Y. J. Am. Chem. Soc. 2007;129:11684. doi: 10.1021/ja074607s. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Takenobu T, Mori S, Fujiwara A. and Iwasa Y. Appl. Phys. Lett. 2003;82:4581. doi: 10.1063/1.1577383. [DOI] [Google Scholar]
- Anthopoulos T D, Tanase C, Setayesh S, Meijer E J, Hummelen J C, Blom P W M. and de Leeuw D M. Adv. Mater. 2004;16:2174. doi: 10.1002/adma.200400309. [DOI] [Google Scholar]
- Wöbkenberg P H, Ball J, Bradley D D C, Anthopoulos T D, Kooistra F, Hummelen J C. and de Leeuw D M. Appl. Phys. Lett. 2008;92:143310. doi: 10.1063/1.2907348. [DOI] [Google Scholar]
- Sakamoto Y, Suzuki T, Kobayashi M, Gao Y, Fukai Y, Inoue Y, Sato F. and Tokito S. J. Am. Chem. Soc. 2004;126:8138. doi: 10.1021/ja0476258. [DOI] [PubMed] [Google Scholar]
- Tang M L, Reichardt A D, Miyaki N, Stoltenberg R M. and Bao Z. J. Am. Chem. Soc. 2008;130:6064. doi: 10.1021/ja8005918. [DOI] [PubMed] [Google Scholar]
- Bao Z, Lovinger A J. and Brown J. J. Am. Chem. Soc. 1998;120:207. doi: 10.1021/ja9727629. [DOI] [Google Scholar]
- Facchetti A, Mushrush M, Katz H E. and Marks T J. Adv. Mater. 2003;15:33. doi: 10.1002/adma.200390003. [DOI] [Google Scholar]
- Yoon M-H, Facchetti A, Stem C L. and Marks T J. J. Am. Chem. Soc. 2006;128:5792. doi: 10.1021/ja060016a. [DOI] [PubMed] [Google Scholar]
- Yoon M-H, DiBenedetto S, Facchetti A. and Marks T J. J. Am. Chem. Soc. 2005;127:1348. doi: 10.1021/ja045124g. [DOI] [PubMed] [Google Scholar]
- Letizia J A, Facchetti A, Stem C L, Ratner M A. and Marks T J. J. Am. Chem. Soc. 2005;127:13476. doi: 10.1021/ja054276o. [DOI] [PubMed] [Google Scholar]
- Ando S, Nishida J, Tada H, Inoue Y, Tokito S. and Yamashita Y. J. Am. Chem. Soc. 2005;127:5336. doi: 10.1021/ja042219+. [DOI] [PubMed] [Google Scholar]
- Kumaki D, Ando S, Shimono S, Yamashita Y, Umeda T. and Tokito S. Appl. Phys. Lett. 2007;90:053506. doi: 10.1063/1.2436641. [DOI] [Google Scholar]
- Ando S, Murakami R, Nishida J, Tada H, Inoue Y, Tokito S. and Yamashita Y. J. Am. Chem. Soc. 2005;127:14996. doi: 10.1021/ja055686f. [DOI] [PubMed] [Google Scholar]
- Mamada M, Nishida J, Kumaki D, Tokito S. and Yamashita Y. Chem. Mater. 2007;19:5404. doi: 10.1021/cm071505s. [DOI] [Google Scholar]
- Kono T, Kumaki D, Nishida J, Sakanoue T, Kakita M, Tada H, Tokito S. and Yamashita Y. Chem. Mater. 2007;19:1218. doi: 10.1021/cm062889+. [DOI] [Google Scholar]
- Naraso Nishida J, Kumaki D, Tokito S. and Yamashita Y. J. Am. Chem. Soc. 2006;128:9898. doi: 10.1021/ja0630083. [DOI] [PubMed] [Google Scholar]


