Abstract
The effect of starting powders on the sintering of nanostructured tetragonal zirconia was evaluated. Suspensions were prepared with a concentration of 10 vol.% by mixing a bicomponent mixture of commercial powders (97 mol.% monoclinic zirconia with 3 mol.% yttria) and by dispersing commercially available tetragonal zirconia (3YTZ, Tosoh). The preparation of the slurry by bead-milling was optimized. Colloidal processing using 50 μm zirconia beads at 4000 rpm generated a fully deagglomerated suspension leading to the formation of high-density consolidated compacts (62% of the theoretical density (TD) for the bicomponent suspension). Optimum colloidal processing of the bicomponent suspension followed by the sintering of yttria and zirconia allowed us to obtain nanostructured tetragonal zirconia. Three different sintering techniques were investigated: normal sintering, two-step sintering and spark plasma sintering. The inhibition of grain growth in the bicomponent mixed powders in comparison with 3YTZ was demonstrated. The inhibition of the grain growth may have been caused by inter-diffusion of cations during the sintering.
Keywords: nanostructured zirconia, colloidal processing, two-step sintering, spark plasma sintering (SPS)
Introduction
Zirconia ceramics have been widely studied for many years because of their numerous applications [1–5]. However, many of their properties such as superplasticity [6, 7], transparency [8] and ionic conductivity [9, 10] are still subject to improvements. To achieve this, it is very important to have good control of the microstructure at the nanoscale level [11–13].
A fine grain size and full densification are two of the most important factors for obtaining reproducible and improved properties. One challenging goal in ceramic processing is suitability for industrial application. Many recent results on nanostructured ceramics have been achieved by producing initial nanopowders in the laboratory, however the methods used may not be suitable on a large scale. For this reason, it is very important to produce nanoceramics through the processing of commercially available zirconia powders.
Kingery and Francois [14] have proposed that during sintering the pores disappear when their coordination numbers are lower than a critical value. The idea of colloidal processing is to produce, by the achievement of an excellent dispersion, a green compact with a narrow pore size distribution. Colloidal processing can control and improve the properties of the final compact [15, 16].
Bead-milling makes it possible to obtain well-dispersed suspensions of heavily agglomerated particles that cannot be broken by normal methods such as ultrasonication [17–19]. In addition, bead-milling is a technique suitable for the large-scale production of suspensions because it can be operated as a continuous process. For these reasons, in this study we used bead-milling equipment with small beads, less than 50 μm diameter to obtain well-dispersed aqueous suspensions of zirconia nanoparticles.
Pressureless normal sintering involves heating to the sintering temperature, followed by soaking and cooling. Dense nanostructured ZrO2 ceramics can be sintered from a synthesized powder of 40 nm particle size at a low temperature of 1150 °C by this method [20, 21]. Recently, Trunec and Maca [22] also reported the low-temperature sintering of tetragonal nanosized zirconia (1100 °C) from a synthesized powder of 10 nm particle size.
Two-step sintering is an effective method of producing nanosized ceramics that was developed by Chen and Wang [23]. Using this technique, it is possible to sinter fully dense nanoceramics without the final grain growth by suppressing grain boundary migration while keeping grain boundary diffusion active. This principle is called ‘kinetic window’. In two-step sintering, it is necessary that the grains at the end of the first step have an intermediate density to render the pores unstable. The relative density obtained at the end of the first step varies in the range of 70–96% of the theoretical density (TD). The two-step sintering of zirconia samples was successfully performed by Binner et al [24], who sintered 16 nm particles at a temperature of 1050 °C during the second step. Recently, Mazaheri et al [25] reported fully dense commercial tetragonal zirconia (3YTZ) by two-step sintering at 1150 °C. In the second stage, they produced a final microstructure with an average grain size of 110 nm.
Spark plasma sintering (SPS) is an important technique for producing fully dense nanoceramics by inhibiting grain growth for a short time at low temperatures [26, 27]. The heat is supplied at a very high rate using an electric current under a desired pressure in a vacuum chamber. The spark plasma sintering of tetragonal zirconia has been widely reported, but full densification was not achieved at low temperatures in the previous studies, but we have achieved it using commercial powders, as reported in this study. Bernard-Granger and Guizard [13] sintered 3YTZ at 1200 °C to produce grains of 100 nm. Basu et al [28] used the same sintering temperature of 1200 °C for 3YTZ; and Li and Gao [29] also obtained a fully dense compact with 80 nm grains by SPS of the synthesized tetragonal zirconia at 1180 °C. In this study, we report here a lower sintering temperature with a final nanostructure of 90 nm grains obtained from a commercial starting material with a particle size of 75 nm.
The solid mixing of zirconia with yttria has been reported to be a very effective technique of forming tetragonal zirconia [30, 31]. The formed 3YTZ was reported to exhibit high sinterability with excellent mechanical properties. Ohnishi et al [31] reported that the samples prepared by powder mixing exhibit a considerable improvement in fracture toughness as a result of stress-induced transformation toughening and microcrack-toughening.
The most noteworthy achievements in this study are the colloidal processing of commercially available powders and the determination of the effect of the starting materials on sintering. In addition, we present an analysis of the optimal operational conditions of the bead-milling equipment, under which full deagglomeration and a well-dispersed ceramic slurry can be achieved. The possibility of obtaining nanostructured ceramics with different sintering techniques makes the results of this study suitable for industrial scale application.
Experimental procedure
The starting materials used were high-purity monoclinic zirconia (TZ0 Tosoh Co., Tokyo, Japan, particle size 74.8 nm, specific Brunauer–Emmett–Teller (BET) surface area 13.6 m2 g-1), commercial Y2O3 powder (NanoTek yttrium oxide, particle size 28 nm) and 3 mol.% Y2O3 stabilized tetragonal ZrO2 (3YTZ; Tosoh, particle size 65 nm, specific BET surface area 15.3 m2 g-1).
Aqueous suspensions containing 10 vol.% solid loading were prepared by mixing TZ0 with yttria (3 mol.% Y2O3 and 97 mol.% monoclinic zirconia). We also prepared a 10 vol.% suspension of 3YTZ. The powders were dispersed in distilled water with 1.3 wt.% polyelectrolyte (poly(ammonium carboxylate), ALON A-6114, Toagosei Co., Tokyo, Japan). The ceramic powders were dispersed and ultrasonicated in water using an ultrasonic stirrer (Nissei model USS-1, Nihonseiki, Japan) operated at 35 W, 40 kHz and 300 rpm for 15 min. The pH of the suspensions was adjusted to 8–8.5. Then the suspensions were exposed for 10 min to an ultrasonic homogenizer (Nissei model 1200 T, Nihonseiki), operated at 1200 W and a frequency of 19.6 kHz to redisperse the agglomerated particles. The zeta potential was studied for suspensions with 1.0 wt.% of dispersant using a laser electrophoresis analyzer (LEZA-600, Otsuka Electronics Co., Japan).
A bead mill (Ultra Apex Mill UAM-015, Kotobuki Industries Co. Ltd., Kure, Japan) was applied to redisperse the heavily agglomerated particles using ZrO2 beads with a diameter of 50 μm at a rotational speed of 4000 rpm; this resulted in well-mixed and fully dispersed suspensions. Suspensions of 3YTZ and a mixture of 97 mol.% monoclinic zirconia with 3 mol.% yttria were treated by bead-milling.
The particle size distribution was measured using a UPA-UT151 NANOTRAC® system (Nikkiso, Japan).
The suspension was evacuated in a desiccator to eliminate air bubbles and slip-cast over plaster molds. The slip-cast samples were dried and isostatically cold-pressed at 392 MPa.
Samples with a volume of 0.7–1.0 cm3 were sintered by normal sintering, two-step sintering and SPS (100kN SPS-1050, Syntex Inc., Japan). Normal sintering was performed at a heating rate of 3 °C min−1 and a holding time of 5 h at the final temperature. Two-step sintering was performed with five different sintering programs at different step temperatures TEMP 1 and TEMP 2, as shown in table 1. The temperature decrease between those two steps before the holding step is due to natural cooling. A schematic of a two-step sintering program is presented in figure 1.
Table 1.
Different two-step sintering programs performed in this study. The initial heating rate to TEMP 1 was 5 °C min−1. TD is the theoretical density.
| Program | TEMP 1 | Time 1 | TEMP 2 | Time 2 | Density | % of TD |
|---|---|---|---|---|---|---|
| (°C) | (min) | (°C) | (h) | (g cm−3) | ||
| 1 | 1300 | 1 | 1100 | 15 | 5.84 | 96.4 |
| 2 | 1300 | 1 | 1200 | 15 | 6.05 | 99.9 |
| 3 | 1200 | 1 | 1100 | 15 | 4.94 | 81.6 |
| 4 | 1400 | 1 | 1150 | 15 | 5.69 | 93.9 |
| 5 | 1400 | 1 | 1200 | 15 | 5.88 | 97.1 |
Figure 1.
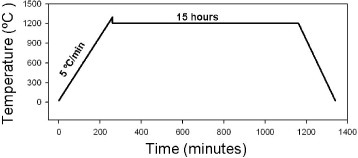
Schematic program for two-step sintering.
In SPS, owing to the good dispersion and high green density achieved in this study, we were able to sinter samples at a low temperature of 1150 °C with a heating rate of 300 °C min−1 and a pressure of 150 MPa. The holding time was 30 min to complete the transformation from a monoclinic to tetragonal structure and to achieve full densification.
The sintered density was measured by the Archimedes method using water. Results of mixed yttria and TZ0 were compared with those for commercial 3YTZ powder.
X-ray diffraction (XRD) patterns were obtained with a JDX-3500d diffractometer (JEOL, Japan) using CuKα radiation with a Ni filter and a graphite monochromator (35 kV, 300 mA).
The microstructure and the dried suspensions were observed using a field emission scanning electron microscope (FE-SEM, JSM-840F, JEOL). The grain size of the sintered samples was measured by the linear intersection method using the Scion Image Beta 4.03 program [32].
Results
Dispersion of 3 mol.% yttria and 97 mol.% monoclinic zirconia
Figure 2 shows the zeta potential curves of yttria, 3YTZ and TZ0, and a mixture of TZ0 with yttria dispersed in water by adding polyelectrolyte. The zeta potential plot shows that at a pH higher than 7, the zeta potentials are sufficiently high to maintain all the powders in suspension, including the mixed dispersed powder. However, at a pH higher than 9 the mixed powder suspension flocculates after several minutes. Thus, the optimal pH is between 8–8.5 for the preparation of this type of suspension.
Figure 2.
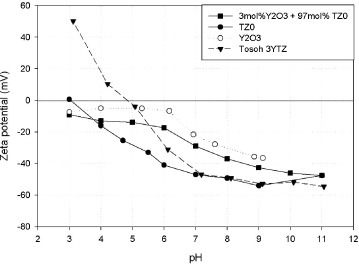
Zeta potential–pH curves of the tetragonal zirconia (Tosoh 3YTZ), monoclinic zirconia (TZ0), yttria (Y2O3) and a mixture of TZ0 and yttria with 1.0 wt.% dispersant added.
Optimal operation conditions
The bead-milling equipment can be operated at different rotation speeds and with different bead sizes. To determine the optimal conditions for dispersing a system of two components, such as yttria and zirconia, it is necessary to investigate bead-milling under different experimental conditions.
The suspension of 3 mol.% yttria with 97 mol.% zirconia was subjected to bead-milling with different bead sizes in each experiment. The results shown in figure 3 reveal that the agglomerates are more difficult to break in the case of a smaller bead size. The 50 μm beads can disperse the initially agglomerated particles most effectively. The effect of rotation speed on the dispersion of 3 mol.% yttria and 97 mol.% zirconia suspensions was then evaluated using 50 μm beads by changing the rotating speed for each suspension once it reached a constant particle size. The results are shown in figure 4. It can be seen that the average particles size decreases gradually with increasing rotational speed. At each speed, the suspension reaches a constant median particle size. At 2000 rpm, the median particle size approaches 140 nm. At 3000 rpm, the particle size remains constant at 115 nm, and at a higher rotation speed of 4000 rpm, the average particle size can be reduced to 100 nm.
Figure 3.
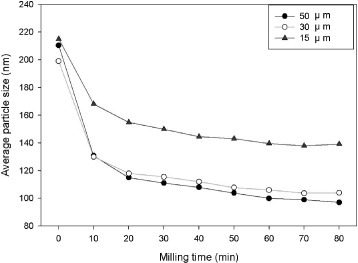
Average particle size versus milling time using different bead sizes for the suspension of 3 mol.% yttria and 97 mol.% zirconia.
Figure 4.
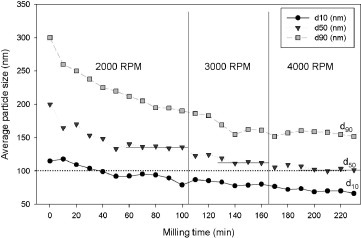
Average particle size versus milling time for the suspension of 3 mol.% yttria+97 mol.% zirconia at different rotation speeds.
Dispersion
Figure 4 also shows the evolution of the particle size distribution with milling. It can be seen that at 4000 rpm with 50 μm beads, the suspension can be considered as a fully dispersed suspension, because the final particle size (100 nm) is close to the particle size (75 nm) calculated from the BET surface area of monoclinic zirconia. In our previous study [33] it was demonstrated that all the particles started with a diameter distributed between 90 and 270 nm, and after 60 min of treatment, the final diameter was between 60 and 140 nm.
Figure 5 shows SEM images of a dried suspension of Y2O3 and TZ0 before and after bead-milling. No agglomeration was observed in the suspension after this treatment. These results demonstrate that bead-milling is a very effective technique for dispersing nanopowders. In previous papers [17–19, 34, 35] it was also reported that bead-milling enables the dispersion of primary particles. The well-dispersed suspensions were submitted to casting, followed by cold isostatic pressing, which resulted in the formation of samples with green densities of 3.60 g cm−3 (62% of TD) for the mixed powders and 3.64 g cm−3 (60% of TD) for 3YTZ. The theoretical density of 3YTZ is 6.06 g cm−3, whereas the theoretical density of the mixture was calculated from the densities of monoclinic zirconia (97 mol.%, 5.836 g cm−3 [36]) and Y2O3 (3 mol.%, 5.032 g cm−3).
Figure 5.
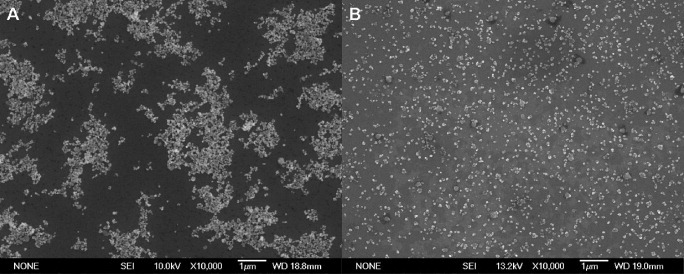
SEM micrograph of the suspension containing Y2O3 and ZrO2. Before (A) and after bead-milling (B).
Sintering
Normal sintering.
The bicomponent sample requires a step in the sintering process where the cations interdiffuse, forming a zirconia solid solution with a stable tetragonal structure. To study this process, the evolution of the (1 1 1) peak for tetragonal ZrO2 and the (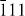 ) and (1 1 1) peaks for the monoclinic form during the sintering was examined in one of our previous studies [33]. During 3 h of soaking at 1300 °C the monoclinic phase transformed completely into the tetragonal phase. In this study, normal sintering was performed with optimum results being obtained for 5 h soaking at 1250 °C.
) and (1 1 1) peaks for the monoclinic form during the sintering was examined in one of our previous studies [33]. During 3 h of soaking at 1300 °C the monoclinic phase transformed completely into the tetragonal phase. In this study, normal sintering was performed with optimum results being obtained for 5 h soaking at 1250 °C.
Figure 6 shows the results of densification and grain growth in the normal sintering experiments with a heating rate of 3 °C min−1 and 5 h soaking time, for the mixed powder and 3YTZ samples. The 3YTZ powder has better initial sinterability up to 1200 °C (at this point the densities of the samples are nearly the same), after this, the behavior of the two samples is similar up to the final full densification. At a temperature of 1250 °C, both samples are considered fully dense (>99.5% of TD), although there is a clear difference in the average particle size; 3YTZ is 175 nm and the mixed powder is 113 nm. Figure 7(a) shows the microstructure of the mixed powder sintered by normal sintering at 1250 °C; no cracks or porosity is apparent throughout the sample.
Figure 6.
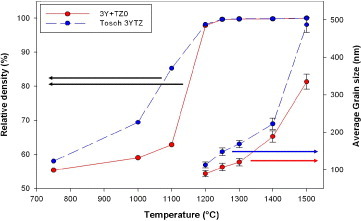
Sinterability of commercial Tosoh 3YTZ and tetragonal zirconia obtained by sintering the bicomponent mixed powder.
Figure 7.
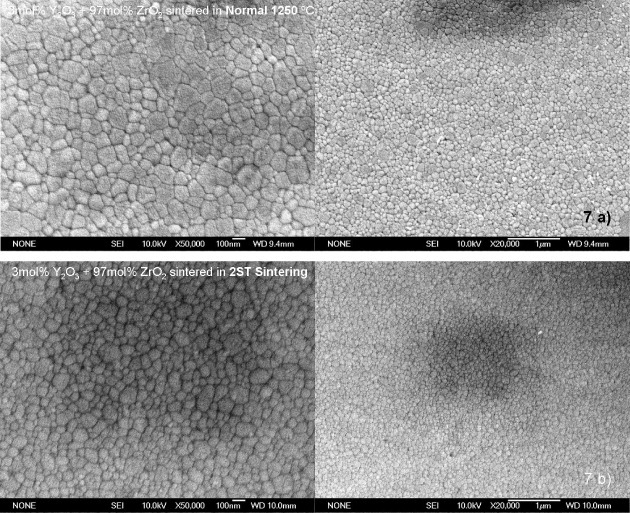
SEM micrograph of mixed powder sintered by normal sintering at 1250 °C (a) and two-step sintering (b).
Two-step sintering.
Other researchers have used different sintering schedules with different densities after the first step: Laberty-Robert et al [37] obtained 80% of TD after the first sintering step of 8YSZ, and Binner et al [24] obtained 85% of TD after the first step. In our study, after the first step at 1300 °C with no holding time, density measurements showed that 87% of TD had been achieved. We performed different two-step sintering programs, as presented in table 1, to establish the optimal two-step sintering process. The ideal conditions are those that result in full densification at a lower final temperature. Program 2 has an initial heating rate of 5 °C min−1 up to 1300 °C without soaking followed by rapid cooling to 1200 °C then soaking for 15 h.
The two-step sintering program performed in this study with the mixed powder allowed us to achieve a fully dense compact (>99.5% of TD) with a nanostructured distribution of grains. Figure 7(b) shows an SEM micrograph of the polished and thermally etched mixed powder sample, after sintering by the two-step sintering method, showing the absence of cracks or porosity. The grain size is approximately 95 nm with a very good homogeneity throughout the sample. For samples of Tosoh tetragonal zirconia sintered under the same conditions, the final microstructure has an average grain size of 125 nm.
SPS.
SPS was performed at 1150 °C with a soaking time of 30 min. This relatively long soaking time for SPS was necessary to complete the transformation from monoclinic to tetragonal zirconia. The XRD pattern of the mixed powder after sintering by SPS is that of tetragonal zirconia. Figure 8 shows the final microstructure of a fully dense (>99.5% of TD) sample sintered by this technique. The grain size remains at the nanoscale with an average grain size of 90 nm. Tosoh 3YTZ, subjected to SPS under the same conditions, also exhibited full densification, but the grain size was 115 nm.
Figure 8.

SEM micrograph of fully dense tetragonal zirconia obtained by SPS of mixed powder at 1150 °C and 150 MPa.
Discussion
Effect of dispersion on sinterability
Optimum colloidal processing enhances the sinterability of ceramics. For this purpose, the use of an optimum amount of dispersant is very important. It has been reported that for similar zirconia samples and the same dispersant, the addition of 0.65 wt.% dispersant results in optimal dispersion [38]. We used a larger amount of dispersant (1.3 wt.%) to cover whole surface area of the finer particles obtained by the bead-milling. This excess of dispersant also prevents the flocculation of the nanoparticles in the suspension, as shown in figure 5.
When slip-casting is performed in a fully dispersed suspension, the formed compacts exhibit high green density, as shown in this study. The stability of the suspension during slip-casting is very important for producing homogeneous samples. Long-term stability is very difficult to maintain in binary aqueous suspensions containing yttria [39, 40]. Yttria can dissolve in water and generate different species that can be adsorbed onto zirconia and cause flocculation. However, by controlling the pH between 8 and 8.5, before, during and after bead-milling, it is possible to obtain an equilibrium point where the suspension is stable and can be slip-cast without segregation or flocculation.
The 3YTZ sample was cast from a suspension of fully dispersed particles with an average particle size of 65 nm and a green density of 60% of TD. For mixed powder samples, the green density is 62% of TD. The suspension was a fully dispersed binary compound consisting of 97 mol.% of monoclinic zirconia (average particle size 75 nm) and 3 mol.% yttria (average particle size 28 nm). The combination of a good dispersion and fully deagglomerated particles results in the excellent packing of TZ0 with the space between the grains occupied by yttria. This enhances the green density and reduces the sintering temperature by improving the physical contact between the particles.
Effect of starting powders
One of the most important differences between the sintering of the mixed powders and the commercial Tosoh 3YTZ is that, when synthesized by the co-precipitation method, the latter forms below the martensitic phase transformation temperature. In the bicomponent sample, the tetragonal solid solution is formed at a higher temperature. Burger et al [30] demonstrated that this transformation occurs very slowly, because it depends on the diffusion rate of yttria, and the cation diffusion rate in yttria and zirconia is very low [41, 42]. The experimental results presented by Burger et al showed that the diffusion-controlled phase transformation plays an important role in the densification behavior and in the inhibition of grain growth.
Table 2 shows the average grain size after each sintering process for Tosoh 3YTZ and tetragonal zirconia prepared by solid mixing. A clear difference is observed between the sintered compacts obtained by sintering the bicomponent sample and the Tosoh tetragonal zirconia. The grain size remains small when the compact is obtained from a combination of TZ0 and yttria for all three types of sintering.
Table 2.
Grain size of samples obtained from sintering Tosoh 3YTZ and a mixture of TZ0 with 3 mol% yttria by different sintering methods.
| Sample | Average grain size (nm) | |
|---|---|---|
| Tosoh | 1250 °C | 175 |
| 2ST | 125 | |
| SPS | 115 | |
| 3Y+TZ0 | 1250 °C | 113 |
| 2ST | 95 | |
| SPS | 90 |
Figure 9 shows the grain sizes and relative densities obtained after sintering at different temperatures. In all cases, the grain size of the sintered bicomponent samples of zirconia remains smaller than that of Tosoh tetragonal powder after normal sintering. The mixed powder has smaller average grain size under all sintering conditions. Both Tosoh tetragonal zirconia and the mixed powder (ZrO2+Y2O3) produce a fully dense microstructure, indicating that the only factor causing the suppression of grain growth is the initial composition of bicomponent mixed powders.
Figure 9.
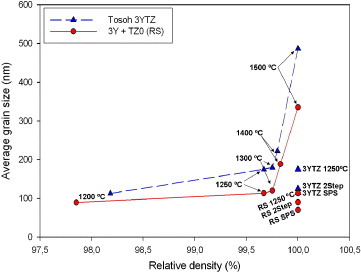
Average grain size of samples sintered at different temperatures. RS: bicomponent mixed powder of 97 mol.% TZ0 with 3 mol.% yttria; 3YTZ: Tosoh 3YTZ raw powder.
It is known that sintering causes the formation of solid bonds between particles when they are heated. The bonds reduce the surface energy by removing the free surface; this is followed by a secondary stage of grain growth due to grain boundary elimination. When sintering the mixed powders, yttria acts as a secondary phase, reducing the sintering temperature; this is an important factor in the improved sintering demonstrated by Burger et al [30]. Furthermore, during the sintering, the initial enrichment of yttria occurs in the grain boundary region with the constant interdiffusion of cations to stabilize the tetragonal phase of zirconia. The excess yttria at the grain boundary is reported to be responsible for the inhibition of grain growth [29]. Furthermore, the yttria diffusion produces a concentration gradient between the grain boundaries and the centers of grains [30]. The excess yttria and the diffusion of yttria to stabilize the tetragonal phase are responsible for retarding the secondary stage of grain growth. The fact of initially having a use of a two-phase system improves the sinterability enabling a higher density to be reached at lower temperatures with the suppression of grain growth.
Sintering
The advantages of two-step sintering are well known; it is not the aim of this study to remark this point. However, table 2 shows the advantages of using a combination of powders in the initial suspension. Normal sintering produces a large difference in the grain size; the bicomponent mixture can be sintered at the same temperature as 3YTZ with a smaller resulting grain size. This observation supports the idea that excess yttria in the grain boundary region inhibits the grain growth.
Chen and Mayo [43] reported that the rate of heating rate during the sintering of 3YTZ nanoparticles is inversely proportional to the final density. Skandan et al [44] demonstrated that the final density improved with increasing pressure. These ideas led us to decrease the heating rate as well as the sintering temperature to produce a small final grain size. SPS makes it possible to obtain nanostructured ceramics owing to the combination of fast joule heating, the thermal effect produced by the current, and the high pressure [45].
Conclusions
The effect of the starting powder composition on the sintering of tetragonal zirconia was studied. The bead-milling process was also studied and optimized. The fully dispersed and well-mixed suspensions were slip-cast. The green density was 60% of TD for Tosoh 3YTZ and 62% of TD for the bicomponent sample.
Sintering of the mixed powder sample proved to be effective for inducing the transformation from monoclinic zirconia and yttria to tetragonal zirconia and for inhibiting the grain growth stage. The optimal sintering temperature was the lowest possible temperature at which fully dense samples with nanosized grains are possible to obtain.
Low-temperature sintering and full densification were achieved by three different sintering techniques: (i) normal sintering at 1250 °C; (ii) two-step sintering with a first step at 1300 °C with no holding time and a second step at 1200 °C with 15 h holding; (iii) SPS of tetragonal zirconia at a temperature as low as 1150 °C for 30 min under a 150 MPa pressure load. The grain size for all sintering techniques was approximately 100 nm.
References
- Garvie R C, Hannink R H. and Pascoe R T. Nature. 1975;258:703. doi: 10.1038/258703a0. [DOI] [Google Scholar]
- Minh N Q. J. Am. Ceram. Soc. 1993;76:563. doi: 10.1111/j.1151-2916.1993.tb03645.x. [DOI] [Google Scholar]
- Heuer A H. and Hobbs F L W. Advances in Ceramics. vol 3. Columbus, OH: American Ceramic Society; 1981. [Google Scholar]
- Zender H H, Leistner H. and Searle H R. Interceramics. 1990;39:33. [Google Scholar]
- Wu X, Wu X, Liang Q, Fan J, Weng D, Xie Z. and Wei S. Solid State Sci. 2007;9:636. doi: 10.1016/j.solidstatesciences.2007.04.016. [DOI] [Google Scholar]
- Sakka Y, Suzuki T S, Morita K, Nakano K. and Hiraga K. Scr. Mater. 2001;44:2075. doi: 10.1016/S1359-6462(01)00889-2. [DOI] [Google Scholar]
- Hiraga K, Kim B-N, Morita K, Yoshida H, Suzuki T S. and Sakka Y. Sci. Technol. Adv. Mater. 2007;8:578. doi: 10.1016/j.stam.2007.09.006. [DOI] [Google Scholar]
- Anselmi-Tamburini U, Woolman J N. and Munir Z A. Adv. Funct. Mater. 2007;17:3267. doi: 10.1002/adfm.200600959. [DOI] [Google Scholar]
- Bernard-Granger G, Guizard C, Surble S, Baldinozzi G. and Addad A. Acta Mater. 2008;56:4658. doi: 10.1016/j.actamat.2008.05.031. [DOI] [Google Scholar]
- Omata T, Goto Y. and Otsuka-Yao-Matsuo S. Sci. Technol. Adv. Mater. 2007;8:524. doi: 10.1016/j.stam.2007.09.001. [DOI] [Google Scholar]
- Sakka Y, Hiraga K, Tanaka K, Ishigaki T. and Hirosaki H. Sci. Technol. Adv. Mater. 2007;8:571. doi: 10.1016/j.stam.2007.09.007. [DOI] [Google Scholar]
- Lu K. Int. Mater. Rev. 2008;53:21. doi: 10.1179/174328008X254358. [DOI] [Google Scholar]
- Bernard-Granger G. and Guizard C. Acta Mater. 2008;55:3493. doi: 10.1016/j.actamat.2007.01.048. [DOI] [Google Scholar]
- Kingery W D. and Francois B. In: Sintering and Related Phenomena. Kuczynski G C, , Hooton N A, Gibbon C F, , editors. New York: Gordon and Breach; 1967. p. p 471. [Google Scholar]
- Sakka Y. J. Ceram. Soc. Japan. 2006;114:371. doi: 10.2109/jcersj.114.371. [DOI] [Google Scholar]
- Sakka Y. and Hiraga K. Nippon Kagaku Kaishi. 1999;8:2489. [Google Scholar]
- Choi J, Hahn B-D, Park D-S, Yoon W-H, Lee J-H, Jang J-H, Ko K-H. and Park C. J. Am. Ceram. Soc. 2007;90:388. doi: 10.1111/j.1551-2916.2006.01400.x. [DOI] [Google Scholar]
- Qiu J-Y, Hotta Y, Watari K, Mitsuishi K. and Yamazaki M. J. Eur. Ceram. Soc. 2006;26:385. doi: 10.1016/j.jeurceramsoc.2005.06.016. [DOI] [Google Scholar]
- Sakka Y, Suzuki T S. and Uchikoshi T. J. Eur. Ceram. Soc. 2008;28:935. doi: 10.1016/j.jeurceramsoc.2007.09.039. [DOI] [Google Scholar]
- Vasylkiv O. and Sakka Y. J. Am. Ceram. Soc. 2001;84:2489. doi: 10.1111/j.1151-2916.2001.tb01041.x. [DOI] [Google Scholar]
- Vasylkiv O, Sakka Y. and Skorokhod V. J. Am. Ceram. Soc. 2003;86:299. [Google Scholar]
- Trunec M. and Maca K. J. Am. Ceram. Soc. 2007;90:2735. doi: 10.1111/j.1551-2916.2007.01781.x. [DOI] [Google Scholar]
- Chen W. and Wang X H. Nature. 2000;404:168. doi: 10.1038/35004548. [DOI] [PubMed] [Google Scholar]
- Binner J, Annapoorani K, Paul A, Santacruz I. and Vaidhyanathan B. J. Eur. Ceram. Soc. 2008;28:973. doi: 10.1016/j.jeurceramsoc.2007.09.002. [DOI] [Google Scholar]
- Mazaheri M, Simchi A. and Golestani-Fard F. J. Eur. Ceram. Soc. 2008;28:2933. doi: 10.1016/j.jeurceramsoc.2008.04.030. [DOI] [Google Scholar]
- Orru R, Licheri R, Locci A M, Cincotti A and Cao G. 2009. Mater. Sci. Eng. R 63 127 10.1016/j.mser.2008.09.003 [DOI] [Google Scholar]
- Maizza G, Grasso S, Sakka Y, Noda T. and Ohashi S. Sci. Technol. Adv. Mater. 2007;8:644. doi: 10.1016/j.stam.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu B, Lee J-H. and Kim D-Y. J. Am. Ceram. Soc. 2004;87:1771. doi: 10.1111/j.1551-2916.2004.00317.x. [DOI] [Google Scholar]
- Li W. and Gao L. J. Eur. Ceram. Soc. 2000;20:2441. doi: 10.1016/S0955-2219(00)00152-7. [DOI] [Google Scholar]
- Burger W, Richter H G, Piconi C, Vatteroni R, Cittadini A. and Boccalari M. J. Mater. Sci: Mater. Med. 1997;8:113. doi: 10.1023/A:1018562917779. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Naka H, Sekino T, Ikuhara Y. and Nihara K. J. Ceram. Soc. Japan. 2008;116:1270. doi: 10.2109/jcersj2.116.1270. [DOI] [Google Scholar]
- Suárez G, Albano M P, Garrido L B. and Aglietti E F. Ceram. Int. 2007;33:925. doi: 10.1016/j.ceramint.2006.02.003. [DOI] [Google Scholar]
- Suárez G, Sakka Y, Suzuki T S, Uchikoshi T and Aglietti E F. 2009. Mater. Res. Bull. doi: 10.1016/j.materresbull.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez G, Sakka Y, Suzuki T S, Uchikoshi T. and Aglietti E F. J. Ceram. Soc. Japan. 2009;117:470. doi: 10.2109/jcersj2.117.470. [DOI] [Google Scholar]
- Osawa E. Diam. Relat. Mater. 2007;16:2018. doi: 10.1016/j.diamond.2007.08.008. [DOI] [Google Scholar]
- Adam J. and Rogers M D. Acta Crystallogr. 1959;12:951. doi: 10.1107/S0365110X59002742. [DOI] [Google Scholar]
- Laberty-Robert C, Ansart F, Deloget C, Gaudon M. and Rousset A. Ceram. Int. 2003;29:151. doi: 10.1016/S0272-8842(02)00099-8. [DOI] [Google Scholar]
- Uchikoshi T, Sakka Y, Ozawa K. and Hiraga K. J. Eur. Ceram. Soc. 1998;18:669. doi: 10.1016/S0955-2219(97)00171-4. [DOI] [Google Scholar]
- Yaserbi M, Zimek-Moroz M, Kemp W. and Sturgis D H. J. Am. Ceram. Soc. 1996;79:1223. doi: 10.1111/j.1151-2916.1996.tb08576.x. [DOI] [Google Scholar]
- Stubican V S, Hink R C. and Ray S P. J. Am. Ceram. Soc. 1978;61:112. doi: 10.1111/j.1151-2916.1978.tb09220.x. [DOI] [Google Scholar]
- Sakka Y, Oishi Y, Ando K. and Morita S. J. Am. Ceram. Soc. 1991;74:2610. doi: 10.1111/j.1151-2916.1991.tb06808.x. [DOI] [Google Scholar]
- Sakka Y, Oishi Y, Ando K. and Masuda M. J. Am. Ceram. Soc. 1989;72:2121. doi: 10.1111/j.1151-2916.1989.tb06042.x. [DOI] [Google Scholar]
- Chen D J. and Mayo M J. J. Am. Ceram. Soc. 1996;79:906. doi: 10.1111/j.1151-2916.1996.tb08524.x. [DOI] [Google Scholar]
- Skandan G, Hahn H, Kear B H, Roddy M. and Cannon W R. Mater. Lett. 1994;20:305. doi: 10.1016/0167-577X(94)90035-3. [DOI] [Google Scholar]
- Munir Z A, Anselmi-Tamburini U. and Ohyanagi M. J. Mater. Sci. 2006;41:763. doi: 10.1007/s10853-006-6555-2. [DOI] [Google Scholar]


