Abstract
In this study, a novel artificial small bone consisting of ZrO2-biphasic calcium phosphate/polymethylmethacrylate-polycaprolactone-hydroxyapatite (ZrO2-BCP/PMMA-PCL-HAp) was fabricated using a combination of sponge replica and electrospinning methods. To mimic the cancellous bone, the ZrO2/BCP scaffold was composed of three layers, ZrO2, ZrO2/BCP and BCP, fabricated by the sponge replica method. The PMMA-PCL fibers loaded with HAp powder were wrapped around the ZrO2/BCP scaffold using the electrospinning process. To imitate the Haversian canal region of the bone, HAp-loaded PMMA-PCL fibers were wrapped around a steel wire of 0.3 mm diameter. As a result, the bundles of fiber wrapped around the wires imitated the osteon structure of the cortical bone. Finally, the ZrO2/BCP scaffold was surrounded by HAp-loaded PMMA-PCL composite bundles. After removal of the steel wires, the ZrO2/BCP scaffold and bundles of HAp-loaded PMMA-PCL formed an interconnected structure resembling the human bone. Its diameter, compressive strength and porosity were approximately 12 mm, 5 MPa and 70%, respectively, and the viability of MG-63 osteoblast-like cells was determined to be over 90% by the MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay. This artificial bone shows excellent cytocompatibility and is a promising bone regeneration material.
Keywords: artificial bone, sponge replica, electrospinning, ZrO2-BCP/PMMA-PCL-HAp
Introduction
The rapid ageing of the population makes bone loss and fracture a major worldwide problem and stimulates bone regeneration research. On the basis of microstructure, human bones are classified into cancellous and cortical bones. The cancellous bones contain numerous interconnected pores and therefore have low strength and are often referred to as sponge bones [1]. In contrast, the cortical bones are strong owing to their low porosity and laminated fibrous osteon structure [2]. They consist of many fibrous osteons and Haversian canals with diameters of 100–500 μm and ∼50 μm, respectively [1, 3, 4]. In addition, the Volkmann's canal is connected to the Haversian canals through blood vessels and nerves. Through the Volkmann's canal, nutrients from the blood can be transferred to the Haversian canals. Nutrients also move to the cancellous and cortical bones through the periosteum. Bone formation and remodeling occur continuously, through interactions between human osteoblast/osteoclast cells and nutrient deposition [5].
Calcium phosphate based ceramics such as hydroxyapatite (HAp, Ca10(PO4)6(OH)2), tricalcium phosphate (TCP, Ca3(PO4)2) and biphasic calcium phosphate (BCP, a mixture of HAp and TCP) are common bone-replacement materials owing to their excellent biocompatibility and osteoconductivity. They are often reinforced by zirconia (ZrO2), which has good mechanical properties and biocompatibility [6].
Polymer-based biomaterials have also been widely used owing to their biodegradable and biocompatible properties. One such example is polymethylmethacrylate (PMMA), which was combined with other polymers to enhance the mechanical strength and biocompatibility of biomaterials [7, 8]. Another widely used biocompatible polymer is polycaprolactone (PCL) [9, 10]. Low mechanical strength is the main drawback of using biopolymers for hard tissue applications such as bone regeneration.
Many methods have been developed to fabricate bone substitute. The sponge replica method has been widely used for bone scaffolds because it can produce a highly porous interconnected structure that mimics the cancellous bone [11, 12]. Electrospinning is an alternative technique that can be used to produce fibrous scaffolds with a structure similar to that of the extracellular matrix [13, 14]. These scaffolds have a porous structure and the diameter of the constituting fibers can be controlled from the nano- to micrometer scale.
The purpose of this work was to investigate the microstructure of a novel artificial small bone that was fabricated using a combination of the sponge replica and electrospinning methods to imitate the human bone.
Materials and methods
Materials
The ZrO2/BCP scaffold was fabricated using tetragonal ZrO2 (3 mol% Y2O3, TZ-3Y, Tosoh, Japan), polyvinylbutyral (PVB, Acros, USA) and polyurethane sponge (60ppi, HD sponge, Korea). HAp and BCP powders were synthesized by the microwave method described in [15].
The starting materials for electrospinning were PMMA (LG chemical, Korea) and PCL (Sigma, USA) dissolved in nitromethane (Duksan Pure Chemicals, Korea) and acetone (SK Chemicals, Korea), respectively.
Fabrication of ZrO2/BCP scaffold using the sponge replica method
To mimic cancellous bones, the polyurethane sponges were immersed in a ZrO2 slurry that contained 5 wt% PVB and 10 vol% ZrO2 powder. After green ZrO2 scaffolds have formed, they were sintered in a microwave furnace (UMF-01, 2.45 GHz, Unicera, Korea) at 1500 °C for 10 min at a heating rate of 100 °C min−1. The ZrO2 scaffolds were immersed in the BCP/ZrO2 slurry to fabricate ZrO2/BCP-ZrO2 scaffolds. Finally, the ZrO2/BCP-ZrO2 scaffolds were again immersed in the BCP slurry and sintered under the same conditions used for the ZrO2 scaffolds to obtain a three-layer ZrO2/BCP-ZrO2/BCP structure [16].
Electrospinning PMMA-PCL-HAp fiber
The Haversian and osteon structure of cortical bone was imitated using the electrospinning technique as follows. PMMA (20% w/v) and PCL (12% w/v) were dissolved in their respective solvents by ultrasonication. After mixing the PMMA and PCL solutions, HAp powder (30 wt%) was added to it. The fibers composed of HAp and PMMA:PCL at 1:1 ratio were wrapped around a steel wire with a diameter of 0.3 mm. The ZrO2/BCP scaffold was also wrapped with the electrospun PMMA-PCL-HAp fibers, which contained a 3:7 ratio of PMMA:PCL. Electrospinning was carried out at a voltage of 25 kV and a solution flow rate of 1 ml min−1.
Combination of mimicked cancellous bone and cortical bone
The ZrO2/BCP scaffold was first wrapped with the PMMA-PCL-HAp composite fibers using the electrospinning method. The wires that were used to mimic the osteon structure were individually wrapped with PMMA-PCL-HAp, bundled, and again wrapped with electrospun PMMA-PCL-HAp fibers. The osteon-like structure was gathered to surround the coated ZrO2/BCP scaffold and was electrospun with the PMMA-PCL-HAp fibers to create the final scaffold.
Characterization of the artificial bone
The microstructure and composition were studied by scanning electron microscopy (SEM, JEOL, JSM-6701F, Japan) and energy dispersive spectroscopy (EDS). The composites were also analyzed by Fourier-transform infrared spectroscopy (FTIR, Spectrum GX, PerkinElmer, USA). The mechanical property and porosity were measured using a universal testing machine (Unitech, R&B, Korea) and a mercury porosimeter (PoreMaster, Quantachrome Instrument, FL, USA).
Cytotoxicity test
Osteoblast-like MG-63 cells were obtained from the Korea Cell Line Bank and used to investigate the cytocompatibility of the artificial bone. The cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM: Hyclone, USA) with 10% fetal bovine serum and 1% penicillin/streptomycin antibiotics. The extraction media were obtained by immersing the specimens in DMEM at 37 °C for 24 h. MG-63 cells were seeded in a 96-well culture plate at a density of 1000 cells per well and then incubated at 37 °C for 24 h. Then, the cell culture media were replaced with various concentrations of extraction media (12.5, 25, 50 and 100%) and incubated for an additional 72 h. Furthermore, MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) was added to the media. After 4 h incubation, dimethyl sulfoxide was added after the removal of the media and the absorbance was measured with a microplate reader (Infinite F50, Tecan, Switzerland).
Results
Figure 1 shows a photograph and SEM images of the ZrO2/BCP scaffold, which was produced by the sponge replica method to mimic the cancellous bone structure. As shown in figures 1(a) and (b), the ZrO2/BCP scaffold had a cylindrical shape with a diameter of ∼4 mm and an interconnected porous structure similar to that of the cancellous bone. Figures 1(c) and (d) show surface and cross-sectional SEM images of the ZrO2/BCP scaffold, respectively. Numerous pores can be seen on the surface. The cross-sectional image reveals three layers that were identified by EDS as ZrO2, ZrO2/BCP and BCP (see figure 1(e1, e2, e3)).
Figure 1.
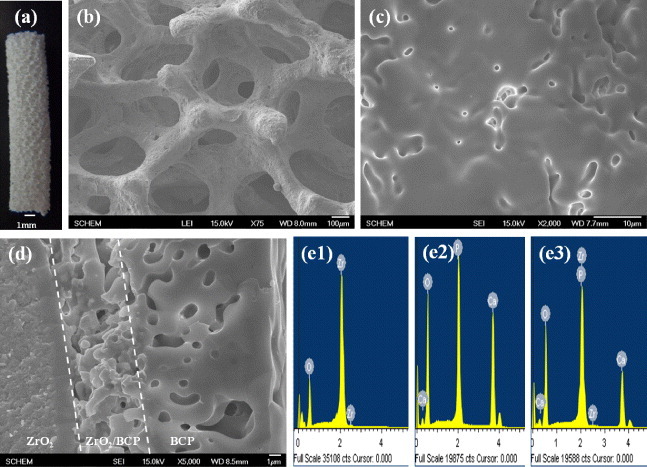
Photograph (a) and SEM images of the surface (b, c) and cross section (d) of the ZrO2/BCP scaffold. Also shown are EDS spectra of the three layers in (d), namely, ZrO2 (e1), ZrO2/BCP (e2) and BCP (e3).
Figure 2 shows an SEM image of the ZrO2/BCP scaffold wrapped with HAp-loaded PMMA-PCL fibers, which have a diameter of about 2 μm.
Figure 2.
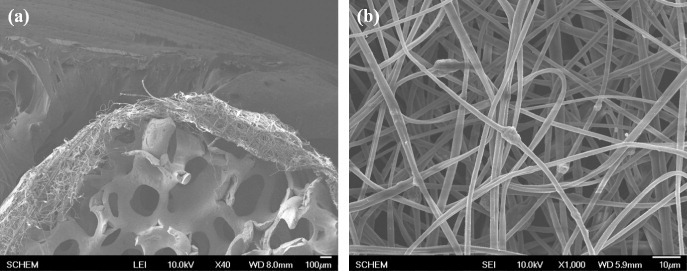
SEM images of the ZrO2/BCP scaffold wrapped with PMMA-PCL-HAp fibers (a) and of the fibers themselves (b).
Figure 3 shows XRD profiles of the ZrO2/BCP scaffold (a) and HAp-loaded PMMA-PCL (b), together with the raw materials. HAp, α-TCP and tetragonal ZrO2 phases were detected in the XRD profile of the ZrO2/BCP scaffold. However, β-TCP peaks that appeared in the raw BCP powder were not detected in the composite owing to the phase transformation that occurred at a high sintering temperature. On the other hand, the pattern of the HAp-loaded PMMA-PCL was consistent with a combination of the individual PMMA, PCL and HAp contributions, as shown in figure 3(b).
Figure 3.
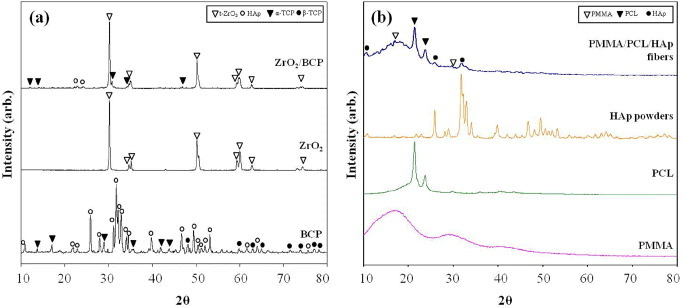
XRD profiles of the ZrO2/BCP scaffold (a) and PMMA-PCL-HAp fibers (b).
The incorporation of the HAp powder to the fibers was verified in the FTIR spectrum (figure 4). The spectra of PMMA-PCL fibers contained both the PMMA and PCL peaks (figures 4(b)-(d)). Furthermore, the PMMA-PCL-HAp fibers contained an HAp peak at 563 cm−1 (figure 4(a)) [17].
Figure 4.

FTIR spectra of electrospun mats: PMMA-PCL-HAp (a), PMMA-PCL (b), PCL (c) and PMMA (d).
By the electrospinning method, steel wires of 0.3 mm diameter were wrapped with PMMA-PCL-HAp fibers as indicated in figure 5(e: step 2). Eight PMMA-PCL-HAp—wrapped steel wires were then tightly wrapped with the PMMA-PCL-HAp fibers, where the PMMA:PCL ratio was 3:7. Finally, a bundle of wires of about 2.5 mm diameter was obtained, which mimicked the osteon structure (figures 5(b) and (e: step 3)). This bundle was attached to the ceramic scaffold that was wrapped with PMMA-PCL-HAp fibers (figure 5(a)). The eight-wire bundles were surrounded with the ZrO2/BCP scaffold, which mimicked the sponge bone and osteon structures as shown in figure 5(b). To stabilize this structure, the PMMA-PCL-HAp fibers were wrapped again using the electrospinning process (figures 5(c, e: step 4)). The steel wires were then removed to create hollow structures similar to the Haversian canal. The diameter of the final scaffold was approximately 12 mm (figures 5(d, e: step 5)).
Figure 5.
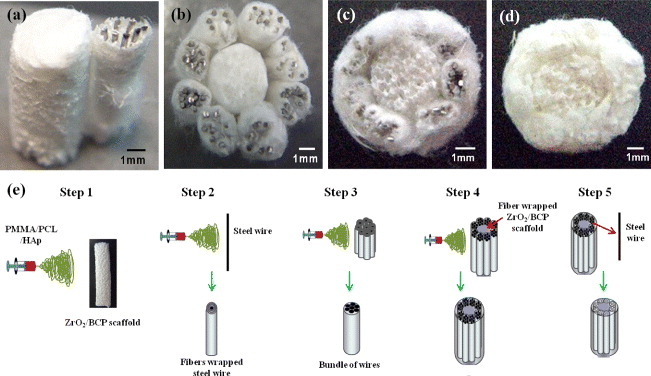
Photographs (a–d) and schematic production diagram (e) of artificial bone: (a) A bundle of wires attached to the ZrO2/BCP scaffold, (b) ZrO2/BCP scaffold surrounded by bundles, (c) bundles surrounded by PCL/PMMA/HAp, (d) after the steel wires were removed.
Figure 6 shows the compressive strength and porosity of the ZrO2/BCP scaffold and ZrO2-BCP/PMMA-PCL-HAp artificial bone. Both the compressive strength and porosity of the scaffold increased after wrapping with the PMMA-PCL-HAp fiber; the increased porosity could be related to the surrounding fiber bundles.
Figure 6.
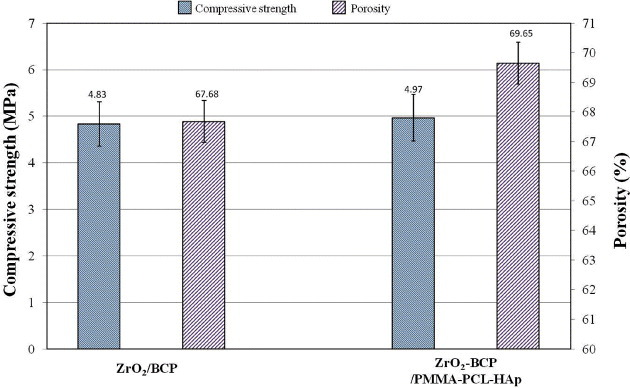
Compressive strength and porosity of the ZrO2/BCP scaffold and ZrO2-BCP/PMMA-PCL-HAp artificial bone.
Cell viability on the ZrO2/BCP scaffold and ZrO2-BCP/PMMA-PCL-HAp artificial bones was measured using the MTT assay. MG-63 osteoblast-like cells demonstrated superior viability when cultured in extraction media obtained from the two specimens. Even though the ZrO2-BCP/PMMA-PCL-HAp composite contained various materials, it showed a similar MG-63 cell viability (90%) as the control sample.
Discussion
Biomimetic approaches to making artificial implants have attracted much attention recently. They usually use porous materials to aid the healing, but often ignore the complex architecture of the natural bone. The healing process depends heavily on the natural regeneration process, which is mediated by osteoblast and osteoclast cells and on their interactions with the implant material. A suitable preform that mimics the architecture and regeneration ability of the natural bone may be a more progressive approach to bone healing.
The sponge replica method produces an interconnected porous morphology that mimics the cancellous bone architecture. However, the mechanical strength of the resulting scaffold is too low for hard bone applications. Thus, in this work, we used the ZrO2/BCP scaffold having a three-layer ZrO2/BCP-ZrO2/BCP structure to avoid the thermal expansion mismatch.
Electrospinning is mostly used for the fabrication of polymer-based scaffolds. Electrospun mats have an interconnected porous structure and various shapes. Even though the human bone consists of both the cancellous and cortical bones, most studies have only focused on the cancellous bone. The cortical bone has a complicated structure and contains nano- and microscaled structures including the Haversian canal, Volkman's canal and osteon structure.
In this work, the sponge replica and electrospinning methods were used to develop a bone scaffold that mimics the cancellous and cortical bone. This technique can be used to fabricate an interconnected porous structure, which can ensure a suitable local environment for artificial bone applications [11, 14]. The interconnected porous structure of the ZrO2/BCP scaffold was fabricated using the sponge replica method.
The ZrO2/BCP scaffold was surrounded with the PMMA-PCL-HAp composite fibers using the electrospinning method as shown in figure 2. The HAp powders were then loaded into the PMMA-PCL fibers (figures 2, 3(b) and 4). Owing to the excellent osteoconductivity of HAp, it can accelerate the bone-forming ability of bone scaffolds [18]. This fibrous wrapping was necessary to integrate the osteon-like structure, which was fabricated separately. To create the Haversian canal-like structure, PMMA-PCL-HAp fibers were wrapped around a steel wire. The osteon structure was fabricated by bundling eight individually wrapped steel wires and winding an additional layer of PMMA-PCL-HAp fibers around the bundle (figure 5).
After wrapping, the steel wires were removed from the osteon-like structure. PMMA and PCL were mixed together because of the opposing material properties of PMMA and PCL fibers: while the PCL fibers were wrapped very tightly around the steel wire, the PMMA fibers were wrapped very loosely. The steel wires were wrapped with the PMMA-PCL-HAp fibers at a PMMA:PCL ratio of 1:1 to retain the interconnected porous structure, and then the wires were removed. The ZrO2/BCP scaffold was wrapped with electrospun mats consisting of PMMA-PCL at a ratio of 3:7 to stabilize the structure.
After the removal of the steel wires, channels were created resembling the Haversian canal of the bone, and the bundled fibers mimicked the osteon architecture. The ZrO2/BCP scaffold and PMMA-PCL-HAp bundles were firmly attached and the porous structure was interconnected through the adjoining fibrous mat. This feature allowed connecting the sponge bone-like central ZrO2/BCP scaffold to the osteon-like structure, which is very important for bone regeneration. The apposite compressive strength and porosity of the scaffold were also significant. Wrapping the ZrO2/BCP scaffold with fibers increased the mechanical strength and resulted in a porosity of about 70%, which is similar to that of the natural bone [19] (figure 6). Furthermore, the cytocompatibility of the artificial bone resulted in excellent cell viability (figure 7). Thus, a combination of the sponge replica and electrospinning methods was successful in fabricating a biomaterial that mimicked the structure of the natural bone. However, more research is needed to evaluate the biological properties of this material both in vitro and in vivo.
Figure 7.
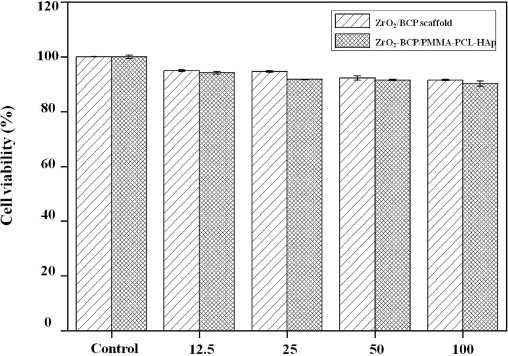
Viability of MG-63 osteoblast-like cells on the ZrO2/BCP scaffold and ZrO2-BCP/PMMA-PCL-HAp for several extraction media concentrations.
Conclusions
We reported a successful fabrication of an artificial bone using a combination of the sponge replica and electrospinning methods. The ZrO2/BCP scaffold was produced by the sponge replica method to mimic the cancellous bone. Structures similar to the Haversian canals and osteon structures were fabricated by wrapping bundled steel wires with electrospun fibers and removing the wires. Dense covering by electrospun fibers mimicked the structure of the cortical bone. This approach can be applied to the development of small artificial bones such as the finger or toe bone.
Acknowledgments
This work was supported by the Mid-career Researcher Program through NRF grant funded by the MEST (No. 2009-0092808), Republic of Korea. The authors are grateful to Min-Sung Kim for his assistance in performing the experiments outlined in this work.
References
- Fyhrie D P. and Kimura J H. J. Biomech. 1999;32:1139. doi: 10.1016/S0021-9290(99)00114-1. [DOI] [PubMed] [Google Scholar]
- Fyhrie D P. and Vashishth D. Bone. 2000;26:169. doi: 10.1016/S8756-3282(99)00246-X. [DOI] [PubMed] [Google Scholar]
- Rho J-Y, Kuhn-Spearing L. and Zioupos P. Med. Eng. Phys. 1998;20:92. doi: 10.1016/S1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- Bigley R F, Griffin L V, Christensen L. and Vandenbosch R. J. Biomech. 2006;39:1629. doi: 10.1016/j.jbiomech.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Tobin F L, Greller L D, Cho C R. and Suva L J. J. Theor. Biol. 2004;229:293. doi: 10.1016/j.jtbi.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Han J-K, Saito F. and Lee B-T. Mater. Lett. 2004;58:2181. doi: 10.1016/j.matlet.2004.01.023. [DOI] [Google Scholar]
- Mousa W F, Kobayashi M, Shinzato S, Kamimura M, Neo M, Yoshihara S. and Nakamura T. Biomaterials. 2000;21:2137. doi: 10.1016/S0142-9612(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Fini M, Giavaresi G, Aldini N N, Torricelli P, Botter R, Beruto D. and Giardino R. Biomaterials. 2002;23:4523. doi: 10.1016/S0142-9612(02)00196-5. [DOI] [PubMed] [Google Scholar]
- Zhao J, Guo L Y, Yang X B. and Weng J. Appl. Surf. Sci. 2008;255:2942. doi: 10.1016/j.apsusc.2008.08.056. [DOI] [Google Scholar]
- Oh S H, Park I K, Kim J M. and Lee J H. Biomaterials. 2007;28:1664. doi: 10.1016/j.biomaterials.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Kim M S, Jang D W, Min Y K, Yang H M, Song H Y. and Lee B T. Mater. Sci. Forum. 2010;654–656:2245. doi: 10.4028/www.scientific.net/MSF.654-656. [DOI] [Google Scholar]
- Muhamad Nor M A A, Hong L C, Arifin Ahmad Z. and Md Akil H. J. Mater. Process. Technol. 2008;207:235. doi: 10.1016/j.jmatprotec.2007.12.099. [DOI] [Google Scholar]
- Linh N T B, Min Y K, Song H-Y. and Lee B-T. J. Biomed. Mater. Res. B: Appl. Biomater. 2010;95B:184. doi: 10.1002/jbm.b.31701. [DOI] [PubMed] [Google Scholar]
- Teng S-H, Lee E-J, Wang P. and Kim H-E. Mater. Lett. 2008;62:3055. doi: 10.1016/j.matlet.2008.01.104. [DOI] [Google Scholar]
- Lee B-T, Youn M-H, Paul R K, Lee K-H. and Song H-Y. Mater. Chem. Phys. 2007;104:249. doi: 10.1016/j.matchemphys.2007.02.009. [DOI] [Google Scholar]
- Kim M S, Franco R A. and Lee B-T. J. Eur. Ceram. Soc. 2011;31:1541. doi: 10.1016/j.jeurceramsoc.2011.03.019. [DOI] [Google Scholar]
- Chang M C, Ko C-C. and Douglas W H. Biomaterials. 2003;24:3087. doi: 10.1016/S0142-9612(03)00150-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Venugopal J R, El-Turki A, Ramakrishna S, Su B. and Lim C T. Biomaterials. 2008;29:4314. doi: 10.1016/j.biomaterials.2008.07.038. [DOI] [PubMed] [Google Scholar]
- Schaffler M B. and Burr D B. J. Biomech. 1988;21:13. doi: 10.1016/0021-9290(88)90186-8. [DOI] [PubMed] [Google Scholar]


