Abstract
We have developed a novel self-heating, temperature-responsive chromatography system for the effective separation of biomolecules. Temperature-responsive poly(N-isopropylacrylamide-co-N-hydroxymethylacrylamide), poly(NIPAAm-co-HMAAm), was covalently grafted onto the surface of magnetite/silica composites as ‘on-off’ switchable surface traps. The lower critical solution temperature (LCST) of the poly(NIPAAm-co-HMAAm)s was controlled from 35 to 55 °C by varying the HMAAm content. Using the heat generated by magnetic particles in an alternating magnetic field (AMF) we were able to induce the hydrophilic to hydrophobic phase separation of the grafted temperature-responsive polymers. To assess the feasibility of the poly(NIPAAm-co-HMAAm)-grafted magnetite/silica particles as the stationary phase for chromatography, we packed the particles into the glass column of a liquid chromatography system and analyzed the elusion profiles for steroids. The retention time for hydrophobic steroids markedly increased in the AMF, because the hydrophobic interaction was enhanced via self-heating of the grafted magnetite/silica particles, and this effect could be controlled by changing the AMF irradiation time. Turning off the AMF shortened the total analysis time for steroids. The proposed system is useful for separating bioactive compounds because their elution profiles can be easily controlled by an AMF.
Keywords: smart polymer, poly(N-isopropylacrylamide), magnetic particle, alternating magnetic field (AMF), high-performance liquid chromatography (HPLC)
Introduction
Smart polymers, also known as stimuli-responsive polymers, have become an important class of materials and their range of applications is steadily increasing. Their properties can be changed by external stimuli such as temperature [1, 2], light [3, 4], electric and magnetic fields [5–7], ionic strength [8], pH [9] and chemicals [10, 11]. Therefore, smart polymers are used in biotechnology, medicine and engineering, in such applications as drug delivery systems [12–14], chemical separation [15, 16], sensors [17] and actuators [18, 19]. The smart polymer that has been studied most extensively is poly(N-isopropylacrylamide) (PNIPAAm) [20–25]. PNIPAAm is soluble in water below its lower critical solution temperature (LCST) of 32 °C and precipitates rapidly at higher temperatures. PNIPAAm can be easily functionalized by derivatization of the side chains or end groups to form reactive groups for surface modification or chemical conjugation [26–30].
These grafting or conjugation processes can produce ‘smart’ surfaces or ‘smart’ conjugates [31–34]. The grafting of PNIPAAm onto solid surfaces, for example, makes them switchable traps, where the surface energy can be controlled by temperature. Kanazawa et al developed an environmentally friendly chromatography system using PNIPAAm-grafted aminopropyl-silica beads as the stationary phase [35, 36]. The polarity of the grafted surface could be altered from hydrophilic to hydrophobic by heating of the mobile phase. Yakushiji et al found that the graft conformation of PNIPAAm on silica beads greatly affected their wettability and hydrophobic interaction with steroids [37]. Kobayashi et al realized an ion-exchange chromatography system using temperature-responsive anionic poly(NIPAAm-co-acrylic acid)-grafted silica beads [38, 39]. Because this type of ionic copolymer is known to shift its pKa in response to temperature changes, the silica beads exhibited simultaneous changes in wettability and charge density under thermal stimuli. These systems can be cost-effective and environmentally friendly, because the elution profiles can be controlled using only an aqueous solution as a mobile phase; however, their performance is adversely affected by the heating/cooling process of the entire mobile phase [40–42].
From these perspectives, we have developed a self-heating packing material composed of magnetite nanoparticles as the stationary phase for temperature-responsive chromatography (figure 1). A ferrimagnetic magnetite (Fe3O4) particle generates heat when subjected to an alternating magnetic field (AMF) because of magnetic hysteresis [43]. Magnetite nanoparticles therefore offer a promising approach for various biomedical applications such as hyperthermia-mediated cancer therapies [44, 45] and magnetic resonance imaging (MRI) [46, 47]. In our previous study, PNIPAAm-based copolymers were successfully immobilized onto magnetite nanoparticles and the hydrophilic/hydrophobic phase separation of the grafted polymer was controlled by switching an AMF on and off [48–50].
Figure 1.
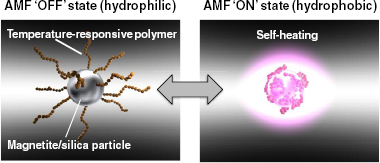
Schematic illustrations of the surface processes in temperature-responsive polymer-modified particles.
In this study, we explored the effect of AMF application time on the interaction with hydrophobic steroids using poly(NIPAAm-co-HMAAm)-grafted magnetite particles. Hydroxymethylacrylamide (HMAAm) was used to control the LCST of the grafted polymer, while the heating profile in the column was varied by changing the magnetite composition or AMF power [50]. A ‘grafting-from’ approach with a silane-coupling agent was adopted because it resulted in a versatile, one-step method, and was much simpler than the ‘grafting-to’ approach. The successful surface grafting was confirmed by thermogravimetric analysis (TGA) and x-ray photoelectron spectroscopy (XPS). The resulting magnetite particles were packed into the glass column of an aqueous liquid chromatography system connected to a high-frequency power unit. The elution profiles for steroids were analyzed as a function of AMF application time.
Materials and methods
Materials
NIPAAm was supplied by Kohjin (Tokyo, Japan) and was recrystallized with benzene. HMAAm and 2,2′-azobis-(isobutyronitrile) (AIBN) were purchased from Wako (Tokyo, Japan) and were recrystallized with ethanol. Silica particles (Wakogel (R) C-200, particle size 75–150 μm), iron(II) chloride (FeCl2·4H2O), iron(III) chloride (FeCl3·6H2O), toluene and 28% ammonia solution were purchased from Wako (Tokyo, Japan). A silane coupling agent, 3-(methacryloxypropyl)triethoxysilane (MPTES), was obtained from Tokyo Chemical Industry (Tokyo, Japan) and used after purification. The model drugs purchased from Wako (Tokyo, Japan) were hydrocortisone (HCO), dexamethasone (DEX) and testosterone (TES). All other chemicals and solvents were commercially available and of analytical grade. Ultrapure Milli-Q water was used in the experiments.
Synthesis of magnetite/silica composite particles
A mixture of 9.0 g of FeCl2·4H2O, 24.0 g of FeCl3·6H2O, and 5.0 g of silica particles with a molar ratio Fe(II) : Fe(III) of 2 : 1 was dispersed into 50 ml of deionized water at 75 °C. The suspension was precipitated by adding 100 ml of aqueous ammonia solution (28%), and the reaction was allowed to continue for 30 min while stirring at 400 rpm (figure 1(a)). The precipitated magnetite/silica particles were then washed thoroughly with distilled water and ethanol and dried in vacuum for 24 h.
Surface grafting of poly(NIPAAm-co-HMAAm)s on magnetite/silica particles
The prepared magnetite/silica composite (7.5 g) was dispersed in 75 ml of toluene. Then a 2.5 ml aliquot of MPTES was added dropwise to the solution, which was stirred at 400 rpm at room temperature for 12 h (scheme 1(b)). The resulting particles were washed sequentially with toluene and ethanol to remove any unreacted compounds. After drying in vacuum for 24 h, the MPTES-modified magnetite/silica particles were recovered as a black powder.
Scheme 1.
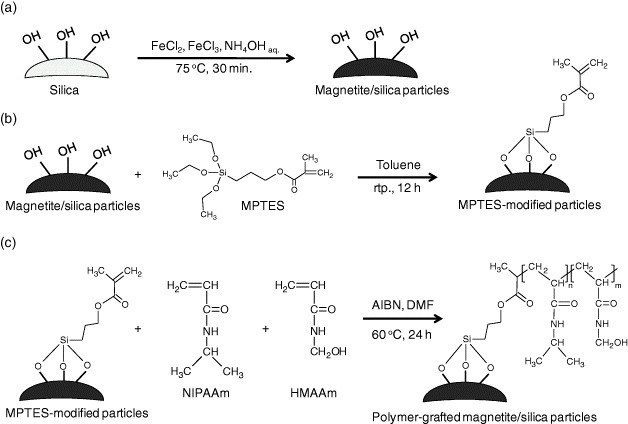
Preparation schemes of the column packing materials. (a) Synthesis of magnetite/silica composite particles. (b) Silanization of magnetite/silica particles with MPTES. (c) Grafting of temperature-responsive polymers from MPTES-modified particles.
Next, the surfaces of magnetite/silica particles were modified with poly(NIPAAm-co-HMAAm)s by the free-radical copolymerization of NIPAAm with HMAAm of various compositions as shown in scheme 1(c). Briefly, 1.1 g of monomers and 1.0 g of MPTES-magnetite/silica particles were added to 10 ml of dehydrated dimethylformamide containing 1.0 mol% of AIBN. The mixture was degassed under N2 gas by freezing and thawing and stirred at 60 °C for 24 h. The obtained poly(NIPAAm-co-HMAAm)-grafted particles were collected by magnetic separation and washed several times to remove any residual monomers and unreacted compounds. The particles were dried under reduced pressure for 24 h. For LCST measurements, the supernatant solutions were collected and purified by dialysis in ethanol and deionized water for 1 week and then lyophilized for 3 days to obtain free copolymers of poly(NIPAAm-co-HMAAm)s. The resulting poly(NIPAAm-co-HMAAm)s were abbreviated to N100-xHx, where N, H, and x are the initial concentrations of NIPAAm, HMAAm and HMAAm in mol%, respectively.
Surface characterizations by TGA and XPS
Each preparation step was evaluated via the weight loss as a function of temperature by TGA (EXSTAR6000 TG/DTA, SII Nanotechnology, Tokyo, Japan). Approximately 2.0 mg of a sample was placed in an Al pan and heated at a rate of 10 °C min−1 from 20 to 500 °C. The presence of organic compounds on the surface of the particles was determined from the weight loss between 200 and 500 °C. Surface modification was also confirmed by XPS (ESCA-1000, Shimadzu, Kyoto, Japan) through the N1 s (410–390 eV) peak.
LCST measurement
The LCST of aqueous solutions (0.1 wt/v%) of poly(NIPAAm-co-HMAAm)s was defined as the temperature at which the transmittance became 50%. The transmittance was measured at 500 nm using a UV-Vis spectrophotometer (Jasco V-550, Tokyo, Japan) equipped with a temperature controller (heating rate 1.0 °C min−1).
Self-heating property
Self-heating was used to investigate the temperature response of the modified magnetite/silica particles. The particles were dispersed in distilled water at a concentration of 100 g l−1. The suspension was placed in a cylindrical tube of 15 mm inner diameter wrapped with a copper coil (three turns with inside and outside diameters of 5 and 6 cm, respectively). Heating was induced by the AMF generated by passing alternating current (400 A, 280 kHz frequency, 2 kW power) through the coil (HOTSHOT2, Ameritherm, NY, USA). The temperature profile of the suspension was monitored using a thermometer.
AMF-induced elution profiles of steroids
Poly(NIPAAm-co-HMAAm)-modified magnetite/silica particles were suspended in Milli-Q water. The particles were then packed into a glass column (3.0×10.0 mm, Tokyo Rikakikai, Tokyo, Japan) and installed into a high-performance liquid chromatography system (LaChrom Elite, Hitachi, Tokyo, Japan) equipped with a UV sensor detecting 254 nm light. The glass column was wrapped with the above-mentioned three-turn coil with inside and outside diameters of 5 and 6 cm, respectively. The mobile phase was set to flow at a rate of 0.5 ml min−1. The three model steroids, HCO (0.1 g l−1), DEX (0.1 g l−1), and TES (0.05 g l−1), were dissolved in Milli-Q water. The elution behaviors of the steroids were recorded both with and without an AMF. The AMF was generated by passing alternating current (400 A, 280 kHz frequency, 2 kW power) through the coil for 0, 3, 5, 10, 15 or 30 min. To investigate the interaction of the stationary phase with steroids at different temperatures, the elusion behavior of the steroids at different temperatures in the range 20–60 °C were also monitored without an AMF. The column temperature was controlled using a thermostated water bath (Tokyo Rikakikai, Tokyo, Japan).
Results and discussion
Synthesis and characterizations of self-heating magnetite/silica particles with a switchable surface
To develop a novel self-heating, temperature-responsive chromatography system, a smart packing material was prepared as the stationary phase. The magnetite/silica particles were first synthesized by a co-precipitation method, which enabled the fabrication of magnetite (Fe3O4) particles through the co-precipitation of Fe(II) and Fe(III) after the addition of ammonia [50]. This reaction was performed in the presence of silica particles to obtain the magnetite/silica composites. In this study, a ‘grafting-from’ approach with MPTES was used for the surface modification because it was more simple and versatile than the ‘grafting-to’ approach. Briefly, the silica surface of the composites was modified with MPTES to introduce vinyl groups. Subsequent polymerization with NIPAAm, HMAAm and AIBN as an initiator produced poly(NIPAAm-co-HMAAm)-grafted particles, N90H10, N80H20 and N70H30, respectively.
The successful modification of the polymers on the silica surfaces was confirmed by TGA and XPS. Figure 2 shows the weight loss of the modified particles when heated to 500 °C. The weight loss below 100 °C was due to the evaporation of water from the surfaces (figure 2(a)). A further weight decrease above 100 °C was related to the decomposition of organic and silanol groups. MPTES-modified particles exhibited a weight loss of 5.02% between 200 and 500 °C (figure 2(b)), whereas the weight loss for the polymer-modified particles N90H10, N80H20 and N70H30 was 10.87, 10.70 and 9.86%, respectively (figures 2(c)–(e)). These results indicate that the particle surfaces were successfully modified with poly(NIPAAm-co-HMAAm)s. No significant variation in the weight loss with the HMAAm content was observed. The surfaces were analyzed by XPS before and after modification, and an increase in the nitrogen N1 s peak intensity was observed after the grafting of poly(NIPAAm-co-HMAAm)s, as shown in figure 3. We also measured the IR spectra (FT/IR-4200, Jasco, Tokyo, Japan) and observed increases in absorption at 1544 and 1655 cm−1 (amide I and II vibrations, respectively), 2977 cm−1 (C–H stretching vibration), and 1632 cm−1 ( vibration), confirming the successful modification of the particles with polymers (see figure S in supporting material available from stacks.iop.org/STAM/12/044609).
vibration), confirming the successful modification of the particles with polymers (see figure S in supporting material available from stacks.iop.org/STAM/12/044609).
Figure 2.
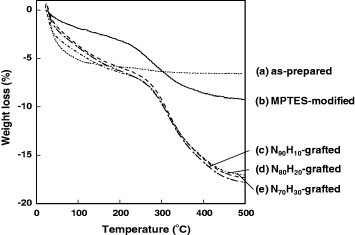
Thermogravimetry curves of magnetite/silica particles: (a) as-prepared, (b) MPTES-modified, (c) N90H10-grafted, (d) N80H20-grafted and (e) N70H30-grafted.
Figure 3.
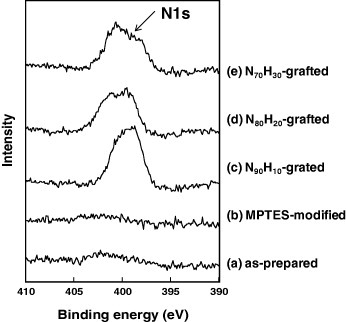
N1S XPS spectra of magnetite/silica particles: (a) as-prepared, (b) MPTES-modified, (c) N90H10-grafted, (d) N80H20-grafted and (e) N70H30-grafted.
Figure 4 shows the self-heating profiles of the polymer-modified magnetite/silica particles when they were subjected to an AMF. A transient temperature increase was observed during the irradiation with the AMF because of the heat generated in the magnetic particles via the hysteresis loss [34]. The temperature exceeded 40 °C in all magnetite/silica samples within 3 min of AMF exposure, while the heating was negligible for pure silica particles. Further increasing the AMF application time to 15 min yielded a temperature increase of up to 63.0 °C for the magnetite/silica particles. Although the heating was slightly less for the surface-modified particles than for the ungrafted particles, the temperatures in the MPTES- and N90H10-modified samples reached 60.5 and 57.8 °C, respectively, which are higher than the LCSTs of the grafted polymers. Similar self-heating profiles were observed for the magnetite/silica particles modified with N80H20 or N70H30 (data not shown).
Figure 4.
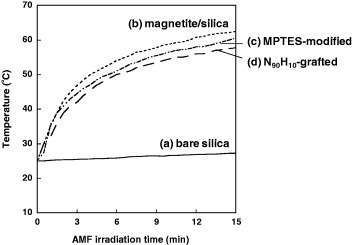
Self-heating profiles for (a) bare silica particles, (b) magnetite/silica particles, (c) MPTES-modified magnetite/silica particles and (d) N90H10-grafted magnetite/silica particles.
Temperature-dependent elution control of steroids
To obtain a better understanding of the temperature dependence of the interaction between the particles and steroids, the elution behavior of the steroids from the N90H10, N80H20 and N70H30 columns was monitored at different temperatures without applying an AMF. First, the LCSTs of the poly(NIPAAm-co-HMAAm)s were measured using ungrafted free polymers collected from the supernatant solutions after the surface modification. Figure 5 shows the temperature dependences of transmittance at 500 nm. The transmittance of N90H10, N80H20 and N70H30 decreased at 36.1, 45.5 and 56.0 °C, respectively, revealing that the LCSTs of the grafted copolymers could be easily varied by controlling the amount of HMAAm.
Figure 5.
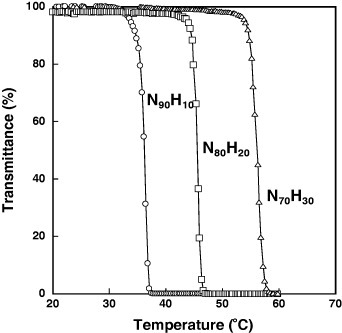
Temperature dependences of optical transmittance at 500 nm for poly(NIPAAm-co-HMAAm)s in aqueous solutions.
Figure 6 shows the retention time for the three steroids on the N90H10, N80H20 and N70H30 columns at different temperatures in the range 20–60 °C without the use of an AMF. The column temperature was controlled using a thermostated water bath. The hydrophobic parameter (log P) for HCO, DEX and TES was 1.61, 1.83 and 3.32, respectively. The retention time for steroids on the N90H10, N80H20 and N70H30 columns increased around the LCSTs, in agreement with the optical data (see figure 5). In particular, the retention behavior of TES, which is the most hydrophobic steroid in this study, changed dramatically. These results can be explained by the enhancement of the hydrophobic interaction between the collapsed polymers and the steroids above the LCSTs.
Figure 6.
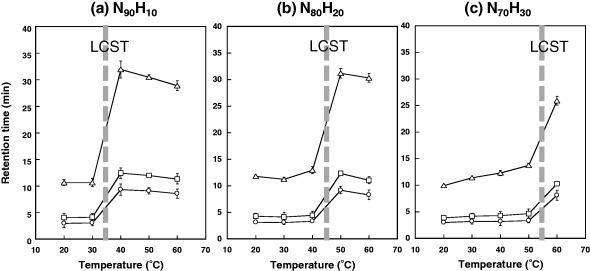
Temperature dependences of retention time of steroids on (a) N90H10, (b) N80H20 and (c) N70H30 columns: circles, HCO; squares, DEX; triangles, TES.
AMF-induced switchable elution control of steroids
We attempted to control the elution of steroids by heating the magnetite/silica particles using AMF. Figure 7 shows the changes in retention time for the three steroids on the N90H10, N80H20 and N70H30 columns as a function of the AMF irradiation time. The retention time increased in all cases owing to the enhanced hydrophobic interaction in the AMF. The retention time for the N90H10 column was nearly constant for up to 10 min of irradiation and rapidly increased after that. Although the temperature exceeded the LCST of N90H10 within a few minutes of irradiation under a no-flow condition (see figure 4), here the temperature profile in the column might be different because of the flow inside the column. Temperature monitoring during the elution experiment was difficult, but it is plausible to assume that the dehydration of the grafted polymers takes a longer time with a flow than without it. An increase in the retention time on the N80H20 column was observed after 15 min of irradiation. There was no significant difference between the retention times for the N80H20 and N70H30 columns. This is because the temperature in the column immediately decreases when the AMF is turned off. In other words, the proposed self-heating system requires only a few minutes of AMF irradiation to induce the phase transition of the grafted polymers on the stationary phase. The cooling performance of this system increases with the flow rate of the mobile phase; it also depends on the heat capacity of the particles, which increases with the amount of magnetite particles.
Figure 7.
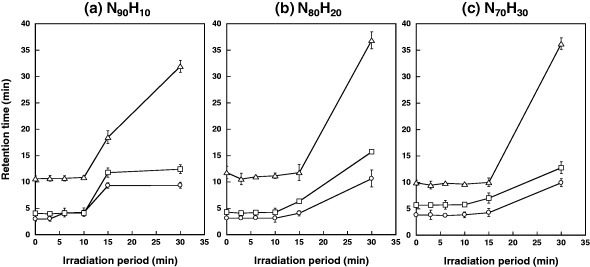
Retention time in steroids on (a) N90H10, (b) N80H20 and (c) N70H30 columns as a function of AMF irradiation time: circles, HCO; squares, DEX; triangles, TES.
To assess the feasibility of the AMF system for the effective separation of steroids, we varied the AMF irradiation time during the elution process. Figure 8 shows the effect of turning off the AMF 16 min after the start of the elution of HCO and TES on the N90H10 column. While the HCO elution peak remained unchanged, the retention time of TES decreased from 30.9 to 19.9 min and the elution peak became sharper. In conventional chromatography, elution is modulated by the addition of organic solvents or increasing the salt concentration in the mobile phase, or by heating/cooling the entire mobile phase [40–42]. The proposed AMF system instead relies on the self-heating of the stationary phase, which is controlled by switching the AMF on and off. In this method, the local temperature inside the column can be precisely controlled by adjusting the AMF power and the composition of the magnetite particles.
Figure 8.
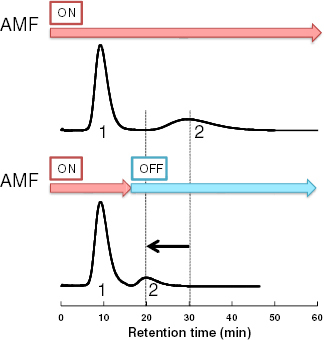
Effect of AMF irradiation time on elution of HCO and TES on the N90H10 column. Peaks: 1, HCO; 2, TES.
Conclusions
We have developed a novel packing material composed of self-heating, temperature-responsive magnetite/silica particles for use as the stationary phase in temperature-responsive chromatography. The ability of magnetic particles to generate heat when subjected to an AMF allowed us to control the hydrophilic to hydrophobic phase separation in the grafted polymer by simply switching the AMF on and off. To assess the feasibility of the poly(NIPAAm-co-HMAAm)-grafted magnetite/silica particles as the stationary phase, the grafted particles were packed into a glass column of an aqueous liquid chromatography system and the elusion profiles of steroids were analyzed. The retention times of the hydrophobic steroids markedly increased under AMF irradiation owing to the enhancement of the hydrophobic interaction. Furthermore, turning off the AMF during the elution process shortened the total analysis time for steroids. By optimizing the LCST of the grafted polymers, the AMF power and the magnetite composition, this system will enable the more accurate, prompt and simple control of the elution profiles for hydrophobic molecules because it does not require heating and cooling of the entire mobile phase.
Acknowledgments
The authors would like to thank Professor Allan S Hoffman (University of Washington) for his valuable comments and discussion. This project was financially supported by the Japan Science and Technology Agency (JST).
References
- Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y. and Okano T. Nature. 1995;374:240. doi: 10.1038/374240a0. [DOI] [Google Scholar]
- Asoh T, Matsusaki M, Kaneko T. and Akashi M. Adv. Mater. 2008;20:2080. doi: 10.1002/(ISSN)1521-4095. [DOI] [Google Scholar]
- Suzuki A. and Tanaka T. Nature. 1990;346:345. doi: 10.1038/346345a0. [DOI] [Google Scholar]
- Gorelikov I, Field L M. and Kumacheva E. J. Am. Chem. Soc. 2004;126:15938. doi: 10.1021/ja0448869. [DOI] [PubMed] [Google Scholar]
- Osada Y, Okuzaki H. and Hori H. Nature. 1992;355:242. doi: 10.1038/355242a0. [DOI] [Google Scholar]
- Lai J J, Hoffman J M, Ebara M, Hoffman A S, Estournes C, Wattiaux A. and Stayton P S. Langmuir. 2007;23:7385. doi: 10.1021/la062527g. [DOI] [PubMed] [Google Scholar]
- Lahann J, Mitragotri S, Tran T N, Kaido H, Sundaram J, Choi I S, Hoffer S, Somorjai G A. and Langer R. Science. 2003;299:371. doi: 10.1126/science.1078933. [DOI] [PubMed] [Google Scholar]
- Genzer J. and Efimenko K. Science. 2000;290:2130. doi: 10.1126/science.290.5499.2130. [DOI] [PubMed] [Google Scholar]
- Bergbreiter D E, Case B L, Liu Y S. and Caraway J W. Macromolecules. 1998;31:6053. doi: 10.1021/ma980836a. [DOI] [Google Scholar]
- Ding Z, Fong R B, Long C J, Stayton P S. and Hoffman A S. Nature. 2001;411:59. doi: 10.1038/35075028. [DOI] [PubMed] [Google Scholar]
- Vallet-Regí M, Balas F. and Arcos D. Angew. Chem., Int. Ed. Engl. 2007;46:7548. doi: 10.1002/(ISSN)1521-3773. [DOI] [PubMed] [Google Scholar]
- Jessica C G, Elina M, Stayton P S. and Charles E M. Biomaterials. 2011;32:2407. doi: 10.1016/j.biomaterials.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppimath K S, Liu L H, Seow W Y, Lin S Q, Powell R, Chan P. and Yang Y Y. Adv. Funct. Mater. 2007;17:355. doi: 10.1002/(ISSN)1616-3028. [DOI] [Google Scholar]
- Kotsuchibashi Y, Ebara M, Yamamoto K and Aoyagi T. 2010. J. Polym. Sci. A 48 4390 10.1002/pola.24226 [DOI] [Google Scholar]
- Bulmus V, Ding Z, Long C J, Stayton P S. and Hoffman A S. Bioconj. Chem. 2000;11:78. doi: 10.1021/bc9901043. [DOI] [PubMed] [Google Scholar]
- Boyer C, Bulmus V, Liu J, Davis T P, Stenzel M H. and Barner-Kowollik C. J. Am. Chem. Soc. 2007;129:7145. doi: 10.1021/ja070956a. [DOI] [PubMed] [Google Scholar]
- Volden S, Kj⊘niksen A L, Zhu K, Genzer J, Nyström B. and Glomm W R. ACS Nano. 2010;4:1187. doi: 10.1021/nn901517u. [DOI] [PubMed] [Google Scholar]
- Maeda S, Hara Y, Sakai T, Yoshida R. and Hashimoto S. Adv. Mater. 2007;19:3480. doi: 10.1002/(ISSN)1521-4095. [DOI] [Google Scholar]
- Murase Y, Maeda S, Hashimoto S. and Yoshida R. Langmuir. 2009;25:483. doi: 10.1021/la8029006. [DOI] [PubMed] [Google Scholar]
- Ebara M, Yamato M, Aoyagi T, Kikuchi A, Sakai K. and Okano T. Adv. Mater. 2008;20:3034. doi: 10.1002/adma.v20:16. [DOI] [Google Scholar]
- Maeda T, Takenouchi M, Yamamoto K. and Aoyagi T. Polym. J. 2009;41:181. doi: 10.1295/polymj.PJ2008201. [DOI] [Google Scholar]
- Matsukuma D, Yamamoto K. and Aoyagi T. Langmuir. 2006;22:5911. doi: 10.1021/la060438y. [DOI] [PubMed] [Google Scholar]
- Yamato M, Konno C, Utsumi M, Kikuchi A. and Okano T. Biomaterials. 2002;23:561. doi: 10.1016/S0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A. and Okano T. Tissue Eng. 2001;7:473. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- Idota N, Tsukahara T, Sato K, Okano T. and Kitamori T. Biomaterials. 2009;30:2095. doi: 10.1016/j.biomaterials.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Aoyagi T, Ebara M, Sakai K, Sakurai Y. and Okano T. J. Biomat. Sci.—Polym. Edn. 2000;11:101. doi: 10.1163/156856200743526. [DOI] [PubMed] [Google Scholar]
- Ebara M, Aoyagi T, Sakai K. and Okano T. Macromolecules. 2000;33:8312. doi: 10.1021/ma000121j. [DOI] [Google Scholar]
- Ebara M, Aoyagi T, Sakai K and Okano T. 2001. J. Polym. Sci. A 39 335 10.1002/(ISSN)1099-0518 [DOI] [PubMed] [Google Scholar]
- Maeda T, Yamamoto K. and Aoyagi T. J. Colloid Interface Sci. 2006;302:647. doi: 10.1016/j.jcis.2006.06.047. [DOI] [PubMed] [Google Scholar]
- Maeda T, Takenouchi M, Yamamoto K. and Aoyagi T. Biomacromolecules. 2006;7:2230. doi: 10.1021/bm060261m. [DOI] [PubMed] [Google Scholar]
- Narain R, Gonzales M, Hoffman A S, Stayton P S. and Krishnan K M. Langmuir. 2007;23:6299. doi: 10.1021/la700268g. [DOI] [PubMed] [Google Scholar]
- Fong R B, Ding Z, Long C J, Hoffman A S. and Stayton P S. Bioconj. Chem. 1999;10:720. doi: 10.1021/bc980151f. [DOI] [PubMed] [Google Scholar]
- Ebara M, Hoffman J M, Hoffman A S. and Stayton P S. Lab chip. 2006;6:843. doi: 10.1039/b515128g. [DOI] [PubMed] [Google Scholar]
- Ebara M, Hoffman J M, Stayton P S. and Hoffman A S. Rad. Phys. Chem. 2007;76:1409. doi: 10.1016/j.radphyschem.2007.02.072. [DOI] [Google Scholar]
- Kanazawa H, Yamamoto K, Matsushima Y, Takai N, Kikuchi A, Sakurai Y. and Okano T. Anal. Chem. 1996;68:100. doi: 10.1021/ac950359j. [DOI] [PubMed] [Google Scholar]
- Kanazawa H, Kashiwase Y, Yamamoto K, Matsushima Y, Kikuchi A, Sakurai Y. and Okano T. Anal. Chem. 1997;69:823. doi: 10.1021/ac961024k. [DOI] [PubMed] [Google Scholar]
- Yakushiji T, Sakai K, Kikuchi A, Aoyagi T, Sakurai Y. and Okano T. Anal. Chem. 1999;71:1125. doi: 10.1021/ac980677t. [DOI] [Google Scholar]
- Kobayashi J, Kikuchi A, Sakai K. and Okano T. Anal. Chem. 2001;73:2027. doi: 10.1021/ac0013507. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Kikuchi A, Sakai K and Okano T. 2002. J. Chromatogr. A 958 109 10.1016/S0021-9673(02)00388-6 [DOI] [PubMed] [Google Scholar]
- Ayano E, Nambu K, Sakamoto C, Kanazawa H, Kikuchi A and Okano T. 2006. J. Chromatogr. A 1119 58 10.1016/j.chroma.2006.01.068 [DOI] [PubMed] [Google Scholar]
- Nagase K, Kobayashi J, Kikuchi A, Akiyama Y, Kanazawa H. and Okano T. Biomacromolecules. 2008;9:1340. doi: 10.1021/bm701427m. [DOI] [PubMed] [Google Scholar]
- Mizutani A, Nagase K, Kikuchi A, Kanazawa H, Akiyama Y, Kobayashi J, Annaka M and Okano T. 2010. J. Chromatogr. A 1217 5978 10.1016/j.chroma.2010.07.067 [DOI] [PubMed] [Google Scholar]
- Wang X, Gu H. and Yang Z. J. Magn. Magn. Mater. 2005;293:334. doi: 10.1016/j.jmmm.2005.02.028. [DOI] [Google Scholar]
- Ito A, Kuga Y, Honda H, Kikkawa H, Horiuchi A, Watanabe Y. and Kobayashi T. Cancer Lett. 2004;212:167. doi: 10.1016/j.canlet.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Li Z, Kawashita M, Araki N, Mitsumori M, Hiraoka M and Doi M. 2010. Mater. Sci. Eng. C 30 990 10.1016/j.msec.2010.04.016 [DOI] [Google Scholar]
- Sun C, Lee J S H. and Zhang M. Adv. Drug. Deliv. Rev. 2008;60:1252. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai M. J. Biosci. Bioeng. 2002;96:606. [PubMed] [Google Scholar]
- Wakamatsu H, Yamamoto K, Nakao A. and Aoyagi T. J. Magn. Magn. Mater. 2006;302:327. doi: 10.1016/j.jmmm.2005.09.032. [DOI] [Google Scholar]
- Yamamoto K, Matsukuma D, Nanasetani K. and Aoyagi T. Appl. Surf. Sci. 2008;255:384. doi: 10.1016/j.apsusc.2008.06.065. [DOI] [Google Scholar]
- Yagi H, Yamamoto K and Aoyagi T. 2008. J. Chromatogr. B 876 97 10.1016/j.jchromb.2008.10.028 [DOI] [PubMed] [Google Scholar]


