Abstract
Little information is available on chemical forms of heavy metals on integrate plant roots. KNO3 (1 M), 0.05M EDTA at pH6 and 0.01 M HCl were used sequentially to extract the exchangeable, complexed and precipitated forms of Cu(II) and Cd(II) from soybean roots and then to investigate chemical form distribution of Cu(II) and Cd(II) on soybean roots. Cu(II) and Cd(II) adsorbed on soybean roots were mainly exchangeable form, followed by complexed form, while their precipitated forms were very low under acidic conditions. Soybean roots had a higher adsorption affinity to Cu(II) than Cd(II), leading to higher toxic of Cu(II) than Cd(II). An increase in solution pH increased negative charge on soybean and thus increased exchangeable Cu(II) and Cd(II) on the roots. Ca2+, Mg2+ and NH4+ reduced exchangeable Cu(II) and Cd(II) levels on soybean roots and these cations showed greater effects on Cd(II) than Cu(II) due to greater adsorption affinity of the roots to Cu(II) than Cd(II). L-malic and citric acids decreased exchangeable and complexed Cu(II) on soybean roots. In conclusion, Cu(II) and Cd(II) mainly existed as exchangeable and complexed forms on soybean roots. Ca2+ and Mg2+ cations and citric and L-malic acids can potentially alleviate Cu(II) and Cd(II) toxicity to plants.
Industrial activities and agricultural practices, such as the application of chemical fertilizers, liming materials, manure, sewage sludge and composts, lead to soil pollution from heavy metals. Accumulation of heavy metals in soils can cause soil degradation and decreased crop yields, and produce long-term risks to ecosystems and human health1. However, the mobility and bioavailability of heavy metals are known to be closely related to the processes of adsorption and desorption of metals by and from soil constituents. Extensive investigations have been conducted into the adsorption of heavy metals by soils and soil constituents such as clay minerals2,3,4, iron and aluminum oxides5, and organic matter6,7. Electrostatic adsorption, specific adsorption and surface precipitation were main mechanisms for the adsorption of heavy metals by soils8,9. Neutral salt such as KNO3 was used to extract the heavy metals adsorbed by soils electrostatically through cation exchange reaction10. Neutral salt extractants were also used to estimate the phytoavailable trace metals in soils11. Meanwhile, EDTA was used to extract the heavy metals adsorbed specifically by soil organic amendments and crop straw biochars12,13. However, it is difficult to distinguish the specific adsorption of heavy metals from their precipitation in soils quantitatively at present.
Heavy metals in soils reach the root surfaces of plants through the root/soil interface and are then adsorbed and absorbed by plant roots14. The root/soil interface therefore presents a barrier to the movement of heavy metals from the soil into plant tissue. There were some investigations involved in the adsorption of heavy metals of Cu(II), Pb(II), Cd(II), Ni(II), Zn(II) and Cr(III) by fine power of plant root materials15,16,17,18. In these investigations, plant roots were used as bio-adsorbents to remove the heavy metals from wastewater and natural water. Cation exchange and complex formation with functional groups on plant roots were main mechanisms for biosorption of heavy metals by plant root materials16,17. There were extensive investigations on the adsorption of heavy metals by cell walls and cell plasma membranes of plant roots as related to rhizotoxicity and uptake of heavy metals by plant roots19,20,21,22,23,24,25. It was found that electric potential induced by surface charge on plasma membranes was one of main factors determining ion activities of heavy metals on plasma membranes and their toxicity to plants20,21,22,23. However, the investigations on the adsorption of heavy metals by entire plant roots as related to their toxicity and uptake to and by plants were rarely found in literature26,27. The relative abundance of the various chemical forms of heavy metal cations on plant roots affects the toxicity of metals to plants and the uptake of these metals by plant roots. While, few studies have investigated this topic. The mechanisms by which heavy metals are adsorbed by plant roots and the relative abundance of the chemical forms of adsorbed heavy metals on the roots need to be better understood.
Cu(II) is mainly adsorbed by soils and soil components specifically28, while Cd(II) is adsorbed mainly through electrostatic mechanisms and is found in the exchangeable form on soil particles29. The different chemical forms of heavy metals exhibit differing bioavailability30. Similarly, Nishizono et al.31 found that the cell walls of plant roots showed a higher affinity to Cu2+ than that to Zn2+ and Cd2+ 31. The cations of the various heavy metals may also be adsorbed by plant roots through different mechanisms. Cu(II) and Cd(II) were selected for this study to examine the relative abundance of their various chemical forms on soybean roots after adsorption.
Carboxyl and phenolic groups are commonly found on plant roots32. The deprotonation of these acidic groups gives plant roots a negative charge and enables roots to adsorb heavy metals through electrostatic interaction. In addition, these acidic groups have a strong complexing ability and thus can adsorb heavy metals specifically through the formation of surface complexes with metal cations on the plant root surfaces33. The degree of dissociation of weak acids increases with increasing system pH, so that pH not only affects the charge characteristics of a root surface, but can also affect the hydrolysis of heavy metal cations in solution, thereby affecting their reaction with functional groups on the root surface.
Environmental stress causes plant roots to actively or passively release soluble organic acids into the rhizosphere. Organic acids contain a large quantity of carboxyl functional groups and form soluble complexes with heavy metal cations34. Consequently, organic acids may affect adsorption and combinations of heavy metals form on the root surface. Exogenous cations of Ca2+, Mg2+ and NH4+ effectively inhibit absorption of Mn(II) by barley and thus reduce Mn(II) toxicity to the plant35. Cations such as Ca2+, Mg2+ and NH4+ compete with Al3+ for adsorption sites and inhibit adsorption of Al3+ by the roots of soybean and maize33. Exogenous cations may also inhibit the adsorption of heavy metal cations by plant roots and affect the relative abundance of the chemical forms of heavy metals on plant roots. However, the effects of cations and organic acids on the relative abundance of the chemical forms of heavy metals on plant roots requires further research.
The objectives of this study were: (1) to investigate the relative abundance of the various chemical forms of Cu(II) and Cd(II) on soybean roots after adsorption by the roots; and (2) to examine the effects of exogenous cations and organic acids on the relative abundance of the chemical forms of Cu(II) and Cd(II) on soybean roots. The findings should improve our understanding of the chemical behaviors of these heavy metals at the root/soil interface, and suggest means of reducing the toxicity of heavy metals to plants.
Neutral salts were previously used to extract exchangeable heavy metals adsorbed on soils and exchangeable Al from plant roots10,33. EDTA was used to extract heavy metal cations adsorbed specifically by soil organic matter and crop straw biochars in previous studies12,13. The mechanism for specific adsorption of Cu(II) and Cd(II) on soybean roots was formation of surface complexes of the metals with functional groups on the roots, similar to the specific adsorption of heavy metals by organic matter and biochars. HCl was used to extract the precipitates of Al hydroxides from plant roots in previous studies33,36. Cu(II) and Cd(II) mainly formed the precipitates of hydroxides on soybean roots in present study. Therefore, the neutral salt of KNO3, EDTA, and HCl were used sequentially to extract the exchangeable, complexed and precipitated forms of Cu(II) and Cd(II) from soybean roots in this study.
Results and Discussion
The relative abundance of the chemical forms of Cu(II) and Cd(II) on soybean roots
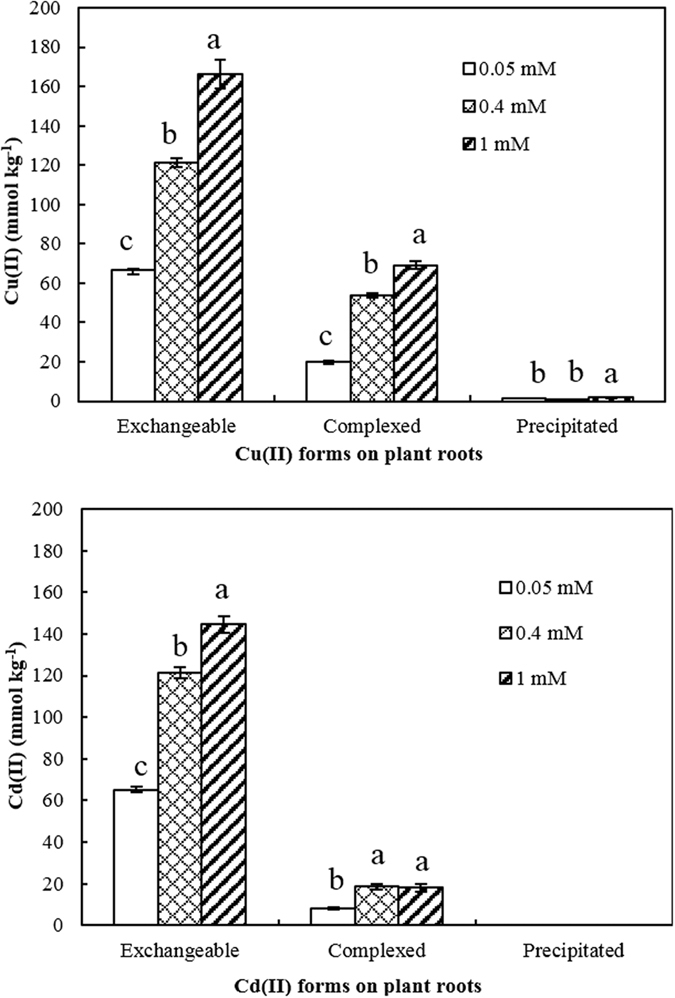
Cu(II) and Cd(II) adsorbed onto soybean roots could be differentiated into exchangeable, complexed, and precipitated forms. Under strong acidic conditions of pH 4.2, Cu(II) and Cd(II) was mainly found on soybean roots in the exchangeable forms, followed by complexed forms, while the amounts of precipitated Cu(II) and Cd(II) were very low (Fig. 1). For example, when the initial concentration of Cu(II) and Cd(II) was 0.4 mM, the exchangeable Cu(II) and Cd(II) accounted for 68.9% and 86.8% of total adsorbed Cu(II) and Cd(II), and the complexed Cu(II) and Cd(II) accounted for 30.5% and 13.2% of total adsorbed Cu(II) and Cd(II), respectively. Both the exchangeable and complexed Cu(II) increased significantly with increasing initial Cu(II) concentration (P < 0.05). Similarly, the exchangeable Cd(II) was significantly increased (P < 0.05) as the initial concentration of Cd(II) rose. The complexed Cd(II) increased significantly as its initial concentration increased from 0.05 mM to 0.4 mM (P < 0.05), while the increase was not significant when the initial concentration increased from 0.4 mM to 1.0 mM. Comparing the two heavy metals, the amount of exchangeable Cu(II) was almost the same as that of the exchangeable Cd(II) at initial concentrations of 0.05 and 0.4 mM. However, the amount of complexed Cu(II) was much higher than that of complexed Cd(II) (Fig. 1). For example, at initial concentrations of 0.05, 0.4 and 1.0 mM, the amount of exchangeable Cu was 1.02, 0.99, and 1.15 times the content of exchangeable Cd(II) respectively, while the content of complexed Cu(II) was 2.43, 2.89, 3.82 times the content of complexed Cd(II), respectively. These results suggest that the adsorption affinity of soybean roots to Cu(II) was much greater than to Cd(II), leading to the higher specific adsorption capacity of the roots to Cu(II) than to Cd(II). This resembles the Cu(II) and Cd(II) adsorption capacity of soil organic matter and crop straw biochars13,37, caused by the stronger complexing ability of organic functional groups with Cu(II) than with Cd(II)38.
Figure 1. Effects of the initial concentration of Cu(II) and Cd(II) on their adsorption and chemical forms on 14-day-old roots of soybean at pH 4.2.
The effect of Cu(II) and Cd(II) on the zeta potential of soybean roots provides evidence for the differences between the formation of surface complexes of Cu(II) and Cd(II) with organic functional groups on soybean roots. The zeta potential of soybean roots was negative (Fig. 2), suggesting that soybean roots carry a negative charge. The adsorption of Cu(II) and Cd(II) by soybean roots reduced the negativity of the zeta potential, suggesting that surface complexes of Cu2+ and Cd2+ with functional groups form on the root surfaces. This resembles the effect of the formation of surface complexes of Cu2+ with surface functional groups on the zeta potential observed on crop straw biochars39. The adsorption of Cu(II) led to a greater change in zeta potential of soybean roots than did the adsorption of Cd(II) (Fig. 2), suggesting that a greater number of Cu(II) complexes formed than those of Cd(II) on soybean roots. This was consistent with the relative abundance of complexed Cu(II) and Cd(II) on soybean roots (Fig. 1).
Figure 2. Zeta potential of 14-day-old soybean roots with and without adsorbed Cu(II) and Cd(II).
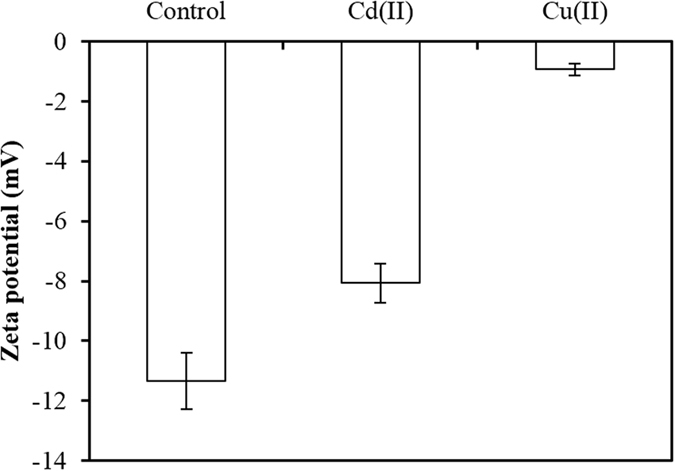
Zeta potential was measured using the streaming potential method at pH 4.2 and NaCl was used as the electrolyte solution.
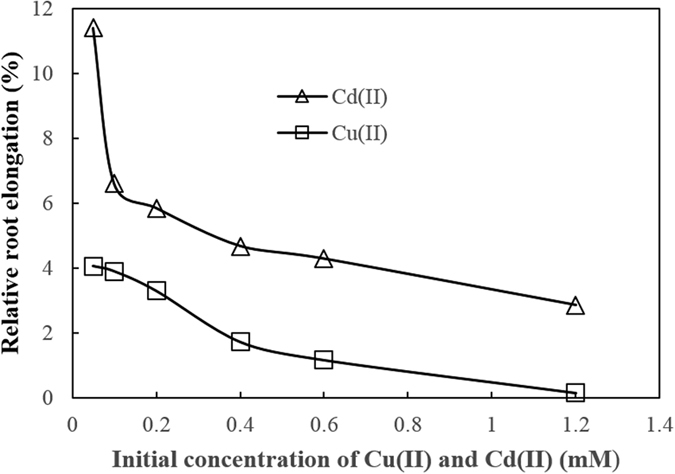
The abundance of the chemical forms of Cu(II) and Cd(II) on soybean roots may be related to their toxicities to the plant. The effects of different concentrations of Cu(II) and Cd(II) on soybean root elongation were compared and the results are shown in Fig. 3. Root elongation was significantly inhibited with the increasing concentrations of these heavy metals, suggesting that increasing amounts of exchangeable and complexed Cu(II) and Cd(II) on soybean roots increased their toxicities to the plant and the resulting inhibition of soybean root growth. The inhibiting effect of Cu(II) on root elongation was greater than that of Cd(II) at the same concentration, suggesting that Cu(II) was more toxic to the plant than Cd(II). This was consistent with previous observations by other researchers40. The amount of exchangeable Cu(II) on soybean roots was almost the same as exchangeable Cd(II), but complexed Cu(II) was much more abundant than complexed Cd(II), which may account for the greater toxicity of Cu(II) to soybean than Cd(II).
Figure 3. Effects of Cu(II) and Cd(II) on the elongation of soybean roots in solution culture conditions.
Effect of pH on the relative abundance of the chemical forms of Cu(II) and Cd(II)
As the pH increased, the amount of exchangeable Cu(II) on soybean roots increased significantly (P < 0.05) (Fig. 4), suggesting that the increased negative charge on the roots induced by increasing pH led to an increase in exchangeable Cu(II). The amount of complexed Cu(II) also increased with increasing pH, but the increase was not significant (Fig. 4). Increasing pH enhanced dissociation of acidic functional groups on the roots and hence the complexing ability of the functional groups with Cu(II). The amount of exchangeable Cd(II) also increased with increasing pH, although not to a significant degree. The increase in pH did not affect the complexed Cd(II) on the roots. The effect of pH on Cu(II) adsorption by soybean roots was greater than on Cd(II) adsorption, suggesting that Cu(II) adsorption was more sensitive to pH change than Cd(II) adsorption.
Figure 4. Effect of solution pH on the adsorption of Cu(II) and Cd(II) and their chemical forms on 14-day-old roots of soybean (Initial concentration of Cu(II) and Cd(II) was 0.4 mM).
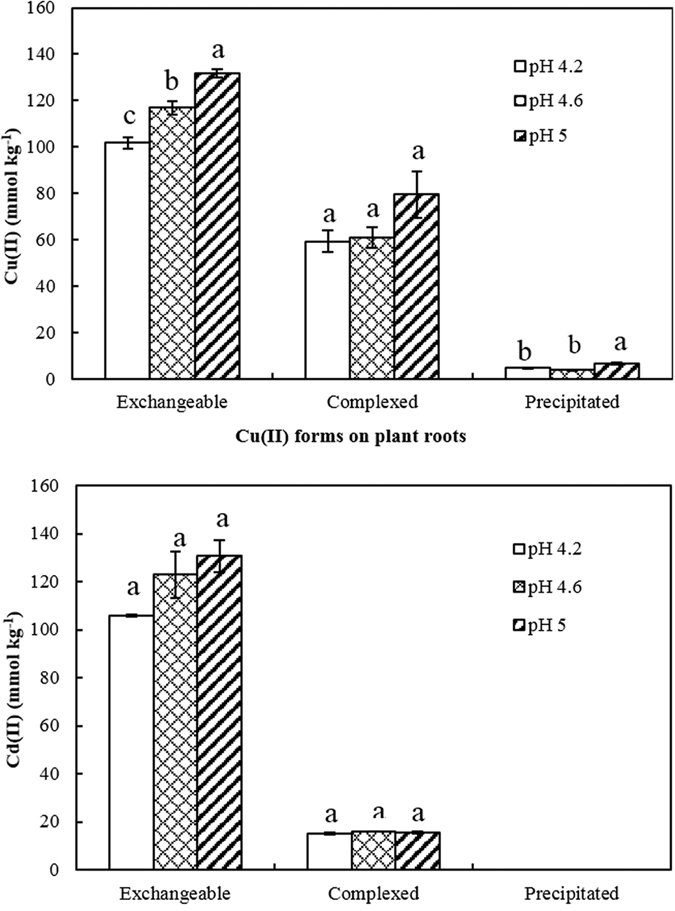
Figure 5 indicates that the deprotonation of the acidic functional groups on soybean roots increased with increasing pH, increasing the negative charge on the roots and resulting in increased negative zeta potential of the roots. This provides evidence for the increases of exchangeable Cu(II) and Cd(II) on soybean roots with increasing pH, as shown in Fig. 4.
Figure 5. Zeta potential of 14-day-old soybean roots measured using the streaming potential method at various pH levels.
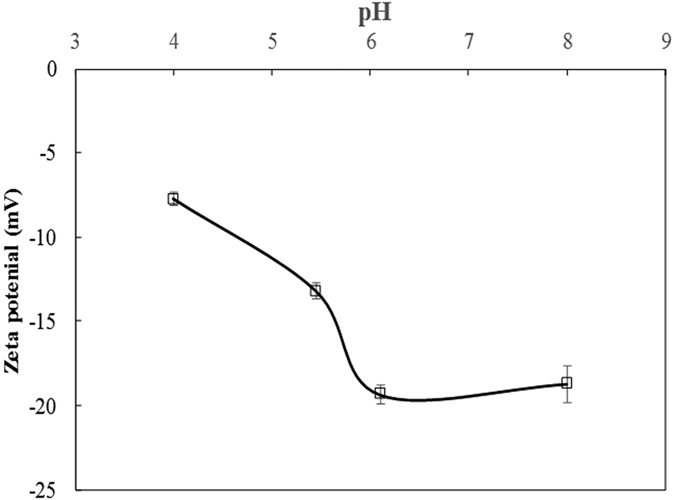
Figure 4 also shows a greater amount of precipitated Cu(II) on soybean roots than precipitated Cd(II), due to the greater hydrolyzing ability of Cu2+ compared with Cd2+. The hydrolysis constant (pK) is an important parameter in characterizing the hydrolyzing ability of heavy metal cations. Smaller pK values indicate stronger hydrolyzing potential. The pK values of Cu2+ and Cd2+ reported in the literature are 6.5 and 9.7, respectively41, indicating that the hydrolyzing ability of Cu2+ is greater than Cd2+. This accounts for the greater amount of precipitated Cu(II) at pH 5 than at pH 4.2 and 4.6.
Cation effects on the relative abundance of the chemical forms of Cu(II) and Cd(II)
The addition of exogenous Ca2+, Mg2+ and NH4+ significantly reduced the exchangeable Cu(II) on soybean roots by 13.2%, 8.8% and 11.0 %, respectively, compared with the control (Fig. 6), but the decrease in exchangeable Cu(II) was not statistically significant. Ca2+ and Mg2+ significantly reduced the exchangeable Cd(II) on soybean roots by 76.1% and 51.1% (P < 0.05). NH4+ decreased the exchangeable Cd(II) by 17.7% compared with the control, but this decrease was not significant. Cations of Ca2+, Mg2+ and NH4+ decreased exchangeable Cu(II) and Cd(II) on soybean roots through competition with Cu(II) and Cd(II) for exchangeable sites on negatively charged roots. The presence of these cations led to a greater decrease in exchangeable Cd(II) than exchangeable Cu(II) due to the greater adsorption affinity of the roots to Cu(II) than Cd(II), as mentioned above. Of the three cations, Ca2+ and Mg2+ had a much greater effect on exchangeable Cd(II) than did NH4+ due to the higher competitive ability for adsorption sites of the bivalent cations of Ca2+ and Mg2+ compared with the monovalent cation NH4+. Ca2+, Mg2+ and NH4+ significantly reduced the amount of complexed Cu(II) by 23.5%, 18.4% and 24.3%, respectively, compared with the control (P < 0.05). Ca2+ significantly reduced the amount of complexed Cd(II), while Mg2+ and NH4+ had no significant effect compared with the control (Fig. 6).
Figure 6. Effects of cations on adsorption of Cu(II) and Cd(II) and their chemical forms on 14-day-old roots of soybean at pH 4.2.
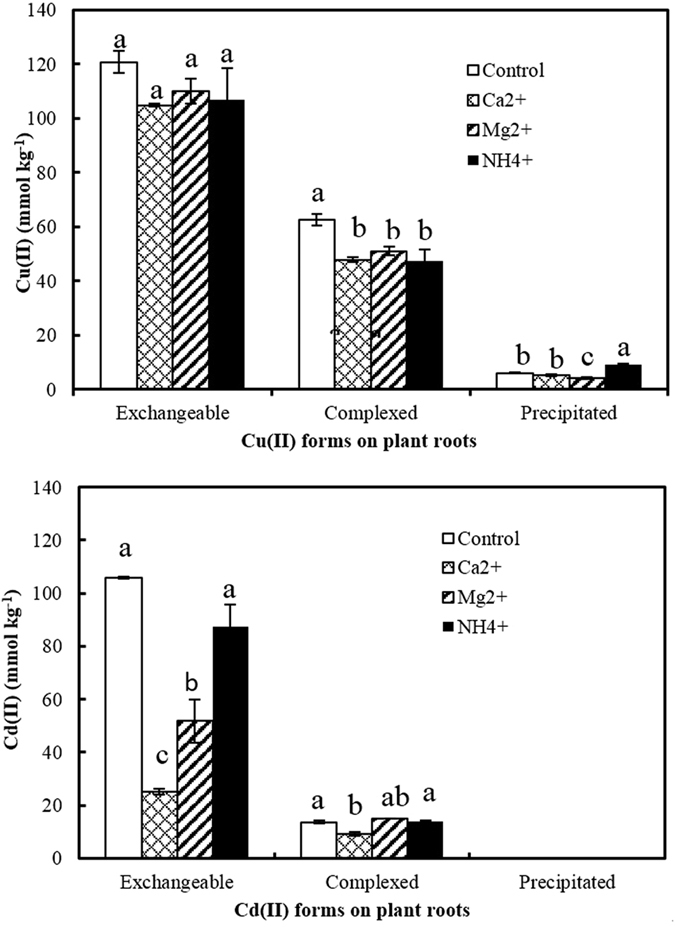
The presence of cations of Ca2+, Mg2+ and NH4+ inhibited the adsorption of Cu(II) and Cd(II) by soybean roots and may therefore decrease the toxicities of these metals to the plant and their absorption by soybean roots. The observations of Hardiman et al.42 support this conclusion42. They found that the presence of base cations inhibited the uptake of Cd(II) by soybean roots and the effects of these cations were ranked: Ca2+ > Mg2+ > K+ > Na+.
Effects of organic acids on the relative abundance of the chemical forms of Cu(II) and Cd(II)
The effects of low-molecular-weight (LMW) organic acids on the relative abundance of the chemical forms of Cu(II) and Cd(II) are shown in Fig. 7. The presence of L-malic and citric acids significantly decreased the amounts of exchangeable and complexed Cu(II) on soybean roots compared with the control (P < 0.05). Lactic acid also reduced the amounts of exchangeable and complexed Cu(II) on soybean roots, but the decrease in exchangeable and complexed Cu(II) was not significant. The three organic acids also decreased the amount of precipitated Cu(II) on soybean roots (Fig. 7). During adsorption experiments of Cu(II) and Cd(II), the solution pH was kept constant at 4.2. Therefore, the effects of these organic acids on the relative abundance of the chemical forms of Cu(II) and Cd(II) on soybean roots were mainly attributed to their complexation with both metal cations in solutions. The stability constants (logK) for complexes of citric, L-malic and lactic acids with Cu2+ are 5.9, 3.33 and 3.02, respectively43,44. Compared with lactic acid, the stronger complexing ability of citric and L-malic acids led to higher levels of Cu(II) remaining in the nutrient solution through the formation of more complexes with Cu(II) and thus increased their inhibitions on Cu(II) adsorption by soybean roots. The formation of complexes between the organic acids and Cu2+ also inhibited the precipitation of Cu(II) on soybean roots (Fig. 7).
Figure 7. Effects of organic acids on adsorption of Cu(II) and Cd(II) and their chemical forms on 14-day-old roots of soybean at pH 4.2.
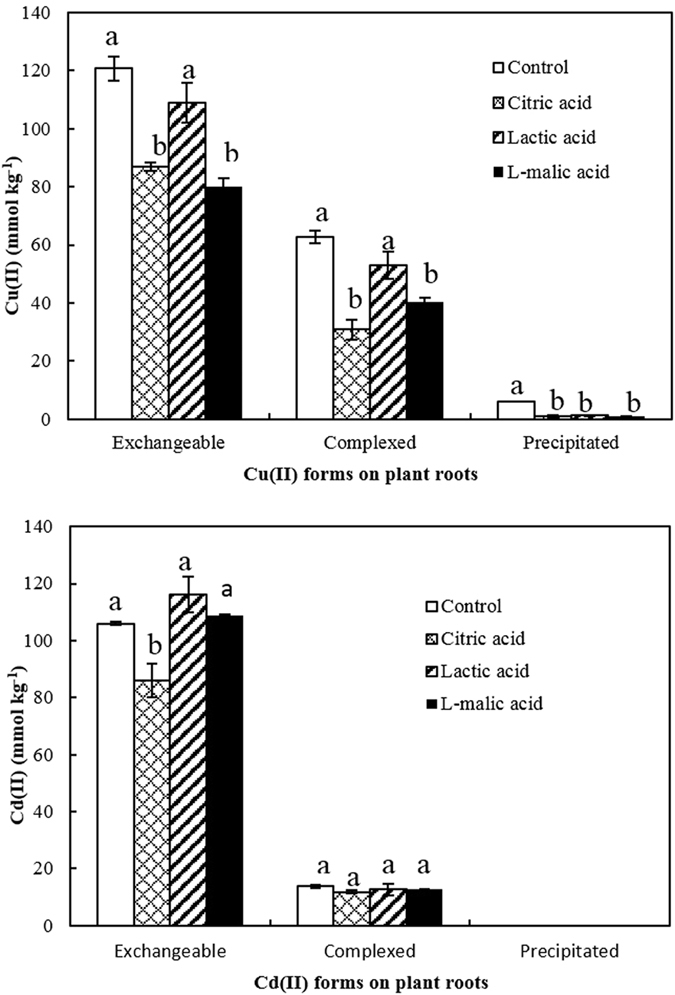
The presence of citric acid significantly decreased the amount of exchangeable Cd(II) (P < 0.05), but did not reduce the amount of complexed Cd(II) significantly, compared with the control (Fig. 7). Both L-malic and lactic acids did not significantly decrease the amounts of exchangeable and complexed Cd(II) on soybean roots. These organic acids showed less effect on the adsorption and relative abundance of the chemical forms of Cd(II) compared with Cu(II), due to their relative lower complexing ability with Cd(II) than with Cu(II). The stability constants (logK) for complexes of citric, L-malic and lactic acids with Cd2+ are 3.15, 1.34 and 1.07, respectively43,45, much lower than these for the complexes with Cu2+. The logK of citric acid with Cd2+ is greater than those of L-malic and lactic acids with Cd2+, and is responsible for the significant decrease in exchangeable Cd(II) caused by citric acid. LMW organic acids arise mainly from the exudates of plant roots and the various stages of decomposition of plant residues in soils34. These organic acids can form complexes with heavy metal cations in soil solutions and thus inhibit the adsorption of heavy metals onto plant roots, which may also decrease the availability and toxic effect of these metals on plants, especially in the case of Cu(II) due to the greater logK of organic acid complexes with Cu2+.
Conclusions
Cu(II) and Cd(II) adsorbed on soybean roots mainly occurred in the exchangeable forms, followed by complexed forms, while the precipitated forms occurred at low levels under acidic conditions. Soybean roots had a higher adsorption affinity to Cu(II) than Cd(II), leading to greater toxicity of Cu(II) to the plant than Cd(II). The formation of complexes of Cu2+ and Cd2+ with functional groups on soybean roots reduced the negative level of the zeta potentials and the adsorption of Cu(II) showed a greater effect on the zeta potential of soybean roots than did the adsorption of Cd(II). The increase in solution pH increased the negative charge on soybean roots and thus increased the amounts of exchangeable Cu(II) and Cd(II) on the roots. Cu(II) adsorption by soybean roots was more affected by pH than was Cd(II) adsorption. The presence of Ca2+, Mg2+ and NH4+ decreased the amounts of exchangeable Cu(II) and Cd(II) on soybean roots through competition for exchangeable sites on negatively charged roots with Cu2+ and Cd2+ and the cations showed greater effects on Cd(II) than Cu(II) due to the greater adsorption affinity of the roots to Cu(II) than Cd(II). Ca2+ showed the greatest effect on exchangeable Cd(II), followed by Mg2+, while NH4+ showed the least effect on exchangeable Cd(II). The presence of L-malic and citric acid decreased the amounts of exchangeable and complexed Cu(II) on soybean roots. Citric acid decreased the amount of exchangeable Cd(II) on soybean roots. In summary, the cations Ca2+ and Mg2+ and the LMW organic acids of citric and L-malic acids can decrease the availability of the heavy metals Cu(II) and Cd(II) and potentially alleviate their toxicity to plants.
The differences in adsorption affinity of soybean roots to Cu(II) and Cd(II) led to the differences in the relative abundance of the chemical forms of both metals and thus affected their toxicity to the plant. The exogenous cations of Ca2+, Mg2+ and NH4+ and organic acids of citric, L-malic and lactic acids decreased the exchangeable and complexed forms of Cu(II) and Cd(II) on soybean roots to various extents, mainly depending on the competing ability of these cations for adsorption sites with the heavy metals and the complexing ability of these organic acids with Cu2+ and Cd2+. These findings are of fundamental significance in understanding the chemical behaviors of heavy metals at the root/soil interface, and suggest means of alleviating the toxicity of heavy metals to plants.
Materials and Methods
Plant materials and cultivation
Soybean (Glycine max cv. Xudou 14) was used in this study. The soybean seeds were surface-sterilized with 10% H2O2 for 10 min, washed with deionized water three times, soaked for 4 h in deionized water, and then germinated in sterilized sand in the dark. From the fourth day, the plant seedlings were cultivated in a nutrient solution (initial pH 4.2) and grown in a controlled environment growth chamber at a temperature of 27 °C, a day-length of 14 h, a light intensity of 375 μmol photonm−2 s−1, and a relative humidity of 70%. The composition of the nutrient solution was as follows: (mM) 2.0 (NH4)2SO4, 12.0 NaNO3, 1.0 NaH2PO4·2H2O, 3.0 K2SO4·5H2O, 2.0 MgSO4·2H2O, 4.0 CaCl2; (μM) 9.15 MnCl2·4H2O, 0.32 CuSO4·5H2O, 0.77 ZnSO4·7H2O, 0.02 (NH4)6Mo7O24·4H2O, 46.26 H3BO3 and 20.0 FeSO4·7H2O-EDTA. The nutrient solution was changed every two days. Fourteen-day-old roots were used for the Cu(II) and Cd(II) adsorption experiments.
Cu(II) and Cd(II) adsorption/desorption experiments with entire roots
Before the adsorption experiments, the plant roots were placed in deionized water for 1 h to remove excess nutrient ions from the root surfaces. The plant roots were then separated from the stems, washed with deionized water three times, and then carefully blotted dry. About 8 g of fresh roots were placed in a 300-mesh nylon bag (preliminary experiments showed that the nylon bag did not adsorb Cu(II) and Cd(II)), then immersed in 1-L Cu(II) and Cd(II) solutions and magnetically stirred. After 2 h, the bag containing the plant roots was removed from the adsorption solution and washed with distilled water three times, and any excess water was removed with filter paper. Then the roots were sequentially placed in 1 L 1 M KNO3, 0.05 M EDTA at pH 6, and 0.01 M HCl for 1 h each to extract the exchangeable, complexed, and precipitated Cu(II) and Cd(II) from the roots. After each extraction, the roots were washed with distilled water three times and the excess water was blotted off in preparation for the next extraction.
The exchangeable heavy metals were absorbed by the soybean roots by electrostatic attraction and can be exchanged with cations of indifferent electrolytes. High concentration KCl has previously been used to extract exchangeable heavy metals from soils46 and from plant roots. The complexed heavy metals comprised the binding state of the metals through the formation of surface complexes with functional groups on the plant roots. The precipitated heavy metals bound to the roots as hydroxyl polymers or oxides formed on the root surfaces. The concentrations of Cu(II) and Cd(II) in the extractants were determined by means of atomic adsorption spectroscopy.
To investigate the effect of the initial concentrations of Cu(II) and Cd(II) on their adsorption and the relative abundance of their chemical forms, 0.05, 0.4 and 1.0 mM of Cu(II) and Cd(II) solutions were used. Similar experimental procedures were then conducted. The solution was kept at pH 4.2 throughout the experiment.
To investigate the effect of pH on the adsorption of Cu(II) and Cd(II) and the relative abundance of their chemical forms, the solution was kept at pH 4.2, 4.6 and 5.0. The initial concentration of Cu(II) and Cd(II) was 0.4 mM. Similar experimental procedures were then conducted.
To investigate the effects of cations on the adsorption of Cu(II) and Cd(II) and the relative abundance of their chemical forms, we prepared solutions containing mixtures of 0.4 mM of Cu(II) or Cd(II) and 1 mM each of Ca2+ (CaCl2), Mg2+ (MgCl2) or NH4+(NH4Cl) and carried out similar experimental procedures. The solution pH was kept at 4.2 throughout the experiment.
The effect of organic acid on the adsorption of Cu(II) and Cd(II) and the relative abundance of their chemical forms was investigated by preparing mixed solutions containing 0.4 mM of Cu(II) or Cd(II) and 1 mM each of L-malic acid, lactic acid or citric acid and carrying out similar experimental procedures. The solution pH was kept at 4.2 throughout the experiment.
The determination of the zeta potential of the root surface
About 10 g of fresh roots were reacted with 0.4 mM solutions of Cu(II) and Cd(II) for 2 h, at a constant solution pH of 4.2. After adsorption of Cu(II) and Cd(II), the roots were washed with distilled water three times and then air-dried. A streaming potential apparatus was used to measure the zeta potential of soybean roots with and without adsorbed Cu(II) and Cd(II)47. The air-dried soybean roots were cut to a length of 2 cm, and 0.25 g of the roots were horizontally loaded into a sample cell. NaCl (1.5 L, electrolyte solution) with a conductivity of 80 μS cm−1 and pH 4.2 was put into an electrolyte container and then flowed through the soybean roots for 40 min using a magnetic pump as a driver. The used electrolyte solution was then discarded, another 1.5 L of the electrolyte solution was put into the electrolyte container and flowed through the soybean roots for 10 min until equilibrium was reached, after which the flow rate of electrolyte was increased stepwise by adjusting the valve and the streaming potential ΔE and pressure difference ΔP were recorded. The zeta potential of the soybean roots was calculated using the Helmholtz–Smoluchowski equation.
Root elongation measurement
The roots of 4-day-old seedlings were exposed for 48 h to 0.5 mM CaCl2 solutions with 0, 10, 20, 40, 100, 200, 1000 μM of Cu(NO3)2 and Cd(NO3)2 at pH 4.2. There were 10 roots in each treatment and their lengths were measured before and after exposure to Cu(II) and Cd(II), and the average lengths were recorded to calculate the relative root elongation (RRE):
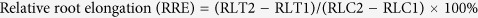 |
Where RLC1 is the initial root length of untreated controls; RLC2 is the final root length of untreated controls after 48 h (control); RLT1 is the initial root length before exposure; RLT2 is the final root length after exposure to Cu(II) and Cd(II) for 48 h.
Statistical analysis
SPSS 20.0 was used for statistical analysis and data processing. A one-way analysis of variance was undertaken for all experiments to identify significant differences between treatments.
Additional Information
How to cite this article: Zhou, Q. et al. Relative abundance of chemical forms of Cu(II) and Cd(II) on soybean roots as influenced by pH, cations and organic acids. Sci. Rep. 6, 36373; doi: 10.1038/srep36373 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (41230855).
Footnotes
Author Contributions R.K.X. and Q.Z. designed the experiments. Q.Z., Z.D.L. and Y.L. performed the experiments. Q.Z. drafted the manuscript. R.K.X. and J.J. revised the manuscript.
References
- Chary N., Kamala C. & Raj D. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotox. Environ. Safe. 69, 513–524 (2008). [DOI] [PubMed] [Google Scholar]
- Spark K. M., Wells J. D. & Johnson B. B. Characterizing trace metal adsorption on kaolinite. Eur. J. Soil Sci. 46, 633–640 (1995). [Google Scholar]
- Al-Qunaibit M. H., Mekhemer W. K. & Zaghloul A. A. The adsorption of Cu(II) ions on bentonite–a kinetic study. J. Colloid Interface Sci. 283, 316–321 (2005). [DOI] [PubMed] [Google Scholar]
- Wang S., Dong Y., He M., Chen L. & Yu X. J. Characterization of GMZ bentonite and its application in the adsorption of Pb(II) from aqueous solutions. Appl. Clay Sci. 43, 164–171 (2009). [Google Scholar]
- Stahl R. S. & James B. R. Zinc sorption by manganese-oxide-coated sand as a function of pH. Soil Sci. Soc. Am. J. 55, 1291–1294 (1991). [Google Scholar]
- Covelo E. F., Vega F. A. & Andrade M. L. Competitive sorption and desorption of heavy metals by individual soil components. J. Hazard. Mater. 140, 308–315 (2007). [DOI] [PubMed] [Google Scholar]
- Gerritse R. G. & Vanel W. The relationship between adsorption of trace metals, organic matter, and pH in temperate soils. J. Environ. Qual. 13, 197–204 (1984). [Google Scholar]
- Xu R. K. Interaction between heavy metals and variable charge surfaces In Molecular environmental soil science (eds Xu J. M. & Sparks D. L.) 193–228 (Springer, 2013). [Google Scholar]
- Sparks D. L. Advances in coupling of kinetics and molecular scale tools to shed light on soil biogeochemical processes. Plant Soil 387, 1–19 (2015) [Google Scholar]
- Xu R. K., Xiao S. C., Zhao A. Z. & Ji G. L. Effect of Cr(VI) on adsorption and desorption behavior of Cu(II) in the colloidal systems of two authentic variable charge soils. J. Colloid Interface Sci. 284, 22–29 (2005). [DOI] [PubMed] [Google Scholar]
- Menzies N. W., Nonn M. J. & Kopittke P. M. Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environ. Pollut. 145, 121–130 (2007) [DOI] [PubMed] [Google Scholar]
- Santos S. et al. Influence of different organic amendments on the potential availability of metals from soil: A study on metal fractionation and extraction kinetics by EDTA. Chemosphere 78, 389–396 (2010) [DOI] [PubMed] [Google Scholar]
- Xu R. K. & Zhao A. Z. Effect of biochars on adsorption of Cu(II), Pb(II) and Cd(II) by three variable charge soils from southern China. Environ. Sci. Pollut. Res. 20, 8491–8501 (2013). [DOI] [PubMed] [Google Scholar]
- Toppi L. S. D. & Gabbrielli R. Response to cadmium in higher plants. Environ. Exp. Bot. 41, 105–130 (1999). [Google Scholar]
- Araú G. C. L., Lemos S. G., Ferreira A. G., Freitas H. & Nogueira A. R. A. Effect of pre-treatment and supporting media on Ni(II), Cu(II), Al(III) and Fe(III) sorption by plant root material. Chemosphere 68, 537–545 (2007). [DOI] [PubMed] [Google Scholar]
- Zheng J. C. et al. Removal of Cu(II) in aqueous media by biosorption using water hyacinth roots as a biosorbent material. J. Hazard. Mater. 171, 780–785 (2009). [DOI] [PubMed] [Google Scholar]
- Li X. S., Liu S. L., Na Z. Y., Lu D. N. & Liu Z. Adsorption, concentration, and recovery of aqueous heavy metal ions with the root powder of Eichhornia crassipes. Ecol. Eng. 60, 160–166 (2013). [Google Scholar]
- Jorgetto A. O. et al. Cassava root husks powder as green adsorbent for the removal of Cu(II) from natural river water. Appl. Surf. Sci. 288, 356–362 (2014). [Google Scholar]
- Vulkan R., Yermiyahu U., Mingelgrin U., Rytwo G. & Kinraide T. B. Sorption of copper and zinc to the plasma membrane of wheat root. J. Membrane Biol. 202, 97–104 (2004). [DOI] [PubMed] [Google Scholar]
- Kopittke P. M., McKenna B. A., Blamey F. P. C., Wehr J. B. & Menzies N. W. Metal-induced cell rupture in elongating roots is associated with metal ion binding strengths. Plant Soil 322, 303–315 (2009). [Google Scholar]
- Wang P., Zhou D. M., Peijnenburg W. J. G. M., Li L. Z. & Weng N. Y. Evaluating mechanisms for plant-ion (Ca2+, Cu2+, Cd2+ or Ni2+) interactions and their effectiveness on rhizotoxicity. Plant Soil 334, 277–288 (2010). [Google Scholar]
- Kopittke P. M., Blamey F. P. C., McKenna B. A., Wang P. & Menzies N. W. Toxicity of metals to roots of cowpea in relation to their binding strength. Environ. Toxicol. Chem. 30, 1827–1833 (2011). [DOI] [PubMed] [Google Scholar]
- Wang Y. M., Kinraide T. B., Wang P., Zhou D. M. & Hao X. Z. Modeling rhizotoxicity and uptake of Zn and Co singly and in binary mixture in wheat in terms of the cell membrane surface electrical potential. Environ. Sci. Technol. 47, 2831–2838 (2013). [DOI] [PubMed] [Google Scholar]
- Guigues S. et al. Isolated cell walls exhibit cation binding properties distinct from those of plant roots. Plant Soil 381, 367–379 (2014). [Google Scholar]
- Keller C. et al. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM Cu. Planta 241, 847–860 (2015). [DOI] [PubMed] [Google Scholar]
- Kalis E. J. J., Temminghoff E. J. M., Town R. M., Unsworth E. R. & van Riemsdijk W. H. Relationship between metal speciation in soil solution and metal adsorption at the root surface of ryegrass. J. Environ. Qual. 37, 2221–2231 (2008). [DOI] [PubMed] [Google Scholar]
- Duffner A., Hoffland E., Weng L. P. & van der Zee S. E. A. T. M. Predicting zinc bioavailability to wheat improves by integrating pH dependent nonlinear root surface adsorption. Plant Soil 373, 919–930 (2013). [Google Scholar]
- Mclaren R. G. & Crawford D. V. Studies on soil copper. J. Soil Sci. 25, 443–452 (1974). [Google Scholar]
- Hickey M. G. & Kittrick J. A. Chemical partitioning of cadmium, copper, nickel and zinc in soils and sediments containing high levels of heavy metals. J. Environ. Qual. 13, 372–376 (1984). [Google Scholar]
- Krishnamurti G. S. R., Huang P. M., van Rees K. C. J., Kozak L. M. & Rostad H. P. M. A new soil test method for the determination of plant-available cadmium in soils. Commun. Soil Sci. Plant Anal. 26, 2857–2867 (1995). [Google Scholar]
- Nishizono H., Ichikawa H., Suziki S. & Ishii F. The role of the root cell wall in the heavy metal tolerance of Athyrium-yokoscense. Plant Soil 101, 15–20 (1987). [Google Scholar]
- Meychik N. R., Nikolaeva J. I. & Yermakov I. P. Ion exchange properties of the root cell walls isolated from the halophyte plants (Suaeda altissima L.) grown under conditions of different salinity. Plant Soil 277, 163–174 (2005). [Google Scholar]
- Liu Y. & Xu R. K. The forms and distribution of aluminum adsorbed onto maize and soybean roots. J. Soils Sediments 15, 491–502 (2015). [Google Scholar]
- Jones D. L. Organic acids in the rhizosphere-a critical review. Plant Soil 205, 25–44 (1998). [Google Scholar]
- Vlamis J. & Williams D. E. Ion competition in manganese uptake by barley plants. Plant Physiol. 37, 650–655 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim A., Luster J., Brunner I., Frey B. & Frossard E. Effects of aluminium treatment on Norway spruce roots: Aluminium binding forms, element distribution, and release of organic substances. Plant Soil 216, 103–116 (2000). [Google Scholar]
- Zhong K., Xu R. K., Zhao A. Z., Jiang J. & Li H. Adsorption and desorption of Cu(II) and Cd(II) in the tropical soils during pedogenesis in the basalt from Hainan, China. Carbonate. Evaporite. 25, 27–34 (2010). [Google Scholar]
- McBride M., Sauve S. & Hendershot W. Solubility control of Cu, Zn, Cd and Pb in contaminated soils. Eur. J. Soil Sci. 48, 337–346 (1997). [Google Scholar]
- Tong X. J., Li J. Y., Yuan J. H. & Xu R. K. Adsorption of Cu(II) by biochars generated from crop straws. Chem. Eng. J. 172, 828–834 (2011). [Google Scholar]
- Soudek P. et al. Effect of heavy metals on inhibition of root elongation in 23 cultivars of flax (Linum usitatissimum L.). Arch. Environ. Contam. Toxicol. 59, 194–203 (2010). [DOI] [PubMed] [Google Scholar]
- Wen Y. K. & Shao J. Ionic polarization and metal ion hydrolysis regularity. Chin. Sci. Bull. 22, 267–268 (1977). [Google Scholar]
- Hardiman R. T. & Jacoby B. Absorption and translocation of Cd in bush beans (Phaseolus Vulgaris). Physiol. Plant. 61, 670–674 (2006). [Google Scholar]
- Martell A. E. & Smith R. M. Critical stability constants (Plenum Press, 1989). [Google Scholar]
- Bastug A. S., Gokturk S. & Sismanoglu T. 1:1 Binary complexes of citric acid with some metal ions: Stability and thermodynamic parameters. Rev. Inorg. Chem. 27, 53–65 (2007). [Google Scholar]
- Wang J., Evangelou B. P., Nielsen M. T. & Qagner G. J. Computer-simulated evaluation of possible mechanisms for quenching heavy metal ion activity in plant vacuoles: I. Cadmium. Plant Physiol. 97, 1154–1160 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. J., Dise N. B. & Gowing D. J. Regional trends in soil acidification and exchangeable metal concentrations in relation to acid deposition rates. Environ. Pollut. 157, 313–319 (2009). [DOI] [PubMed] [Google Scholar]
- Li Z. Y., Liu Y., Zheng Y. Y. & Xu R. K. Zeta potential at the root surfaces of rice characterized by streaming potential measurements. Plant Soil 386, 237–250 (2015). [Google Scholar]


