Abstract
Adaptive wear-resistant coatings produced by physical vapor deposition (PVD) are a relatively new generation of coatings which are attracting attention in the development of nanostructured materials for extreme tribological applications. An excellent example of such extreme operating conditions is high performance machining of hard-to-cut materials. The adaptive characteristics of such coatings develop fully during interaction with the severe environment. Modern adaptive coatings could be regarded as hierarchical surface-engineered nanostructural materials. They exhibit dynamic hierarchy on two major structural scales: (a) nanoscale surface layers of protective tribofilms generated during friction and (b) an underlying nano/microscaled layer. The tribofilms are responsible for some critical nanoscale effects that strongly impact the wear resistance of adaptive coatings. A new direction in nanomaterial research is discussed: compositional and microstructural optimization of the dynamically regenerating nanoscaled tribofilms on the surface of the adaptive coatings during friction. In this review we demonstrate the correlation between the microstructure, physical, chemical and micromechanical properties of hard coatings in their dynamic interaction (adaptation) with environment and the involvement of complex natural processes associated with self-organization during friction. Major physical, chemical and mechanical characteristics of the adaptive coating, which play a significant role in its operating properties, such as enhanced mass transfer, and the ability of the layer to provide dissipation and accumulation of frictional energy during operation are presented as well. Strategies for adaptive nanostructural coating design that enhance beneficial natural processes are outlined. The coatings exhibit emergent behavior during operation when their improved features work as a whole. In this way, as higher-ordered systems, they achieve multifunctionality and high wear resistance under extreme tribological conditions.
Keywords: nano-structured PVD coatings, adaptive materials, multi-functionality, nano-laminates, self-organization, dissipative structures, emergent behavior, nano-tribology, hierarchical materials, surface phenomena, surface engineering, extreme applications
1. Introduction
Traditionally, wear-resistant (hard) coatings were developed using the knowledge obtained from physics and mechanics of solid materials combined with material science of bulk materials [1–3]. Because friction is the process of transformation and dissipation of mechanical energy into other kinds of energy, an energy-based concept looks more generic [4]. It is timely to move further and develop some new principles of hard coating design that are based on modified and probably somewhat new philosophy.
This is especially topical for extreme tribological conditions. Some modern tribological applications show an increasing trend toward harsher operating environments [5]. High-speed, especially ‘green’ (coolant-free) machining of hard to cut materials, such as hardened tool steels and modern aerospace Ni-based superalloys, are classic examples of harsh conditions. From a tribological viewpoint, such processes are far from equilibrium operating conditions and are characterized by strong gradients of different characteristics on the friction surface [6]. Sometimes, for instance in attrition-wear conditions with built up edge formation that are typical for machining of aerospace alloys, this is complicated by the significant instability of operational conditions [7–9]. This is the field where adaptive wear-resistant coatings can develop their full potential.
In this paper, we present a concept on how to move away from self-assembly approaches, occurring in materials under equilibrium conditions, to the complex nanostructured surface-engineered materials with dissipative structures formed as a result of self-organization [10] associated with external stimuli such as high temperatures and severe mechanical constraints. We note the possibilities of a nature-inspired approach for nanomaterials development. This approach focuses on the integrative performance of complex materials such as emergent behavior, which is closely related to self-organization occurring in ‘open’ (i.e. interacting with the environment) tribosystems; the advantage of nanomaterials with a highly nonequilibrium state is that they can function well in unstable operating conditions.
Under extreme tribological conditions associated with machining of hard to cut materials, operators deal with high temperatures (up to 1000 °C and above) and stresses (around 1–3 GPa [7, 8, 11–13]) on the friction surface, which are further complicated by the strong adhesive interaction with the workpiece. This leads to very complex frictional phenomena [14–15]. In addition to intensive rubbing, adhesion, abrasion and tribochemical reactions at these high temperatures, there is also thermal fatigue for some cutting applications [14]. From a tribological viewpoint, this is a catastrophic wear mode [6], which is close to the ‘edge of chaos’ [16] due to excessive wear rate of unprotected (uncoated) friction surfaces. Therefore, effective protection of friction surfaces is required. To address all these phenomena, novel surface engineering methods should undoubtedly be multifunctional [17].
An important step in the development of hard coatings was the introduction of adaptive hard coatings [2, 6, 18, 19]. Adaptation is a process that produces a correlation between the system's structure, behavior and environment [6, 20–23]. Adaptive wear-resistant coatings enable a tribosystem to shift to a milder wear mode instead of sustaining the severe wear mode in the case when non adaptive coatings are used [6, 24]. Adaptation is directly related to self-organization phenomena during friction, and vice versa, self-organization implies adaptation [25, 26]. Self-organization during friction results in reduction of entropy production and correspondingly a wear rate decrease [6]. Adaptive characteristics can be explored by the application of nanostructured coatings. Nanostructuring of the coatings does not automatically improve mechanical properties such as hardness but strongly affects their physical and chemical properties and therefore their ability to interact with the environment [2, 27].
Many modern materials synthesis techniques, including physical vapor deposition (PVD), take advantage of processes that occur far from thermodynamic equilibrium [28]. PVD coatings (with nanoscaled crystalline/composite/laminated structure) impart the initial surface with a high density of lattice imperfections and vastly increased surface/interface area compared to macroscale materials [2, 29]. Many nanomaterials are highly active catalysts [30]. As a result, a surface with a highly nonequilibrium initial state significantly accelerates beneficial physicochemical reactions initiated by friction [6, 31] and provide surface layers with properties that could not be achieved using traditional methods of material fabrication.
Current nanotechnology is mostly focused on creating individual structures (nanoparticles, nanocrystals and so on [32, 33]). However, when integrating them into macroscopic devices a question arises of how to design the interactions between the elements of the system. Integration brings complexity and emergent behavior, which are typical for biosystems. It is expected that eventually engineering systems will behave like biosystems and bioinspired artificial multifunctional materials will be more common [34, 35]. Adaptive nanomaterials and smart and multifunctional materials should become a major focus of advanced technologies [25, 36–39].
Development of adaptive materials and systems requires a clear understanding of the self-organization phenomena. Self-organization during friction develops in processes such as the permanent formation of nanoscaled surface tribofilms with their further destruction and regeneration [6]. Therefore, surface-engineered layers that enhance these processes, for instance nanomultilayered coatings, may be considered as nature-mimicking structures [6, 40, 41]. The nanolaminates are adjusted to enhance these processes of the tribofilms' regeneration. They include the ordered sequence of layers that supply the surface with a controlled flow of matter and energy, and if destroyed or transformed, they enhance the formation of the next generation (which is somewhat similar to biogenerations) of protective tribofilms.
Modern multifunctional wear-resistant nanostructural coatings belong to a category of complex systems. System science employs two approaches to complex system engineering. One is the traditional reductionism (usually used in engineering) and another is an integrative concept of emergence [42–45]. Emergence refers to creation of novel structures and properties during self-organization in complex systems [43, 46]. Using the first (reductionist) approach, one or two properties are usually employed for a wear-resistant coating such as hardness and oxidation resistance [47]. Other properties are largely ignored, and this may limit wear resistance in various applications [24, 48]. An integrative approach where characteristics of the system are combined as a whole would be a new strategy in nanostructured materials design to achieve multifunctionality and adaptive behavior [19, 44].
Complex systems are usually conglomerates of interacting structural components, and features (characteristics), each exhibiting some sort of nonlinear dynamics; they are driven by an influx of energy [49], posses adaptability and self-organization, and are also exploited to tackle the openness and dynamism [50–53]. Thereby, the nature-inspired complex system design outlined above is mostly related to adaptability and emergent behavior.
The concept of emergent properties is the key element of a theoretical and methodological tradition claiming that the whole is more than the sum of its parts [54–56]. While each of the emergent characteristics individually contributes to the system performance, the overall organizational design is intended to create a performance that relies heavily on the interaction and synergy among these characteristics [57]. An emergent phenomenon is a universal mechanism that generates complexity [17, 52, 58, 59]. For complex dynamical systems designed for severe applications, a special interest has to be paid to the emergence of collective behavior near a critical point of a phase transition, either by tuning and/or by self-organization [49–52].
In this paper, we focus on wear-resistant PVD coatings. During the last decade, the Al-rich TiAlN PVD coatings [61] and Al-rich AlCrN-based coatings [62] were widely used for machining of hard to cut materials [3]. Systematic optimization of the coating composition was made by addition of various elements (such as Cr, Si, Y, B and others) to TiAlN [24, 63–65] and corresponding elements to AlCrN-based coatings [62, 66–68]. Various designs of hard coatings have been introduced as well [48, 69–80]. Nanomultilayered coatings [3, 81–83] consisting of alternating nanolayers [70, 84–88] with varied and modulated chemical compositions have been a promising development that improved the coating characteristics and enhanced their wear performance [81–83].
Usually there is a difference between fundamental studies in the field of irreversible thermodynamics, self-organization and adaptation for physical, chemical and biological systems on one hand and applied studies mainly focused on specific applications on the other hand. In this paper we are trying to fill this gap and present some results of long-term interdisciplinary research in the fields of nanomaterials, surface engineering and tribology with the involvement of some elements of irreversible thermodynamics.
2. Two approaches in hard coatings design for extreme tribological applications
In the first approach, the coating is considered as a completely artificial system that has to sustain the environment with minimal changes. In contrast, the second approach regards a coating as a surface-engineered tribological system that combines an artificial system (the coating) and beneficial natural processes associated with friction and interaction with the environment, and results in adaptation to this environment. These two philosophies create different strategies of coatings development. The first approach is to create coatings with a limited number of advanced properties, primarily increased hardness. Nanocomposite coatings are mostly developed based on this approach [2, 27, 47, 89–97]. Recently there have been claims that nanocomposite coatings have strong potential for high-speed machining of hard to cut materials [47, 98].
As outlined above [14] cutting involves very complex phenomena [7], and one or two parameters of the tooling material could not be responsible for tool life improvements. A variety of characteristics is needed, which is why the second approach to hard coatings design holds promise. The second approach is based on the concept that both natural and synthetic processes should be considered during friction. The aim is to control the synthetic processes to encourage the evolution of the natural processes during friction that lead to a minimum wear rate. This could be done by developing adaptive coatings that can weaken the external impact and shift the process to a milder wear mode. As shown below, adaptability of the coating is fully developed under severe or even extreme and somewhat unstable frictional conditions. Under these conditions, properly designed adaptive coatings form nanolayers that efficiently protect/lubricate the surface and control wear resistance. Owing to the formation of these protective and lubricious films, the underlying ‘bulk’ coating stays less damaged. As a result, the coating can sustain the extreme environment and show otherwise unattainable wear resistance.
2.1. Nanocomposite hard coatings
High hardness and thermal stability are important characteristics of the surface-engineered tools that determine their resistance to severe tribological conditions of modern metal cutting associated with high localized stress as well as high temperature on the friction surface [7, 60, 99]. Therefore, the development of hard coatings was mostly focused on the improvement in their hardness [7, 100, 101] and thermal and oxidation stability [47, 102–106], as well as on the reduction of their thermal conductivity [47, 95, 107–109]. A few categories of nanocomposite coatings are used most widely for cutting tools applications [2, 27, 89–93, 96, 110–112]: (i) designed as a combination of various compounds synthesized during coating deposition, such as TiN and AlTiN or AlCrN/Si3N4 [47, 113, 114], as well as TiN/c-BN coatings [97, 98, 115]; (ii) formed as a result of annealing (for example of AlTiN coating with a high Al content of ∼65 at%) [2, 102–106, 116]. Literature indicates the major drawbacks of hard coatings designed in this way. Despite nanocomposite ‘superhard’ coatings, i.e. with hardness above 40 GPa, having excellent ability to diminish crack initiation, they cannot prevent failure of the coating with further severe surface damage of cutting tool [70, 81, 117, 118], as the coatings do not have the same energy dissipative mechanisms available as softer films. Besides, this family of coatings has a relatively high coefficient of friction at high temperatures [1, 113, 119, 120]. Although several studies report successful application of nanocomposite superhard coatings [47, 121–123], there is still a lack of in-depth investigation of their wear performance, and the design of various nanocomposite coatings has to be customized for specific applications [47]. Multifunctionality of these coatings is an open question because it is not quite clear which combination of characteristics allows them to sustain varying operating conditions. These types of coatings are mostly suited for specialized applications, first of all dry cutting applications, such as ball-nose end-milling of hardened tool steels with hardness above 55–60 HRC [47, 110], or applications involving continuous turning of various materials [106].
2.2. Adaptive/smart coatings: previous art
We suggest the whole family of adaptive coatings can be divided into three main groups:
-
(a)
Adaptive nanocomposite coatings that combine in their structure different compounds such as hard phases, lubricating compounds, etc [124]. These coatings change their structure and properties in response to various operating conditions [125–128].
-
(b)
Smart coatings, which are functionally graded systems that may be characterized by the variation in composition and structure gradually over volume, resulting in corresponding changes in the properties depending on the stages of wear process [99, 129–136].
-
(c)
Adaptive coatings that can change their structure and properties as a result of the engineered surface dynamic response to the environmental impact due to formation of surface tribofilms.
In the first two groups, adaptive behavior is given in the initial design of the coating. In the third group, adaptability dynamically develops during operation. The adaptive coatings of the third group possess enhanced ability to generate tribofilms on the friction surface on an on-going basis, which protect and lubricate the surface during operation and critically lower the wear rate.
3. Novel approach for adaptive hard coatings design for severe tribological applications: adaptive coatings as surface-engineered hierarchical materials
Hierarchical materials (both natural and synthetic) exhibit structure on more than one length scale; in some materials, the structural elements themselves have structure [34]. This structural hierarchy can play a major part in determining the material properties. Adaptive hard coatings are a typical example of hierarchical materials.
In recent years, materials scientists have started to consider the complex hierarchical structure of natural materials as a model for the development of new types of high-performance engineering materials [23, 137–145]. In biomaterials, a dynamic strategy is realized. This means that the final result is obtained by an algorithm instead of copying an exact design. Similar features are typical for adaptive coatings as well [6, 40]. The major feature of adaptive wear-resistant coatings that allows one to regard them as nature-inspired hierarchical materials is the formation of various nanoscaled tribofilms on the surface as a result of the self-organization process during friction [36, 40–41].
3.1. Dynamic formation of nanoscaled films on the surface of adaptive hierarchical tribomaterials
3.1.1. Generic concept of self-organization for surface-engineered materials.
Friction is a complex phenomenon that could be considered as an irreversible nonequilibrium process. This process may leap either into chaos or greater complexity and stability due to self-organization with dissipative structures formation [146–149].
To use approach of nonequilibrium thermodynamic [10, 150], some generic features of friction should be outlined. Wear rate can vary over a wide range of magnitudes; a large variety of different wear mechanisms exist, but wear is a fundamental characteristic of friction for any tribosystem. Some researchers [151–153] have also concluded that the other generic characteristic of friction is the formation of tribofilms or secondary (i.e. formed during friction) structures on the surface [153]. Stabilization of the parameters of friction occurring at the running-in stage is accompanied by the formation and stable regeneration of tribofilms [151].
Friction is an irreversible process because energy dissipation takes place [154]. We should be interested not only in the initial and final stage of the friction process, but also in the way this process has developed as well as in the possibilities to optimize this dynamic process. Friction corresponds to the conditions of equilibrium thermodynamics only under marginal conditions [10] that cannot definitely be related to the extreme tribological conditions that are the major focus of this review. In this latter case, intensive gradients of various characteristics occur on the friction surface [155, 156]. That is why traditional thermodynamics could not be applied to this tribological application. According to Prigogine and Kondepudi [149], among many fundamental laws of nature, only the second law of thermodynamics implies a direction and the possibility of evolution. That is why irreversible thermodynamics, as well as the theory of self-organization, is based on the second law of thermodynamics [147, 157].
Structures are generated within the system due to intensive matter and energy flow, i.e. due to intensive irreversible processes. These structures are consistent with the energy flow and cannot exist without it. The structures that spontaneously form under strongly nonequilibrium conditions are called ‘dissipative structures’ and the process of dissipative structure formation and material ordering is self-organization [149]. Dissipative structures differ from the equilibrium ones in that they are the processes. The origins of these structures are intensive spontaneous processes with negative entropy production that previously did not exist in the system. The driving force of self-organization is that the open systems tend to protect and stabilize themselves. This could be related to the decrease in entropy production during nonequilibrium processes. Once the dissipative structures have been formed, the system can transfer more energy almost without any damage as compared to the conditions when these structures were not present. This could be explained by the following: the energy that was spent on the system damage prior to the formation of the dissipative structures is instead spent on their generation. That is why wear rate critically drops in a tribosystem, since the self-organization process has been initiated. The self-organization during friction is usually associated with the formation of energy-rich surface structure. If the phase and/or structure transformation takes place in a solid (in our case in hard coatings), then, since the flows of matter and energy disappear, the dissipative structures disappear as well. However, the metastable structures (tribofilms) still remain on the surface since the friction has been stopped, as a sort of imprint [10].
The concepts of self-organization and irreversible thermodynamics may be successfully used for development of advanced tribomaterials, in particular adaptive hard coatings. As shown below, adaptive wear-resistant nanostructured coatings fully develop their potential under extreme tribological conditions. These conditions are very far from equilibrium and the wear rate of the unprotected friction surface is extremely high. Therefore, we can consider the tribosystem as working close to the ‘edge of chaos’ [16]. Moreover if the operating conditions are characterized by a strong (but in engineering practice, somewhat controlled) instability, the gradient of characteristics on the friction surface is even growing further. Therefore, in complex surface-engineering systems such as adaptive coatings, the probability of self-organization is higher [52, 53, 158]. Tribosystems under these conditions may be close to self-organized critical systems. In physics, self-organized criticality is a critical point at which a system radically changes its behavior or structure and shows avalanche-like behavior [159]. Self-organized criticality could be associated with the stick and slip phenomenon during friction that is similar to avalanche-like behavior [160, 161]. The stick-slip phenomenon is typical in cutting process while the chip slides along the rake surface of the tool [162]. Cutting of hard to cut materials (such as aerospace materials) is an example of severe tribological conditions that has some interesting features. In general, the process is characterized by a high instability. A common feature of aerospace materials machining (such as Ni-based superalloys) is a formation of strong buildup due to intensive adhesive interaction (seizure) of the cutting tool surface with the workpiece material [163, 164]. The formation of a built-up layer is attributed to self-organization of the ‘tool/workpiece’ tribosystem. The built-up layer is a composite ‘third body’, which consists of heavily deformed and refined machining material, as well as various compounds generated during cutting as a result of interaction with the environment [6]. The stability of a built-up layer is very low and leads to unstable attrition wear conditions [7]. The repeated failure of the built-up layer results in intensive wear as well as significant tool surface damage. From a tribological viewpoint, this combination of seizure with deep surface damage is a typical catastrophic wear mode [6]. Similar severity of wear conditions is observed during ultrahigh-speed end-milling of hardened steels. Combination of very large stress and temperatures on the friction surface with thermal cycling also results in extreme and unstable wear conditions [6].
3.1.2. Tribofilms (secondary structures).
During friction the tribofilms are generated from the base surface of the engineered material by structural modification and interaction with the environment (mostly oxygen from air) [6, 165–169]. Tribofilms carry out protective, thermal barrier and lubricious functions.
According to the principle of ‘dissipative heterogeneity’ [130, 170] a phenomenon of structural adaptation of tribomaterials occurs, which leads to the concentration of the larger part of the interactions between frictional bodies within the thin layer of the tribofilms (secondary, i.e. generated during friction structures, SS) [171]. The depth of the layer can be lower by an order of magnitude (or more) than that typically associated with damage phenomena [170].
During machining, most of friction energy transforms to heat and dissipates through various channels: via chip removal, into the workpiece, into the environment, and so on [60]. However, an appreciable fraction of the energy of friction is accumulated within the layer of tribofilms [1, 172, 173]. Note that self-organization is a probabilistic process and it is impossible to make a reliable evaluation of the portion of the energy accumulated in the tribofilm. We can only state that intensity of wear, after self-organization begins, is reduced markedly as demonstrated by the experimental data presented below. Therefore, the layer of tribofilms could be considered as a steady state zone where intensive energy dissipation as well as accumulation is taking place affecting wear resistance under extreme operational conditions.
To understand mechanisms of tribofilm formation, the self-organizing process during friction has to be considered based on the thermodynamic analysis of the cutting tool/workpiece tribosystem [10, 174, 175].
As shown in detail in [174] the change of entropy of a frictional body (dS) can be expressed as
After differentiation with respect to time equation (1) in a stationary condition will look as
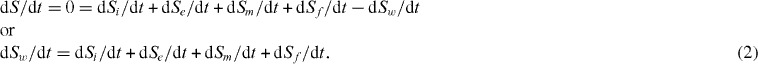 |
Here dSi/dt is entropy production as a result of the distribution of heat and other flows within a body; dSe/dt—flow of entropy due to external impact, which could change due to the transition of heat from a hotter friction surface into a colder body (the cutting tool) and which results in an increase of entropy; dSm/dt—change of entropy due to the formation of chemical compounds on the tool surface due to the entropy of the matter from the contacting frictional body (in our case, it is mostly due to cutting tool–chip interactions with further formation of seizure zones or buildups [165, 176, 177]); dSf/dt—change of entropy due to tribofilm formation; dSw/dt—change of entropy due to wear process (the ‘ − ’ sign is used in the equation because the wear products leave the frictional body with their own entropies). The change of entropy, dSf/dt, could be negative, if nonequilibrium processes are taking place on the surface and entropy of a frictional body is reduced, or positive, if the equilibrium processes are going along [174]. However, the sum of these terms, i.e. dSi/dt + dSf/dt > 0, is the general entropy production [174]. As it follows from (2), lower entropy production corresponds to a lower dSw/dt value. Bearing in mind that entropy is an additive value, we can consider that the lower dSw/dt, the lower the wear rate. Therefore, the entropy production decrease leads to reduction in the wear rate. Nonequilibrium processes on the surface (dSf/dt < 0), with all other conditions being equal, can also reduce the wear rate. Nonequilibrium processes are steadily going along while the dissipative structures are forming during the process of self-organization.
It has been found that there are two types of tribofilms: (i) superductile and lubricious and (ii) triboceramics with thermal barrier properties and increased hardness and strength [130, 166, 169]. Tribofilms (SS) of the first type (SS-I) are observed as a result of structural activation that is marked by an increase in the density of atomic defects at the surface. Tribofilms are supersaturated solid solutions formed by reaction with elements from the environment (usually oxygen). SS-I films are similar to Beilby layers [168, 169]. In these secondary structures, the material may be superplastic (due to a nanoscale-grained or amorphous-like structure) with an elongation up to 2000% [130]. The amorphous-like structure of SS-I may also lead to a decrease in the thermal conductivity of the surface, an important consideration in controlling friction of cutting tools [178]. Secondary structures of this type mostly promote energy dissipation during friction.
Tribofilms of the second type (SS-II or triboceramics with a higher content of elements such as oxygen) are primarily formed by thermal activation processes. SS-II films are usually nonstoichiometric compounds [166]. Depending on chemical composition, secondary structures of this type exhibit either lubricating (such as high-temperature lubricating oxides [6]) or surface-protective properties due to high thermodynamic stability, thermal barrier properties and high hardness [166]. The adaptation of the tribosystem, in this case, relies upon a low-intensity chemical interaction with the workpiece, beneficial heat distribution on the friction surface and the high hardness of the tribofilms. This results in low entropy production during friction and, obviously, leads to a decrease in the wear rate. On the other hand, destruction of these hard films should be prevented by proper surface engineering of the substrate material that ensures effective support of the tribofilms during friction.
Two features of tribofilms formation on the friction surface have to be outlined. The first is the repetitive formation and destruction of the tribofilms during friction. The second is that the formation of tribofilms on the friction surface is an evolutionary process. Self-organization during friction is developing during the initial running-in stage of wear [6]. Despite evolving in a step-by-step fashion, and becoming increasingly complicated, the tribofilms eventually stabilize for a given tribopair and conditions of friction. When the characteristics of the surface layers become optimal, the running-in phase is completed, and the wear rate stabilizes at a significantly lower level [6]. As shown in detail in [179], tribofilm formation starts from generation of unstable intermediate phases and then gradually transforms to the more complex tribofilms that are better adapted to specific operating conditions. According to classical evolution theory [180] the different biospecies are competing with each other in the given environment. Those species that are best adapted to the environment have better chances to survive. Therefore in new generations, the species with spontaneous beneficial deviations will dominate. Over time this is a mechanism ensuring continual long-term advance in the bioworld from the simplest species to the most complex and best-adapted organisms. It is shown below that something similar likely occurs on the friction surface (see table 1 and corresponding text for details).
Table 1.
Types of tribofilms formed on the surface of coated tools in relation to severity of tribological conditions and wear resistance improvement.
| Coating |
Major parameters controlling severity of tribological conditions |
Types of tribofilms formed |
Wear resistance improvement |
||
|---|---|---|---|---|---|
| Cutting speed (m min−1) | Workpiece material | Lubricious amorphous phase and triboceramics | Protective triboceramics | ||
| TiNa | 50–70 | Annealed 1040 steel, HB 220 | Amorphous Ti–O | More than twice compared to uncoated steel [127]. | |
| Nanocrystalline TiAlNb | 250 | Annealed 1045 steel, HB 220 | Amorphous Al–O | Aluminad | More than 4 times compared to commercial TiAlN [130] |
| Nanomultilayered TiAlCrN/WNc | 220 m min−1; dry cut | H13, hardness 53–55 | Amorphous W–O and variety of W–O triboceramics with selective adaptation to the frictional conditions | Aluminad | Almost twice compared to commercial TiAlCrN [65] |
| Nanomultilayered TiAlCrN/NbNc | 300–400 m min−1; dry cut | H13, hardness 53–55 | Variety of Cr–O triboceramics; variety of Nb–O triboceramics with enhanced ability of energy dissipation | Sapphired | Almost four times compared to commercial TiAlCrN [66] |
| Nanocrystalline TiAlCrSiYNc | 500 m min−1; dry cut | H13, hardness 53–55 | Variety of Cr–O triboceramics; Si–O triboceramic, amorphous Al–O | Sapphired Mullite | 1.3 times compared to commercial state-of-art nanocomposite coating; productivity improved almost twice compared to commercial TiAlCrN [12] |
| Nanomultilayered TiAlCrN/TiAlCrNc | 500–700 m min−1; dry cut | H13, hardness 53–55 | Variety of Cr–O triboceramics; Si–O triboceramic, amorphous Al–O | Increased amount of sapphired and mullite | 2.15 times compared to commercial state-of-art nanocomposite coating; productivity improved more than 2.3 times compared to commercial TiAlCrN [5] |
HSS turning inserts.
Carbide inserts.
Carbide ball-nose end mill.
Non-protective rutile tribofilms are observed as well.
Some examples are presented below on various types of tribofilms formed under severe tribological conditions (table 1). Secondary ion mass spectroscopy (SIMS) depth profile of the tribo-oxide layer formed on the surface of the cutting tool shows that its thickness is below 100–150 nm [181]. Comprehensive data on the composition, atomic, electronic and crystal structure of the nanoscaled tribofilms have been obtained [182–185].
The structure of the tribofilm formed on the surface of cutting tools strongly depends of the severity of operating conditions. If high-speed-steel cutters are used with a traditional TiN-based PVD coating at moderate cutting speeds (50–70 m min−1) and tribological conditions are not that severe (machining of annealed 1045 steel) then only amorphous tribofilms form on the friction surface (table 1) [179]. As outlined above, these amorphous films, which are SS-I, have high plasticity and improved lubricating properties [130]. Under more severe cutting conditions (machining of annealed 1045 steel at cutting speeds of 250–450 m min−1) cemented carbide tooling has been used with TiAlN nanocrystalline coatings [186]. A more complex phenomenon takes place on the friction surface. Mostly amorphous (with a minor crystalline fraction) tribofilms are formed on the surface of the TiAlN coating due to higher service temperatures as a result of the thermal activation process [166] (table 1). The only protective-layer material that can withstand these more aggressive cutting conditions is alumina [187]. Crystalline alumina films additionally improve the wear behavior because: (i) they are composed of a chemically stable compound which limits adhesive interaction on the friction surface, (ii) they have thermal barrier properties [188] that prevent heat generated during cutting to be transported into the tool surface and subsurface areas. More severe frictional conditions are associated with dry (coolant free) machining of hardened tool steels (H13, hardness HRC 53–55). Under these conditions, an adaptive nanomultilayered TiAlCrN/WN coating with enhanced high-temperature lubricating ability has been used at a cutting speed of 220 m min−1 (table 1) [86]. For this kind of coating, protective alumina tribofilms form on both flank and rake surfaces. The W–O tribofilms are usually formed on the rake surface of the end mills with a TiAlCrN/WN coating. X-ray photoelectron spectroscopy (XPS) analysis indicates the presence of various W-based triboceramics and amorphous WOx films. Tribofilms based on oxides of heavy metals, such as W, Nb, Ta and others [189], look promising for machining of hard to cut materials, because they have high-temperature lubricous properties [190]. Formation of various tribo-oxides is typical for these heavy-metal compounds [191]. The oxides belong to so called ‘Magneli’ phases [192–195] that possess lubricious properties because they contain crystallographic shear planes with low shear strengths at high temperatures [196]. Therefore, the tribosystem potentially has a higher possibility to select an oxide with a better combination of properties suitable for the specific conditions; this results in additional improvements in tool life and wear behavior [197]. Several W–O tribo-oxides are forming on the friction surface. XPS analysis shows that the strongest peak belongs to WO3, a good high-temperature lubricant [124, 190, 197–199]. As a result of the self-organization process, the tribosystem selects a specific type of tribofilms tailored to given frictional conditions. The preferred configuration of tribofilms (dissipative structures) will be one that allows high energy throughput without significant surface damage [200]; it would reduce friction [86] and thus increase wear resistance. This is one more example of similarity in adaptation between artificial surface-engineered systems and biosystems [201].
When considering more severe tribological conditions, a lubricious tribofilm with a higher thermal stability could be beneficial. For dry machining of hardened tool steels (H13, hardness HRC 53–55) with cutting speeds of 300–400 m min−1 one can use an adaptive nanomultilayered TiAlCrN/NbN coating that has improved high-temperature lubricating properties (table 1) [87].
Under these, more severe tribological conditions the tribofilms formed during friction consist of protective sapphire-like tribo-oxides [31, 96, 191, 197, 202–207] with some nonprotective (at elevated temperatures) rutile-like tribofilms and polyvalent lubricious tribo-oxides of Cr and Nb [87].
Note that for high-performance machining, the Nb-based oxide tribofilms could significantly enhance wear resistance at elevated temperatures, as they have high thermal stability due to strong interatomic bonds in a compound with a heavy atomic nucleus [191]. Thus a TiAlCrN/NbN nanomultilayer coating exhibits its protective functions with growing cutting speeds up to aggressive conditions of 400 m min−1 (table 1).
One more cause of life improvement in the TiAlCrN/NbN coated cutting tool is the ability of Nb–O tribofilms to dissipate energy during friction at elevated temperatures. Literature data show that one of the features of Nb-based oxides is their abnormally high electrical conductivity at high temperatures compared to other tribo-oxides formed on the friction surface, such as alumina, rutile and Cr-based tribo-oxides [197, 204]. This property indicates some metallic character of a Nb-based tribo-oxide. Having metallic properties, this oxide works as a solid lubricant with an increased ability to dissipate energy during friction due to existence of metallic bonds that was proved experimentally using high-resolution electron energy loss spectroscopy (HREELS) [87, 187, 205–208]. It is worth noting that the wear behavior of Nb–O tribofilms is a clear illustration of the term ‘dissipative structure’.
Under extreme conditions, the speeds may increase up to 500 and even 700 m min−1 during dry cutting of hardened H13 tool steel (table 1) [24], and the surface may heat up to 1100 °C, and probably even higher temperatures [48]. Nanostructured TiAlCrSiYN-based coatings have been successfully used in such cases [24].
Selected XPS data of the worn crater surface of the monolayer TiAlCrSiYN coating are shown in table 2. Chromium oxides with various valences are formed during wear (table 2). Titanium has a higher affinity to oxidation than chromium. This leads to formation of a nonstoichiometric titanium oxide films and rutile TiO2 (table 2). XPS indicate formation of sapphire-like [207] triboceramics and nanofilms with a phase composition close to Al6Si2O13 mullite [209–214]. A partial chemical transformation of aluminum in the TiAlCrSiYN monolayer coating has taken place: 28.6% of Al was retained in the complex nitride, 17.6% of aluminum oxidized with the formation of Al–Si–O oxide (mullite) and 8.8% of aluminum oxidized with the formation of trivalent Al2O3 (sapphire, table 2). Formation of sapphire/mullite tribofilms could be attributed to the interaction of two processes: (i) severe, highly nonequilibrium operating conditions with high gradients of characteristics on the friction surface and (ii) enhanced tribo-oxidation of the nanostructured coating with a nonequilibrium structure that promotes its physical and chemical reactivity [215]. If both of these conditions are not fulfilled, for instance tribological conditions are not very severe (moderate cutting speed, table 1) or the coating does not possess a nonequilibrium state (annealed coating for instance [31]) then instead of sapphire a less protective alpha-alumina phase is formed on the friction surface (table 1).
Table 2.
| Coating |
Chemical composition |
|||||||
|---|---|---|---|---|---|---|---|---|
| Al (at%) |
Ti (at%) |
Cr (at%) |
||||||
| Al2O3 (sapphire) tribofilm | Mullite tribofilm | Nitride coating | TiOx tribofilm | TiO2 tribofilm | Nitride coating | CrxOy tribofilm | Nitride coating | |
| TiAlCrSiYN/TiAlCrN multilayer | 29.1 | 6.6 | 19.3 | 7.3 | 5.5 | 7.2 | 15.9 | 4.1 |
| TiAlCrSiYN monolayer | 8.8 | 17.6 | 28.6 | 6.8 | 7.2 | 6.0 | 16.0 | 4.0 |
Introduction of the more advanced TiAlCrSiYN/TiAlCrN nanomultilayer coating with modulated chemical compositions of the nanolayers results in further enhancement of the protective/thermal barrier ability of the surface [17]. That is why this coating can sustain even ultrahigh-speed machining conditions (cutting speed up to 700 m min−1, table 1). This is illustrated by the XPS data shown in table 2. A partial chemical transformation of aluminum in the multilayered coating is taking place: 19.3% of Al is retained in a complex nitride, 6.6% of aluminum oxidizes with formation of a complex Al–Si–O oxide (mullite), and 29.1% oxidizes with the formation of Al2O3 (sapphire) [207–216], which is two times higher compared to the monolayered coating (table 2). XPS data indicates the formation of Al–Si–O bonds in Al6Si2O13 mullite tribofilms [209–212]. The nonstoichiometric titanium tribo-oxide (7.3%) and rutile TiO2 (5.5%, table 2) are also formed on the friction surface. Along with the lubricious Cr–O (table 2), protective and high-temperature lubricous Si–O phases are formed as well [17, 211–218]. The phase composition of tribo-oxides differs for monolayered and nanomultilayered coatings: a considerably higher concentration of Al2O3 is formed during tribo-oxidation of the multilayered coating (table 2). Overall, the amount of protective sapphire and mullite-like tribofilms is significantly higher in the multilayer coating as well. As shown previously [63], the aluminum-rich Ti0.25Al0.65Cr0.1N nanolayers are characterized by a higher oxidizing ability than the Ti0.2Al0.55Cr0.2Si0.03Y0.02N ones. Owing to the contribution of the more aluminum-rich Ti0.25Al0.65Cr0.1N nanolayers in the multilayered coating, the amount of alumina tribofilms on the friction surface grows. The amplification of boundary diffusion caused by the increase of density of interfaces and grain boundaries in nanomultilayer coatings is another important factor [219]. The sapphire phase rapidly grows on the surface of the nanolayered coating as a result of intensified mass transfer of aluminum with further tribo-oxidation at high temperatures [220].
To confirm the XPS results, the atomic structure of the tribofilms was investigated using the electron energy loss fine structure (EELFS) technique. The fine structure of electron loss spectra in the 250 eV range near backscattered peak (E0 = 1000 eV) has been analyzed. The Fourier transforms (an analog to the radial distribution function [28, 29]) are shown in figures 1(a)–(d). Each peak position corresponds to a radius of coordination sphere in the crystal lattice. It is shown in figures 1(a) and (b) that two phases coexist on the surface of the worn crater: sapphire- and mullite-like tribofilms, since the measured lengths of nearest interatomic Al–O, O–O and Si–O bonds are close to the standard crystallography data [216, 221, 222] as well as experimental Fourier transform of R-sapphire and mullite standards (see the scales on the top and bottom in figures 1(a) and (b) [216].
Figure 1.
Fourier transforms of EELFS spectra from various microareas on the surface of worn craters of cutting tools with the TiAlCrSiYN monolayer coating (a, b) and TiAlCrSiYN/TiAlCrN multilayer coating (c, d). The nearest atomic surrounding has characteristics of sapphire (a) and mullite (b) structure; the standard interatomic bonds lengths in sapphire and mullite are presented at the top and the bottom, respectively. (Reproduced with permission from [17, 24], Elsevier, AIP.)
Simultaneous formation of such refractory compounds as sapphire and mullite leads to excellent protection of the surface under severe frictional conditions (with increasing cutting speeds). Sapphire is the hardest oxide possessing an excellent thermal shock resistance at high temperatures [207, 222], and mullite is a refractory compound that has similar high-temperature properties [209–211, 214]. Both materials, especially sapphire, have excellent ability to accumulate energy from external impact [216, 222–224] that leads to reduction of entropy production during friction. Being chemically stable materials, they reduce adhesive interactions at the workpiece/tool interface and therefore heat generation during cutting. This could shift the friction to a much milder mode [99], significantly reducing the wear rate.
The Fourier transforms of the worn surface of the TiAlCrSiYN/TiAlCrN coating are shown in figures 1(c) and (d). The EELFS spectra were obtained from two different places at the worn surface. The structural peaks in the Fourier transform of figure 1(c) correspond to the interatomic distances in the sapphire-like aluminum oxide [216, 221, 222]. The Fourier transform in figure 1(d) was calculated for the other area at the tool surface. In this case it has four wide peaks at 1.0–5.0 Å interatomic distances (figure 1(d)). The first peak is nonstructural. Complex chemical composition of the tribofilms provides a large set of pair interatomic distances. The phase with high concentration makes the biggest input in the radial distribution function. The first intense structural peak is formed by the mullite oxide and the nonoxidized coating (figure 1(c)) [218]. The presence of a Si-Ox phase is also possible [225–228]. One can see in figures 1(c) and (d) that similar to the worn surface of monolayer coating, sapphire- and mullite-like tribofilms are also present on the friction surface [229]. However, if we compare the intensity of peaks of the sapphire phase at the remote atomic distances above 4 Å, they are significantly higher for the multilayered coating (figures 1(a) and (c)) that corresponds to XPS data presented in table 2. A higher amount of the protective crystalline triboceramic phase additionally improves wear behavior resulting in better tool life (table 1).
From experimental data we can see that selection of the elements to optimize an adaptive coating's design should consider the features of tribofilm formation and understanding of their desirable characteristics for specific applications [6]. This selection depends on severity of tribological conditions. If conditions are extreme, such as dry end-milling of hardened steel at 500–700 m min−1, then the following features of tribofilms that ensure high tool life could be outlined: (i) the tribofilms have to act in synergy (as a whole) and in this way present emergent behavior [230] to protect/lubricate the surface at high operating temperatures. (ii) The elements that form high-temperature stable oxides (such as Al, Si, to a lesser degree Cr and other similar elements) can be used for the coating alloying to enhance formation of protective triboceramic films. (iii) The lubricating action could be performed by the oxygen-containing tribofilms that have enhanced oxidation stability at high temperatures (such as Cr-O tribofilms [203] and amorphous-like Al–O, Si–O tribofilms). This would prevent intensification of chemical and oxidative wear during high-speed cutting [231–234]. In contrast, under less aggressive tribological conditions–for instance, at lower cutting speeds (table 1) or during machining of alloys that generate lower temperatures at the tool/chip interface–the formation of stable low-temperature lubricious oxides such as W–O and Nb–O triboceramics significantly improves the wear resistance (table 1).
3.1.3. Nanotribological effect of the thermal-barrier/lubricious tribofilms formation in relation to the adaptive behavior of surface-engineered hierarchical materials.
Adaptive behavior and the related process of self-organization are strongly associated with the gradient of characteristics within the entire system. The coating affects heat flow redistribution in the cutting zone [235]. As shown above, protective tribofilms formed on the surface of TiAlCrSiYN-based coatings have sapphire and mullite crystal structures [17, 24]. Thermal conductivity of these oxide ceramic films at high temperatures is noticeably lower than that of the nitride coating, and this difference grows with temperature [224, 236, 237]. The thermal barrier properties of the hard coating system, therefore, depend on the properties of the coating and, equally importantly, on the properties of the nanoscale layer of the tribofilms formed on the friction surface. Altogether, these characteristics can result in beneficial heat redistribution during cutting.
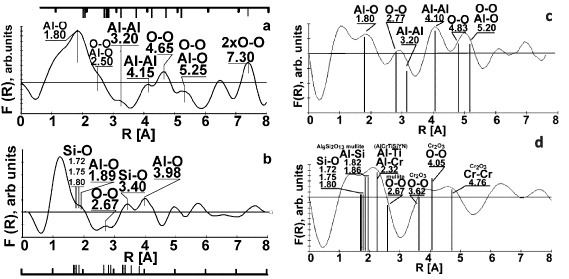
Critical features of tribofilms such as their protective ability and lubricity outlined above (table 1) determine the wear performance of adaptive coatings. Based on the heat redistribution analysis presented in [235], the dependence of heat flows on characteristics of the tribofilms formed on the coated tool surface is schematically shown in figure 2. If there are no tribofilms on the surface (figure 2(a)) then a significant portion of heat flows into the tool, heating the coated tool and rapidly destroying it. A relatively small portion of the heat goes into chips as well. When adaptive self-lubricating coatings are used, the heat flow distribution is different (figure 2(c)). Owing to the formation of lubricious tribofilms with lower coefficients of friction on the coated surface of cutting tool, the chip/tool contact length diminishes, producing more curly chips [186, 238, 239]. This leads to the lower heat generation during operation (figure 2(c)) because heat efficiently dissipates on the friction surface with improved lubricious properties. Less heat flows into the tool and chip and the actual temperature of coated tool body is reduced (figure 2(c)). Formation of the tribofilms with enhanced protective and thermal barrier properties results in a very complex process at the tool/chip interface associated with: (i) lower heat generation due to diminished adhesive interaction at the chip/tool interface [17, 240], and (ii) significant reduction of heat flow into the body of the coated tool (figure 2(b)) [241] that allows operation under more aggressive conditions. The thermal barrier layer of tribofilms raises the temperature at the tool-chip interface because the cutting temperature grows with decreasing thermal conductivity of the tool material (in our case alumina-based tribofilms). On the other hand, experimental studies show that owing to the improvement in thermal behavior of the coating with protective tribofilms, heat is largely transferred into chip, which softens its surface [17]. The metal flow at the tool/chip interface is enhanced. Combined with lower adhesive interaction at the chip/tribofilms interface, frictional conditions are improved and cutting forces are reduced [17]. Data presented in figures 3–5 show that a larger portion of heat flows into the chip and dissipates via chip removal [17]. The thermal barrier tribofilms serve as a nanolayer that accumulates a significant portion of the frictional energy [10, 236] and prevents heat penetration into the body of the tool. With these types of tribofilms formed on the surface, the actual temperature of the coating underneath decreases significantly (see figures 3–5). Under extreme operating conditions, these two methods of beneficial heat redistribution are working in synergy owing to simultaneous formation of two types of tribofilms (lubricious and thermal barrier) [6].
Figure 2.
Beneficial heat redistribution on the surface of coated cutting tools due to formation of various tribofilms: (a) no tribofilms, (b) lubricious tribofilms and (c) protective tribofilms with the thermal barrier properties. Vc—cutting speed, m min−1; Q—heat flow: Qch—heat flow in chips; Qt—heat flow in tool, Vc1 = Vc2 = Vc3.
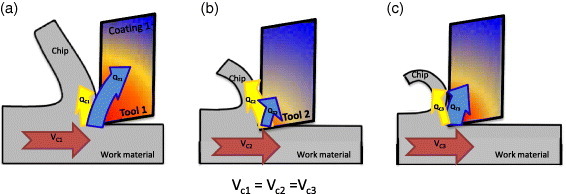
Figure 3.
TEM images (cross-sectional views) of the worn coated ball-nose end mills with (a) TiAlCrSiYN monolayered and (b) TiAlCrSiYN/TiAlCrN multilayered coatings. (Reproduced with permission from [17], Elsevier.)
Figure 5.
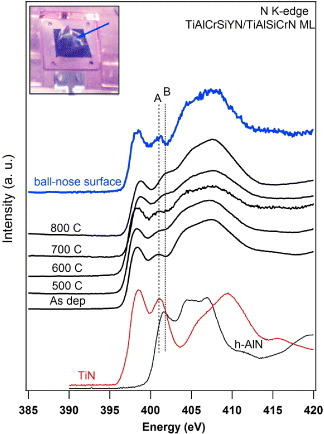
Partial fluorescence yield x-ray absorption spectra of N-K edge after vacuum annealing of the TiAlCrSiYN/TiAlCrN multilayer coating at various temperatures: c-TiN and h-AlN N-K edge standards are added for comparison, to show the evolution in thermal decomposition of the TiAlCrSiYN/TiAlCrN multilayer. Features A and B of the nitrogen signal are related to the first, most prominent peaks of c-TiN and h-AlN, respectively. The inset shows the tip of the worn ball-nose tool that was cut and placed on top of a copper holder to acquire the XANES signal. (Reproduced with permission from [240], AIP.)
With the formation of tribofilms, the adaptive coating transforms to a hierarchical material with different and improved characteristics as compared to the as-deposited state [240]. This is illustrated by transmission electron microscopy (TEM) investigations of the workpiece/tool interface for the cutting tools with adaptive TiAlCrSiYN monolayer and TiAlCrSiYN/TiAlCrN multilayer coating under extreme operating conditions (table 2) [17, 24]. Under high-temperature frictional conditions, when the surface temperature reaches 1000–1200 °C [240], significant surface damage and grain size coarsening are taking place on the surface of the monolayer TiAlCrSiYN coating (figure 3(a)) [24]. In contrast, no significant surface damage is observed in the TEM image of the workpiece/tool interface in the TiAlCrSiYN/TiAlCrN multilayer coating that is protected by sapphire/mullite tribofilms (table 2, figure 3(b)) [17]. Grain size coarsening in this coating on the friction surface is minimal compared to the as-deposited state (figure 3(b)) [17, 241, 242].
A thermally insulating coating, such as TiAlN-based coatings, impedes the heat flow to the substrate [62]. Note that the thermal conductivity of Al-rich TiAlN-based coatings is relatively low due to the presence of the AlN nanophase [223, 236, 238, 243]. However, coatings with a similar amount of Al but different structure have very different ability to form protective oxide films on the surface (table 2). This affects the physical and chemical properties of the coatings responsible for their thermal behavior (see below) and correspondingly controls their wear performance. Therefore, formation of alumina tribofilms on the surface identified by XPS and EELFS mostly controls wear mechanisms under extreme conditions of ultrahigh-speed machining (figure 1). The formation of these films beneficially redistributes the heat flow as shown in figure 2(c), largely preventing heat flow propagation into the body of the tool. This reduces the surface damage (figure 3) and affects phase transformation during friction (figure 4). The as-deposited TiAlCrSiYN/TiAlCrN multilayer coating has only a minor amount of hexagonal AlN (h-AlN) phase [17]. In addition to the low surface damage of this coating, it is hard to identify any h-AlN phase just below the layer of tribofilms (within the surface layer of 40 nm, figures 4(a) and (b)). At a greater depth (of around 80 nm) there is a small amount of a phase that corresponds to h-AlN (100) ∼0.268 nm (figures 4(c) and (d)). This indicates that the temperature of the coating below the layer of the tribofilms is strongly reduced. This marked reduction in the temperature of the coating (probably down to around 600–700 °C) hinders the damage (figure 3) and phase transformations (phase decomposition, figure 4) within the surface layer. TEM results (figure 4) are clearly confirmed by x-ray absorption near-edge structure (XANES) data shown in figure 5. The comparison of the line shape of the nitrogen signal in figure 5 indicates that the inner layer of the worn coating with the tribofilms formed on the friction surface did not reach high temperatures. Feature B, which is related to the first (most prominent peak from the h-AlN standard), is not predominant in the coating on the worn tool. However XANES analysis shows that during in situ heating of the coated layer in vacuum, formation of h-AlN phase at temperatures above 600 °C is quite significant (figure 5). The clear reduction in the formation of the undesirable h-AlN phase beneath the layer of tribofilms indicates a strong temperature gradient (up to 400–600 °C) within this surface layer [240, 241]. This reduces damage at high temperatures and explains why this coating can sustain such extreme service conditions when the cutting speed reaches 500–700 m min−1 (table 1).
Figure 4.
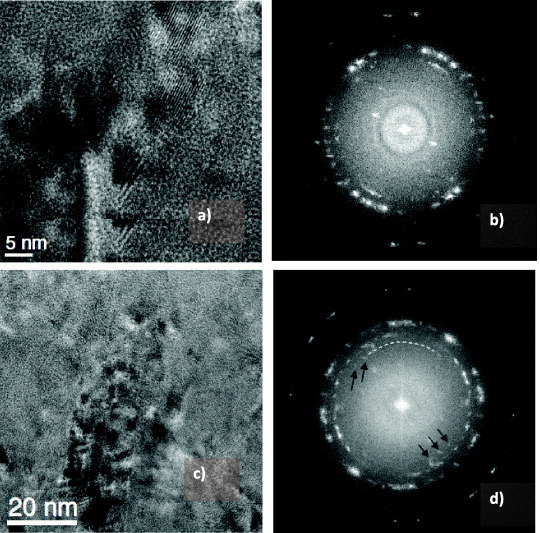
TEM images (a, c) with SAED patterns (b, d) of the worn TiAlCrSiYN/TiAlCrN coating (see figure 3): (a, b) 45 nm from the surface, no evidence of h-AlN; (c, d) 85 nm from the surface, small amount of the h-AlN phase. (Reproduced with permission from [240], AIP.)
3.2. Structural characteristics and major properties of the coating in surface-engineered hierarchical materials
The coating in surface-engineered hierarchical materials is equally important as the nanolayered tribofilms. To the best of our knowledge, few wear-resistant coatings can be regarded as complex systems with emergent behavior. In this section we present such a coating using the unique example of nanomultilayer TiAlCrSiYN/TiAlCrN coating [17]. Structural, physical, chemical and micromechanical characteristics of the coating are considered below (table 3).
Table 3.
| Coating | Microstructure | Microhardness (GPa) |
Maximum impact depth (nm;150 mN load) | Residual stress (GPa) | Weight gain, TG (%) after oxidation in air (0.5 h, 1000 °C) | DTA (μV;at 1000 °C) | |
|---|---|---|---|---|---|---|---|
| 25 °C | 500 °C | ||||||
| TiAl55CrSiYN | Nanocrystalline monolayer | 29.9+3.5 | 19.2+2.8 | 6155+359 | −0.490 | 0.015 | 27.96 |
| TiAl55CrSiYN/TiAlCrN | Nanocrystalline multilayer | 30.0+4.8 | 27.4+4.1 | 5901+207 | −0.520 | −0.020 | −41.74 |
3.2.1. Structural characteristics.
Selection of the structural category of the adaptive coating strongly depends on its field of tribological applications and ensures its ability to sustain specific extreme conditions. As outlined above, efficient coatings that are usually used under these conditions mostly belong to the Al-rich TiAlN family [3]. All these coatings consist of a solid solution of Al in a TiN crystal lattice [3] and have a cubic B1 crystal structure, as revealed by x-ray diffraction (XRD) and TEM [61, 62, 224, 245]. This cubic B1 structure can be stabilized at a higher Al content by introducing Cr to the Ti–Al–N system [63]. The wear-resistant AlCrN family of coatings [62], which are solid solutions of Al in CrN [61, 246], also became popular for severe tribological applications because they possess a combination of high-temperature hardness and oxidation stability [247]. However, comparison of tool life under extreme conditions shows that TiAlCrN coatings have better potential for extreme tribological applications [24, 242, 248].
Nanomultilayer coatings are of special interest in this review because they present some abnormal physical, chemical and micromechanical properties such as high-temperature hardness [241, 249, 250] and improved protective and thermal barrier properties [243] that are best suited for extreme tribological applications [2, 241, 251]. Moreover, nanomultilayer coatings can be considered as complex surface-engineered tribosystems with increased tendency to self-organization and adaptability [17, 52]. An example of such a surface-engineered system is shown below. A family of TiAlCrSiYN/TiAlCrN nanomultilayered coatings has been introduced recently to improve the properties of TiAlCrSiYN-based coatings. These coatings have a modulated chemical composition but similar characteristics of the alternating nanolayers (figure 6) [17, 242] [242]. Figures 6(a) and (b) present high-angle annular dark-field scanning transmission electron microscopy (HAADF-STM) images (cross-sectional view) of the Ti0.2Al0.55Cr0.2Si0.03Y0.02N/Ti0.25Al0.65Cr0.1N nanomultilayered PVD coating. It is possible to distinguish the nanolayers by the atomic number of the elements, which results in the different contrast observed for the alternating nanolayers. The bright nanolayers are Ti0.2Al0.55Cr0.2Si0.03Y0.02N, which contains the heavier element (Y) and is richer in Cr. The dark nanolayers are Ti0.25Al0.65Cr0.1N, which is richer in lighter elements (Al and Ti) and has a lower Cr content. Energy-dispersive x-ray spectroscopy (EDX) profiles of the layers correlate with the dark and white contrast in the HAADF image. The grain size is around 20–40 nm. The selected area electron diffraction (SAED) pattern indicates a B1 crystal structure (figures 6(a)–(c)). The coatings have a complex structure that combines nanomultilayers with modulated composition and columnar features (figures 6(a)–(c)). The continuous columnar growth has been attributed to the alternating layers having the same B1 crystal structure [64]. This complex structure promotes beneficial tribochemical reactions on the friction surface, encouraging self-organization [52] and improvement of some critical micromechanical properties [241, 242].
Figure 6.
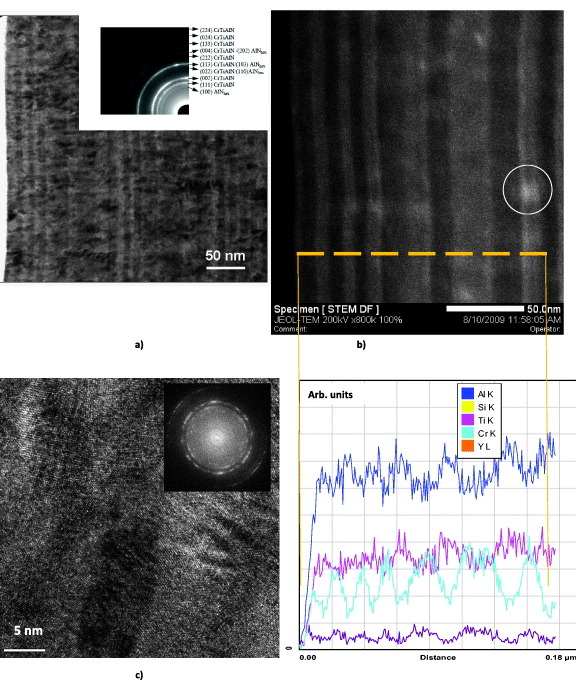
(a) TEM image of the Ti0.2Al0.55Cr0.2Si0.03Y0.02N/ Ti0.25Al0.65Cr0.1N coating (cross-sectional view) with SAED pattern inset, (b) HAADF-STEM image and energy-dispersive spectroscopy profile of the coating and (c) TEM image with SAED pattern inset. (Reproduced with permission from [17], Elsevier.)
3.2.2. Physicochemical properties: adaptive nanostructured coatings as nonequilibrium medium aiming to protect and lubricate the friction surface.
The coating is a surface-engineered hierarchical material intended, first of all, to enhance the mass transfer of desired elements to the friction surface to form protective/lubricious tribofilms and, secondly, to ensure accumulation and dissipation of the frictional energy during operation.
Formation of tribofilms as a result of tribo-oxidation during friction is closely related to the oxidation resistance of the coatings at high temperatures. In operation, the cutting edge is permanently exposed to high temperature due to friction-generated heat. For that reason oxidation wear is one of the dominant wear mechanisms at high temperatures [7] and is considered one of the most important properties of wear-resistant coatings.
Optimization of hard coating composition is a major way to improve oxidation resistance. It was shown that addition of Cr to the TiAlN-based coating strongly promotes its oxidation resistance owing to favorable changes in electronic and crystal structure [63, 252]. Many studies aimed to improve oxidation resistance of various heat-resistant metallurgical systems such as alloys based on MCrAl (M = Ni, Fe, Co) [253] or TiAl [132] by incorporating various foreign elements such as Y [68, 254–256], Hf, Nb, B [257, 258], V [259] or Si [64, 109, 111, 217, 260, 261]. Among these dopants, Si and Y seem to be the most promising for improving the oxidation resistance of hard nitride coatings at relatively small concentrations. The effect of Si and Y addition has been independently studied for nitride coating systems based on TiN [109, 261], TiAlN [111, 217] and AlCrN [68, 262]. The effect of Si on oxidation resistance is twofold. Preferential oxidation of Si is observed after the oxidation test and this Si-rich oxide film acts as a diffusion barrier for further oxidation [217], much like in case of the preferential oxidation of Al in the (Ti,Al)N coating [109, 263]. Addition of Si also results in grain refinement of TiAlCrN coatings [64]. It has been reported that the addition of Y greatly improved oxidation resistance of heat-resistant alloys and several mechanisms have been proposed [245]. Yttrium addition to Si-content TiACrN coating prevents intensive grain coarsening at elevated temperatures [24] and probably increases phase stability [264]. Since Si and Y seem to improve oxidation resistance via different mechanisms, simultaneous incorporation of both elements is effective [262, 265].
Oxidation resistance of hard PVD coatings is greatly enhanced by their nanocrystalline structure. Having a highly nonequilibrium state due to the formation of large numbers of lattice defects in the as-deposited state [2], the nanostructured coating possesses drastically improved mobility and reactivity of the necessary coating elements (such as Al, Si and in lesser degree Cr) at high temperatures and under heavy loads. It can transport these elements quickly (almost in an explosive-like manner) to the friction surface [266, 267]. The predominant formation of protective alumina films on the surface of the nanocrystalline TiAlCrSiYN-based coatings occurs during oxidation over a wide range of temperatures from 800 to 1200 °C. This leads to a very low oxidation rate [65, 242].
Oxidation behavior of TiAlCrSiYN-based monolayer and multilayer nanoscaled coating has been studied within a temperature range of 25–1000 °C in air using thermogravimetric and differential thermal analysis (TG-DTA) [17, 65, 242]. This range of temperatures was selected to mimic the severe cutting conditions during machining of hard to cut materials [7, 48, 163, 164]. TG data for a Ti0.2Al0.55Cr0.2Si0.03Y0.02N monolayer coating showed almost no changes up to 1000 °C with only a slight weight increase observed at higher temperatures (figure 7(a)) [17]. A Ti0.2Al0.55Cr0.2Si0.03Y0.02N/Ti0.25Al0.65Cr0.1N coating is slightly less stable at lower temperatures (figure 7(b)). However, this multilayered coating exhibits a very low weight gain at actual operating temperatures (0.01% at >1000 °C) compared to the already low gain of 0.03% for the monolayered coating at 1100 °C [48]. This is a significant improvement to lower-ordered coatings such as TiAlCrN [63, 86] and TiAlCrSiN [64]. DTA determines the thermal behavior of the coating and indicates formation of oxide films [268] that result in improvement of the thermal performance of the friction surface [269]. The DTA data indicate a phase change with the formation of the oxide. This phase change improves the thermal behavior, which results in a beneficial heat distribution on the friction surface. DTA values of the monolayered Ti0.2Al0.55Cr0.2Si0.03Y0.02N coating increase with temperature (figure 7(a)). In contrast they drastically reduce with temperature for the multilayered coating (figure 7(b)). These DTA data correspond to the nanotribological studies of thermal behavior of the tribofilms during machining (figures 4 and 5).
Figure 7.
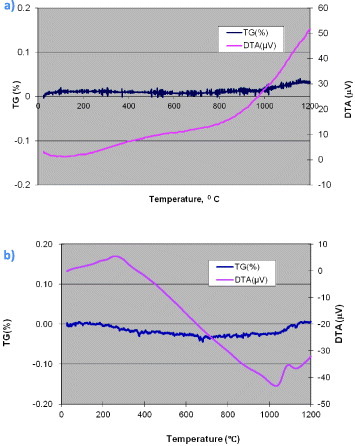
TG-DTA data versus temperature in air for (a) monolayered TiAlCrSiYN coating and (b) multilayered TiAlCrSiYN/TiAlCrN coating. (Reproduced with permission from [17], Elsevier.)
3.2.3. Micromechanical properties: adaptive hard coating as a surface layer providing dissipation and accumulation of frictional energy.
In addition to the ability to supply the surface with necessary elements for the formation of protective/lubricious tribofilms, the ‘bulk’ coating in the hierarchical surface-engineered nanomaterial has to perform two extra functions: (i) promote further energy accumulation and dissipation by the coating—this reduces the surface-damaging process of crack initiation and propagation that eventually increases the wear rate [229, 270]; (ii) ensure stable regeneration of the tribofilms embedded in the surface of the underlying coating.
One of the most efficient ways to do this is by application of the coatings with a nanomultilayered structure that can efficiently accumulate and dissipate thermomechanical energy supplied to the friction surface. As outlined above, this goal can be achieved with multilayer coatings that consist of alternating nanolayers with varied and modulated chemical compositions [3, 81, 83, 271]. Figure 8 presents micromechanical data for TiAlCrSiYN monolayer and TiAlCrSiYN/TiAlCrN multilayer coatings versus temperature up to 600 °C [240]. In the multilayer TiAlCrSiYN/TiAlCrN coating the hardness is stable at 27 GPa up to 500 °C and then drops a little at 600 °C, but still remains quite high at 22 GPa (figure 8(a)). In contrast, the TiAlCrSiYN monolayer coating gradually softens with temperature (figure 8(a)). The stable high-temperature hardness of the TiAlCrSiYN/TiAlCrN coating could be related to the hindering of dislocation movement by the layer interfaces in the nanomultilayer coatings under load [249, 250]. The TiAlCrSiYN/TiAlCrN multilayer coating also has significantly better load support (resistance to plastic deformation that scales with the H3/Er2 ratio [117, 272, 273], which is very high for this coating), especially at elevated temperatures (figure 8(c)). This parameter can show excellent correlation with the impact fatigue fracture resistance [17].
Figure 8.
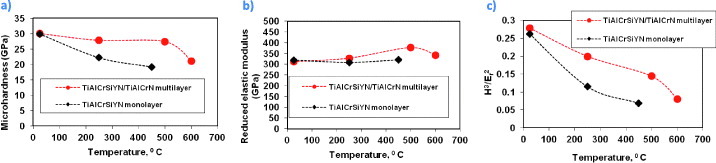
Micromechanical properties of TiAlCrSiYN/TiAlCrN multilayer and TiAlCrSiYN monolayer coatings measured at room and elevated temperatures: (a) microhardness, (b) reduced elastic modulus and (c) H3/Er2 ratio. (Reproduced with permission from [240], AIP.)
The impact fatigue fracture resistance is slightly better for the multilayered coating (figure 9). The impact plots at 150 mN (figure 9) show that the multilayered coating works better due to greater load support [272]. It minimizes the probability of initiating cracks [272], diminishes surface damage and improves wear resistance [17].
Figure 9.
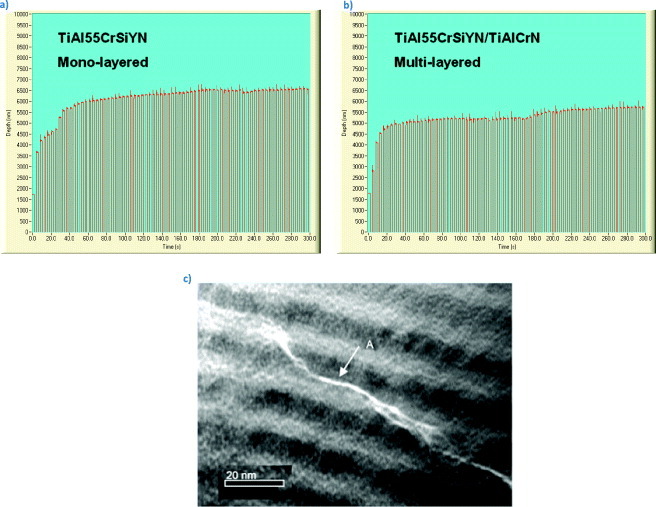
Impact fatigue data of TiAlCrSiYN/TiAlCrN multilayer (b) and TiAlCrSiYN monolayer (a) coatings measured at room temperature; (c) the cross-sectional TEM image shows the propagation of nanocracks in nanomultilayered coating, which could be a possible mechanism of thermomechanical energy dissipation in the studied coating. (Reproduced with permission from [17, 70], Elsevier.)
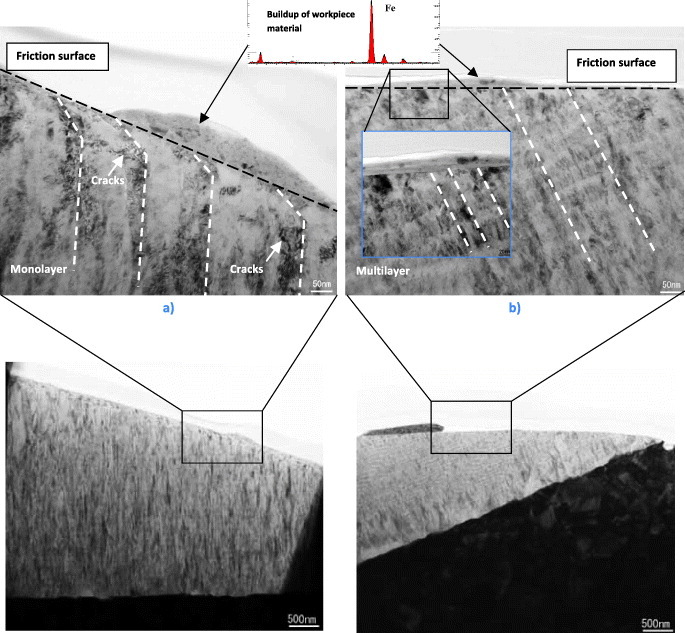
Abrupt fracture is typical for a monolayered Ti0.2Al0.55Cr0.2Si0.03Y0.02N coating. In contrast, cracks are deflected by the nanolayered interfaces in the Ti0.15Al0.6Cr0.2Si0.03Y0.02N/Ti0.25Al0.65Cr0.1N multilayered coating (figure 9(b)) [70, 242]. This mechanism of energy dissipation [251] was observed by TEM studies in multilayer coatings (figure 9(c)) [70]. Results of micromechanical testing are confirmed by progressive wear studies performed for ball-nose end mills with TiAlCrSiYN-based monolayer and multilayer coatings using scanning electron microscopy (SEM, figure 10). Cracks develop within the monolayer coating (figure 3) due to the surface damage induced by friction and intensive thermomechanical shock absorbed by the tools, eventually resulting in its failure at the cutting edge (figure 10(a)). Only after 1.9 m length of cut, the monolayer coating is removed from the very cutting edge (figure 10(a)). Similar results were observed before for different categories of hard coatings [48]. In contrast, the multilayer coating remains on the surface for a much longer period of time and efficiently covers the edge, performing its protective/lubricating functions (figure 10(b)) [17]. The impact fatigue fracture resistance data (figures 9(a) and (b)) presented above along with some literature data (figure 9(c) [70]) reveal that crack propagation can be inhibited by the internal interfaces of the nanolayers. Nanomultilayered coatings deflect cracks and dissipate energy without coating failure. This result is confirmed by TEM/SEM analysis (figures 9(c) and 10) and directly corresponds to the wear rate data [17]. As shown in figure 3 an intensive plastic deformation (bending) of columnar structure and crack formation occur on the worn surface of the monolayer TiAlCrSiYN coating due to friction and thermal cycling. In contrast, no surface damage could be observed in the multilayer TiAlCrSiYN/TiAlCrN coating (figure 3(b)). The multilayered coating with increased fatigue properties and, therefore, improved ability to dissipate energy of friction (figure 10(b)) remains stable on the friction surface. These results show that mechanical properties are a critical factor in providing a stable environment for the hierarchical surface-engineered system to display adaptive behavior. The nanomechanical properties of the underlying layer provide a stable low-wear environment for the tribofilms to form, regenerate and efficiently protect this layer so that it can survive high temperatures and heavy loads during operation [17].
Figure 10.
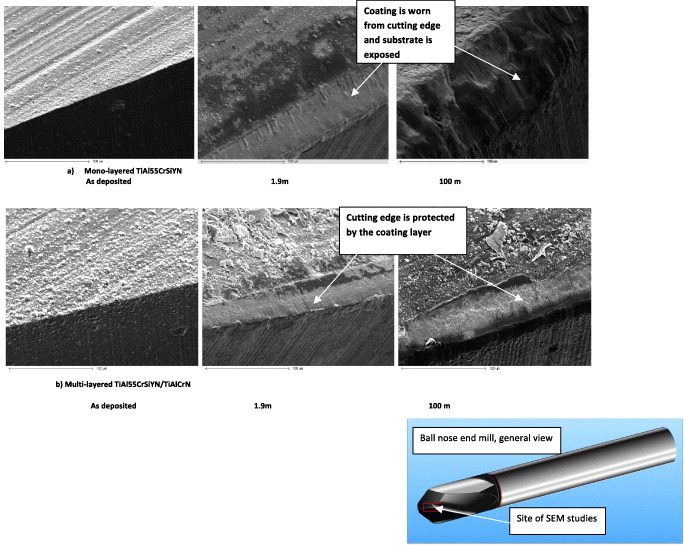
SEM images of surface morphology versus length of cut of the worn coated ball-nose end mills with (a) TiAlCrSiYN monolayered and (b) TiAlCrSiYN/TiAlCrN multilayered coatings. Tool: carbide ball-nose end mill, D = 10 mm; workpiece-H13 tool steel, hardness HRC 53–55, speed: 500 m min−1; feed, 0.06 mm per tooth; axial depth, 5.0 mm; and radial depth, 0.6 mm. (Reproduced with permission from [17], Elsevier.)
The wear resistance of TiAlCrSiYN/TiAlCrN coatings is further improved by the application of a plasma-enhanced arc cathode which noticeably decreases the amount of droplet phases on the surface, improving the surface finish and tribological characteristics of the coating [63].
3.3. Wear performance of the adaptive coatings; their multifunctionality and emergent behavior
3.3.1. Selected examples of wear performance of adaptive coatings under extreme frictional conditions.
The new generation of adaptive hierarchical surface-engineered nanomaterials exhibits various features under different conditions. They therefore have an ability to sustain strongly varying operating conditions. Some examples of this behavior are shown below for the nanomultilayered TiAlCrSiYN/TiAlCrN coating.
A remarkable example of extreme tribological applications under heavy loads and at high temperatures on the friction surface is ultrahigh-speed machining of hardened (hardness HRC 52–55) tool steels. This is a field where adaptive hierarchical surface-engineered materials develop their full potential operating at highly nonequilibrium, extreme tribological conditions [17, 47, 248]. Detailed studies of the tool life and wear behavior of different categories of hard coating show that the adaptive nanostructured TiAlCrN-based family of coatings has a strong potential for this application [24, 135, 229, 274]. Usually commercial and even experimental coatings designed for this application can work efficiently within cutting speeds of 200–300, and even up to 400–500 m min−1 [47–48, 247]. Figures 11(a)–(c) present tool life data for the new TiAlCrSiYN monolayer coating and the nanomultilayer TiAlCrSiYN/TiAlCrN adaptive coatings at various cutting speeds [17]. Even under extreme cutting conditions, which are not widely accessible yet in modern manufacturing practice (cutting speed of 600–700 m min−1), the TiAlCrSiYN/TiAlCrN coating shows a long tool life (figure 11(c)) and does not exhibit signs of linear (rapid) wear (figure 11(c)).
Figure 11.
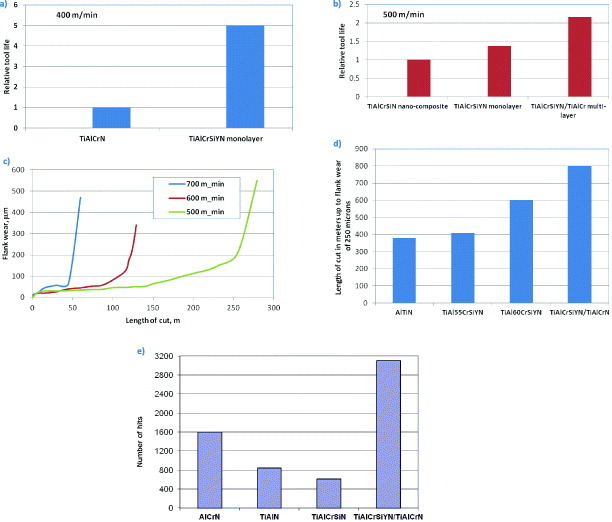
Tool life data for adaptive TiAlCrSiYN-based coatings under strongly varying cutting conditions: (a–c) during dry machining of hardened steels. Tool, carbide ball-nose end mill, D = 10 mm; workpiece-H13 tool steel, hardness HRC 53–55; speed, 400–500 (a) and (b); 500–700 (c) m min−1; feed, 0.06 mm per tooth; axial depth, 5.0 mm; radial depth, 0.6 mm; (d) during turning of Ni-based aerospace DA 718 alloy, hardness HRC 47–48; speed, 40 m min−1; feed, 0.125 mm rev−1; depth of cut, 0.25 mm; (e) during deep hole drilling of PM hardened structural steel, grade MPIF P/F 11C60 (C: 0.4–0.8%, Cu, 1.5–2.5%, Fe-balance; HRC 30–35). Speed, 69.1 m min−1; feed rate, 625 mm min−1; depth of cut, 39 mm.
Figure 11(d) presents lifetime data of the cutting tools with various coatings during turning of Ni-based aerospace superalloy [242, 275]. Machining of such alloys can be regarded as extreme operating conditions due to high temperatures and heavy loads on the friction surface [7, 8]. Note that the same nanomultilayered coating that showed best tool life during ultrahigh-speed dry machining of hardened tool steel demonstrates a significant improvement in tool life during machining of Ni-based superalloy in comparison to the commercial AlTiN coating that is widely used for aerospace machining applications [229].
Figure 11(e) presents tool life data of the cutting tools with various coatings during deep hole drilling of hardened structural steels. Such drilling generates significant amount of heat and leads to buildup edge formation [7]. It is shown in figure 11(e) that an adaptive TiAlCrSiYN/TiAlCrN multilayer coating outperforms other types of coatings under these conditions. This could be explained by the beneficial combination of micromechanical properties of the coating as well as its protective ability. It allows the coating to stay stable on the surface and efficiently perform its protective functions leading to the tool life increase.
3.3.2. Multifunctionality and emergent behavior under extreme tribological conditions.
Multifunctionality of the nanomultilayered TiAl55CrSiYN/TiAlCrN coating develops through the ability to sustain completely different operating conditions because the coating has a complex nanomultilayered structure and various tuned characteristics, and in this way it can exhibit various features under different conditions [17, 242]. When it is necessary to sustain high temperatures and heavy loads under extreme operation conditions (ultrahigh-speed dry machining of hardened tool steel), the coating exhibits its enhanced adaptability, firstly the formation and permanent regeneration of protective tribofilms with thermal barrier properties (figures 2 and 5), and secondly by the ability to remain stable on the surface (figure 10) and to sustain heavy loads during operation (figure 8), exhibiting low cycling fatigue (figure 9) [17]. When it is necessary to sustain unstable attrition wear conditions with intensive adhesive interaction at the tool/workpiece interface and further deep surface damage, complicated by the high-temperature strength and low thermal conductivity of the machined part made of Ni-based superalloys, the coating firstly exhibits increased impact fracture resistance as well as resistance to localized plastic deformation on the friction surface (figure 8) combined with a simultaneous ability to form protective tribofilms (figures 1, 5 and 7) [242]. More remarkably, this class of surface-engineered nanomaterials works well enough under completely different conditions such as deep drilling of hardened structural steels (figure 11(d)). This is also due to the combination of improved fatigue characteristics and ability to form protective tribofilms. So, under the first type of tribological conditions (ultrahigh-speed dry machining of hardened steels) the coating adapts to external impact in one way, mostly by forming protective tribofilms with thermal barrier properties; under the second and third type of conditions (machining of Ni-based superalloys and deep drilling of hardened steels) it adapts in a slightly different way, mostly due to its ability to sustain heavy loads at elevated temperatures and its low cycling fatigue. This is how the multifunctionality works for these coatings under extreme machining conditions.
To understand the causes of improvement in wear performance of the multilayered coating using the example of ultrahigh-speed machining of hardened tool steel (figure 12), we have to consider the combination of all characteristics of the coating (table 3). Tuned characteristics in the complex engineering systems are joined together and interact with each other synergistically, as a whole. The ability of a system to adapt to the strongly changing external environment is significantly improved in this way.
Figure 12.
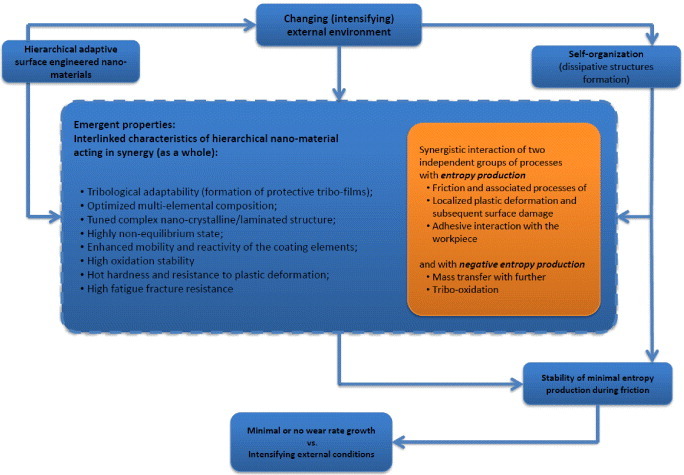
Schematic of emergent behavior of the adaptive coatings under intensifying external conditions.
Critical features of the coating that work in synergy are the following. Initially, rubbing and adhesive interaction between materials in the tribocontact generates frictional energy that mainly transforms to heat, thereby increasing the surface temperature. These processes induce a beneficial mass transfer of the coating's elements such as Al, Si or Cr to the friction surface. The adaptive coating possesses an improved mobility and reactivity of the elements, provided by its complex nanostructure with highly nonequilibrium state (figures 6 and 7). Further beneficial tribo-oxidation as a result of interaction with the environment leads to the formation of dynamically stable tribofilms, which is directly related to the self-organization during friction. Self-organization, and associated tribological adaptation, leads to the situation where further work of the tribosystem proceeds with a minimal entropy production despite intensifying external impact (in our case cutting speed increase) [10]. The stability of minimal entropy production is provided by the complex interaction of different processes outlined above. Growth in entropy production caused by one of the processes, in particular due to the friction under increasingly severe conditions and associated processes of localized plastic deformation, surface damage and adhesive interaction with the workpiece, is being compensated by the reduction in entropy production by the intensification of the other process (in our case, beneficial mass transfer of coating elements such as Al or Si) [31]. This process, combined with interaction with the environment (tribo-oxidation), results in negative entropy production within the tribofilms. Thus, the tribosystem starts working with feedback. A tribosystem with this behavior could accumulate more energy without significant surface damage and wear rate increase. Nonequilibrium processes consume much energy that is spent on these processes instead of surface damage [10]. In this case, the system is capable of sustaining extreme external impact.
The coating also has excellent oxidation stability and thermal barrier properties that are related to the formation of protective tribofilms (table 3; figures 1 and 7). In addition, the coating has micromechanical properties that ensure stable regeneration of tribofilms during friction, such as an increased ability to sustain heavy loads during operation (first of all high-temperature hardness, figure 8). Moreover, the impact fatigue fracture resistance of the coating is improved (figure 9) to resist cyclic loading under milling operations.
The next very important feature of the coating is its nanomultilayered structure, which can efficiently dissipate thermomechanical energy supplied to the friction surface. This reduces the surface damage, which would otherwise increase the wear rate [270]. As outlined in figure 10, no surface damage could be observed in the TiAlCrSiYN/TiAlCrN multilayered coating. The coating with a better ability to dissipate energy of friction due to improved micromechanical properties (figure 9) stays stable on the friction surface and efficiently performs its protective/lubricating functions (figures 10 and 11). In this way, the wear rate has stabilized rapidly at a very low level and stays stable for a long time (see figure 11(c)). So, the advantage in one set of characteristics (micromechanical) affects other categories of characteristics (stable regeneration of protective/lubricious tribofilms) and vice versa: the nanolevel mechanisms of tribofilms formation influence the nano/microlevel effects associated with resistance to plastic deformation as well as cracks formation and propagation within the coatings [19]. In this complex way, the coatings' characteristics are integrated within the system and work in synergy. The other example of complex interrelation of the coatings' characteristics is the formation of alumina-like surface films that affect the thermal properties of the surface layer (figures 1, 4–6), create a strong temperature gradient and prevent temperature increase, surface damage and corresponding wear rate growth. This is a typical case of emergent behavior. A tribosystem with this behavior can accumulate/dissipate more energy. Remarkably, the emergent behavior of the coating could be assessed through various properties of the coating. The properties shown in figures 7–9 are tuned as a whole to prevent advances in one kind of properties at the cost of the other. Only simultaneous advances in several characteristics (such as high-temperature hardness: figure 8, fatigue resistance: figure 9 and thermal properties: figure 7) enhance the emergent behavior (table 3). That is why the adaptive coating with emergent properties can sustain increasingly severe operating conditions [17].
In our opinion, future generations of wear-resistant coatings for extreme tribological applications have to be complex engineering systems that possess emergent properties [17]. Such coatings can perform as a higher-ordered system during operation and therefore achieve long life under extreme tribological conditions.
Further advances in the design of hierarchical surface-engineered materials will be associated with the higher complexity of the surface-engineered layer [52] to increase its multifunctionality and therefore adaptability during operation. They can move in two directions: (i) inventing new ways to accelerate formation and improve service characteristics of beneficial tribofilms on the surface of the hierarchical adaptive nanomaterials [31]; (ii) introduction of more complex designs of adaptive coatings [52], for example, smart/nanolaminated adaptive coatings with programmable characteristics of the surface-engineered layer. These are definitely interesting topics for future research.
4. Conclusions
Modern adaptive wear-resistant coatings may be regarded as hierarchical surface-engineered nanostructured materials. They exhibit dynamic hierarchy on two major structural scales: (a) the nanoscaled surface layers of the tribofilms that perform protective, lubricating and thermal barrier functions and largely control wear resistance of the entire surface-engineered system and (b) the nano/microscaled coating itself with its complex nanocrystalline/multilayered structure and highly nonequilibrium state.
Adaptive wear-resistant coatings develop their full potential under extreme tribological conditions. Such operating conditions are mainly associated with strong gradients of various characteristics occurring on the friction surface. They are far away from equilibrium and the wear rate of the unprotected friction surface is extremely high. Moreover, if the operating conditions are characterized by strong instability (for example, attrition wear mode during machining of aerospace Ni-based alloys or interrupted cut at ultrahigh-speed dry end-milling of hardened steels) then the gradient of characteristics grows even further. Therefore, in complex surface-engineering systems such as adaptive coatings, the probability of self-organization is much higher, resulting in significant improvement of wear resistance.
A major feature of the adaptive hierarchical surface-engineered nanomaterials is the formation of various nanoscaled tribofilms on the surface of the coating as a result of self-organization during friction. These tribofilms are generated from the base surface of the engineered material by structural modification and interaction with the environment (mostly with oxygen from air) leading to tribo-oxidation under heavy load, high temperature conditions. A new direction in nanomaterials research is presented: compositional and structural optimization of the surface tribofilms by tuning the adaptive hard coating design for specific applications. Results of long-term studies on the characteristics of the tribofilms are summarized. Thermodynamic analysis shows that formation of the tribofilms is a strongly nonequilibrium process that leads to a decrease of entropy production. This nonequilibrium process requires much energy, thus the tribosystem diverts energy available for surface damage, which substantially reduces the wear rate. As dissipative structures, the tribofilms have several characteristics best suited to sustain various operating conditions, thus assuring superb protective and/or lubricious functions of the friction surface. Another critical feature of adaptive coatings with enhanced formation of protective/lubricious tribofilms is their improved thermal behavior that creates a strong gradient of temperature at the tool/chip interface and reduces overheating of the coated tool. The concentration of a larger part of the interactions between frictional bodies within the thin nanoscale layer of the tribofilms is taking place. As a result, the surface damage within the coating is significantly reduced. This explains why adaptive coatings can sustain severe and extreme service conditions. Taking control of the structure and properties of nanoscaled tribofilms by optimizing the characteristics of adaptive coatings can be one of the future trends in nanotechnology.
It is shown that the coating in surface-engineered hierarchical materials plays a no less important role than the nanoscaled surface tribofilms. As a nonequilibrium medium, it controls physicochemical properties of the coatings and supplies the desired elements to the friction surface to form the tribofilms with necessary characteristics. It also ensures accumulation and dissipation of the frictional energy during operation. Unique features of nanomultilayer coatings design are outlined that help achieve desirable characteristics of the adaptive coatings.
A new approach to multifunctional adaptive coatings design for severe applications is presented. A critical feature of the surface-engineered hierarchical materials to sustain extreme operating conditions is their emergent behavior that can be achieved by careful tuning of various characteristics of the adaptive coating to promote natural beneficial processes associated with self-organization during friction with dissipative structure formation. Tuned characteristics in the complex engineering systems are joined together and interlink, working as a whole. Following this approach, the ability of the system to adapt to a strongly changing environment is greatly enhanced. The nanomultilayered coatings that represent hierarchical surface-engineered adaptive nanomaterials possess emergent properties through several improved features (synergistic action of protective/lubricious tribofilms, beneficial mass transfer of the necessary coating's elements to the friction surface, improved thermal and micromechanical properties of the coating) that work as a whole. The advantages in one kind of characteristics (micromechanical) affect other characteristics (stable regeneration of protective/lubricious tribofilms) and vice versa: the nanoscale mechanisms of tribofilms formation influence the microscale effects associated with damaging processes within the coatings. In this complex way they are integrated within the system and work in synergy. As a higher-ordered system, they can achieve multifunctionality and high wear resistance under extreme tribological conditions.
References
- Holmberg K. and Matthew A. Coating Tribology: Principles, Techniques and Application in Surface Engineering. Amsterdam Elsevier; 1994. [Google Scholar]
- Mayrhofer P H, Mitterer C, Hultman L. and Clemens H. Prog. Mater. Sci. 2006;51:1032. doi: 10.1016/j.pmatsci.2006.02.002. [DOI] [Google Scholar]
- PalDey S. and Deevi S C. Mater. Sci. Eng. A. 2003;361:1. doi: 10.1016/S0921-5093(03)00473-8. [DOI] [Google Scholar]
- Bushe N. and Kopitko V. Compatibility of Rubbing Surfaces. Moscow Science; 1981. [Google Scholar]
- Brushman B. Tribology Handbook. vol 2. Boca Raton , FL CRC Press; 2005. [Google Scholar]
- Fox-Rabinovich G, and Totten G, , editors. Self-Organization During Friction: Advance Surface Engineered Materials and Systems Design. Boca Raton , FL CRC Press; 2006. [Google Scholar]
- Wright P, and Trent E, , editors. Metal Cutting. 4th edn. Boston, MA Butterworth-Heinemann; 2000. [Google Scholar]
- Ezugwu E O, Bonney J. and Yamane Y. J. Mater. Proc. Technol. 2003;134:233. doi: 10.1016/S0924-0136(02)01042-7. [DOI] [Google Scholar]
- Shuster L. Adhesive Interaction in Metal Solids. Ufa , Russia Gilem; 1999. [Google Scholar]
- Gershman I. and Bushe N. Elements of thermodynamics and self-organization during friction. In: Fox-Rabinovich G, , Totten G, , editors. Self-Organization During Friction: Advanced Surface-Engineered Materials and Systems Design. Boca Raton, FL CRC Press; 2006. [Google Scholar]
- MacGinley T. and Monaghan J. J. Mater. Proc. Technol. 2001;118:293. doi: 10.1016/S0924-0136(01)00969-4. [DOI] [Google Scholar]
- Claudin C, Rech J, Grzesik W. and Zalisz S. Int. J. Mater. Form. 2008;1:511. doi: 10.1007/s12289-008-0172-3. [DOI] [Google Scholar]
- Donachie M. and Donachie S. Superalloys: A Technical Guide. Materials Park , OH ASM International; 2002. [Google Scholar]
- Löffler F H W. Surf. Coat. Technol. 1994;68–69:729. doi: 10.1016/0257-8972(94)90246-1. [DOI] [Google Scholar]
- Alden Kendall A. Friction and Wear of Cutting Tools and Cutting Tool Materials. vol 18. Materials Park, OH ASM International; 1992. [Google Scholar]
- Langton C G. Physica D. 1990;42:12. doi: 10.1016/0167-2789(90)90064-V. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Yamamoto K, Beake B D, Kovalev A I, Aguirre M H, Veldhuis S C, Dosbaeva G K, Wainstein D L, Biksa A. and Rashkovskiy A. Surf. Coat. Technol. 2010;204:3425. doi: 10.1016/j.surfcoat.2010.04.002. [DOI] [Google Scholar]
- Aouadi S M, Luster B, Kohli P, Muratore C. and Voevodin A A. Surf. Coat. Technol. 2009;204:962. doi: 10.1016/j.surfcoat.2009.04.010. [DOI] [Google Scholar]
- Matthews A, S Franklin S. and Holmberg K. J. Phys. D: Appl. Phys. 2007;40:5463. doi: 10.1088/0022-3727/40/18/S07. [DOI] [Google Scholar]
- Andresen K. Design and Use Patterns of Adaptability in Enterprise Systems. Berlin Gito; 2006. [Google Scholar]
- Toussaint M. and von Seelen W. Biosystems. 2007;90:769. doi: 10.1016/j.biosystems.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Martín H J, de Lope J. and Maravall D. Nature Comput. 2009;8:757. doi: 10.1007/s11047-008-9096-6. [DOI] [Google Scholar]
- Vaia R. and Baur J. Science. 2008;319:420. doi: 10.1126/science.1152931. [DOI] [PubMed] [Google Scholar]
- Fox-Rabinovich G S, Veldhuis S C, Dosbaeva G K, Yamamoto K, Kovalev A I, Wainstein D L, Gershman I S, Shuster L S. and Beake B D. J. Appl. Phys. 2008;103:043001. doi: 10.1063/1.2904907. [DOI] [Google Scholar]
- Heylighen F. Proc. 1st European Conf. on System Science; 1989. p. p 23. [Google Scholar]
- Heylighen F. Complexity and self-organization. In: Bates M J, editor. Encyclopedia of Library and Information Sciences. Boca Raton, FL Taylor and Francis; 2008. [Google Scholar]
- Zhang S, Sun D, Fu Y. and Du H. Surf. Coat. Technol. 2003;167:113. doi: 10.1016/S0257-8972(02)00903-9. [DOI] [Google Scholar]
- Hultman L. Vacuum. 2000;57:1. doi: 10.1016/S0042-207X(00)00143-3. [DOI] [Google Scholar]
- Cahn R. and Haasen P. Physical Metallurgy. 4th edn. Cambridge , MA Cambridge University Press; 1996. [Google Scholar]
- Balogh L. and Tomalia D A. J. Am. Chem. Soc. 1998;120:7355. doi: 10.1021/ja980861w. [DOI] [Google Scholar]
- Fox-Rabinovich G, Veldhuis S C, Kovalev A I, Wainstein D L, Gershman I S, Korshunov S, Shuster L S. and Endrino J L. J. Appl. Phys. 2007;102:043001. doi: 10.1063/1.2785947. [DOI] [Google Scholar]
- Mann S. Nature Mater. 2009;8:781. doi: 10.1038/nmat2496. [DOI] [PubMed] [Google Scholar]
- Kabakoff L. 2006 Nanomaterials: reality versus hype NATO Reports Office of Naval Research. [Google Scholar]
- Lakes R. Nature. 1993;361:511. doi: 10.1038/361511a0. [DOI] [Google Scholar]
- Sanchez C, Arribart H. and Giraud Guille M M. Nature Mater. 2005;4:277. doi: 10.1038/nmat1339. [DOI] [PubMed] [Google Scholar]
- Pugno N M. and Carpinteri A. Phil. Mag. Lett. 2008;88:397. doi: 10.1080/09500830802089843. [DOI] [Google Scholar]
- Voevodin A A. and Zabinski J S. Thin Solid Films. 2000;370:223. doi: 10.1016/S0040-6090(00)00917-2. [DOI] [Google Scholar]
- Dosbaeva G K, Veldhuis S C, Yamamoto K, Wilkinson D S, Beake B D, Jenkins N, Elfizy A. and Fox-Rabinovich G S. Int. J. Refract. Met. Hard Mater. 2010;28:133. doi: 10.1016/j.ijrmhm.2009.09.003. [DOI] [Google Scholar]
- Barthelat F. Phil. Trans. R. Soc. A. 2007;365:2907. doi: 10.1098/rsta.2007.0006. [DOI] [PubMed] [Google Scholar]
- Fratzl P. and Weinkamer R. Prog. Mater. Sci. 2007;52:1263. doi: 10.1016/j.pmatsci.2007.06.001. [DOI] [Google Scholar]
- Dabbs D M. and Aksay I A. Annu. Rev. Phys. Chem. 2000;51:601. doi: 10.1146/annurev.physchem.51.1.601. [DOI] [PubMed] [Google Scholar]
- Corning P. Complexity. 2002;7:18. doi: 10.1002/cplx.10043. [DOI] [Google Scholar]
- Goldstein J. Emergence: Complexity Organ. 1999;1:49. [Google Scholar]
- Beckerman L P. Syst. Eng. 2000;3:96. doi: 10.1002/1520-6858(2000)3:2%3C96::AID-SYS4%3E3.0.CO;2-7. [DOI] [Google Scholar]
- Waldrop M. Complexity: The Emerging Science at the Edge of Order and Chaos. New York Touchstone Simon and Schuster; 1992. [Google Scholar]
- Jones R. Nature Nanotechnol. 2008;3:245. doi: 10.1038/nnano.2008.117. [DOI] [PubMed] [Google Scholar]
- Veprek S. and Veprek-Heijman M J G. Surf. Coat. Technol. 2008;202:5063. doi: 10.1016/j.surfcoat.2008.05.038. [DOI] [Google Scholar]
- Ning L, Veldhuis S C. and Yamamoto K. Int. J. Mach. Tools Manuf. 2008;48:656. doi: 10.1016/j.ijmachtools.2007.10.021. [DOI] [Google Scholar]
- Chialvo D R. New Ideas Psychol. 2008;26:158. doi: 10.1016/j.newideapsych.2007.07.013. [DOI] [Google Scholar]
- Casadei M, Gardelli L. and Viroli M. Electron. Notes Theor. Comput. Sci. 2007;175:59. doi: 10.1016/j.entcs.2007.05.022. [DOI] [Google Scholar]
- Maxion R A. Physica D. 1990;42:66. doi: 10.1016/0167-2789(90)90067-Y. [DOI] [Google Scholar]
- Fox-Rabinovich G, Gershman I, Yamamoto K, Biksa A, Veldhuis S, Beake B. and Kovalev A. Entropy. 2010;12:275. doi: 10.3390/e12020275. [DOI] [Google Scholar]
- Sato Y, Akiyama E. and Crutchfield J P. Physica D. 2005;210:21. doi: 10.1016/j.physd.2005.06.031. [DOI] [Google Scholar]
- Sanders J W. and Smith G. Electron. Notes Theor. Comput. Sci. 2009;259:207. doi: 10.1016/j.entcs.2009.12.026. [DOI] [Google Scholar]
- Buchmann M. Emergent properties. In: Smelser N, and Baltes B P, , editors. International Encyclopedia of the Social and Behavioral Sciences. Oxford Pergamon; 2004. [Google Scholar]
- Johnson C W. Reliab. Eng. Syst. Saf. 2006;91:1475. doi: 10.1016/j.ress.2006.01.008. [DOI] [Google Scholar]
- Bennet A B. and Bennet D. Organizational Survival in the New World: The Intelligent Complex Adaptive System. Boston , MA Elsevier; 2004. Relations among emergent properties. [Google Scholar]
- Würtz R, Branke J. and Schmeck H. Organic Computing. vol 21. Heidelberg Springer; 2008. Evolutionary design of emergent behavior. [Google Scholar]
- Haken H. Advanced Synergetics: Instability Hierarchies of Self-Organizing Systems and Devices. New York Springer; 1993. [Google Scholar]
- Astakhov V P. Tribology of Metal Cutting. London Elsevier; 2006. [Google Scholar]
- Endrino J L, Fox-Rabinovich G S. and Gey C. Surf. Coat. Technol. 2006;200:6840. doi: 10.1016/j.surfcoat.2005.10.030. [DOI] [Google Scholar]
- Kalss W, Reiter A, Derflinger V, Gey C. and Endrino J L. Int. J. Refract. Met. Hard Mater. 2006;24:399. doi: 10.1016/j.ijrmhm.2005.11.005. [DOI] [Google Scholar]
- Yamamoto K, Sato T, Takahara K. and Hanaguri K. Surf. Coat. Technol. 2003;174–175:620. doi: 10.1016/S0257-8972(03)00580-2. [DOI] [Google Scholar]
- Yamamoto K, Kujime S. and Takahara K. Surf. Coat. Technol. 2005;200:1383. doi: 10.1016/j.surfcoat.2005.08.025. [DOI] [Google Scholar]
- Yamamoto K, Kujime S. and Fox-Rabinovich G. Surf. Coat. Technol. 2008;203:579. doi: 10.1016/j.surfcoat.2008.05.046. [DOI] [Google Scholar]
- Endrino J L. and Derflinger V. Surf. Coat. Technol. 2005;200:988. doi: 10.1016/j.surfcoat.2005.02.196. [DOI] [Google Scholar]
- Soldán J, Neidhardt J, Sartory B, Kaindl R, Cerstvý R, Mayrhofer P H, Tessadri R, Polcik P, Lechthaler M. and Mitterer C. Surf. Coat. Technol. 2008;202:3555. doi: 10.1016/j.surfcoat.2007.12.041. [DOI] [Google Scholar]
- Rovere F, Mayrhofer P H, Reinholdt A, Mayer J. and Schneider J M. Surf. Coat. Technol. 2008;202:5870. doi: 10.1016/j.surfcoat.2008.06.161. [DOI] [Google Scholar]
- Holleck H. and Schulz H. Thin Solid Films. 1987;153:11. doi: 10.1016/0040-6090(87)90165-9. [DOI] [Google Scholar]
- Yau B-S, Huang J-L, Lu H-H. and Sajgalik P. Surf. Coat. Technol. 2005;194:119. doi: 10.1016/j.surfcoat.2004.05.009. [DOI] [Google Scholar]
- Helmersson U, Todorova S, Barnett S A, Sundgren J E, Markert L C. and Greene J E. J. Appl. Phys. 1987;62:481. doi: 10.1063/1.339770. [DOI] [Google Scholar]
- Sundgren J E, Birch J, Håkansson G, Hultman L. and Helmersson U. Thin Solid Films. 1990;193–194:818. [Google Scholar]
- Knotek O, Löffler F. and Krämer G. Surf. Coat. Technol. 1993;59:14. doi: 10.1016/0257-8972(93)90048-S. [DOI] [Google Scholar]
- Luo Q, Rainforth W M, Donohue L A, Wadsworth I. and Münz W D. Vacuum. 1999;53:123. doi: 10.1016/S0042-207X(98)00406-0. [DOI] [Google Scholar]
- Donohue L A, Smith I J, Münz W D, Petrov I. and Greene J E. Surf. Coat. Technol. 1997;94–95:226. [Google Scholar]
- Donohue L A, Münz W D, Lewis D B, Cawley J, Hurkmans T, Trinh T, Petrov I. and Greene I E. Surf. Coat. Technol. 1997;93:69. doi: 10.1016/S0257-8972(97)00028-5. [DOI] [Google Scholar]
- Chang C-L, Jao J-Y, Ho W-Y. and Wang D-Y. Vacuum. 2007;81:604. doi: 10.1016/j.vacuum.2006.08.003. [DOI] [Google Scholar]
- Lewis D B, Creasey S, Zhou Z, Forsyth J J, Ehiasarian A P, Hovsepian P E, Luo Q, Rainforth W M. and Münz W D. Surf. Coat. Technol. 2004;177–178:252. doi: 10.1016/j.surfcoat.2003.09.041. [DOI] [Google Scholar]
- Nordin M, Larsson M. and Hogmark S. Surf. Coat. Technol. 1998;106:234. doi: 10.1016/S0257-8972(98)00544-1. [DOI] [Google Scholar]
- Prengel H G, Jindal P C, Wendt K H, Santhanam A T, Hegde P L. and Penich R M. Surf. Coat. Technol. 2001;139:25. doi: 10.1016/S0257-8972(00)01080-X. [DOI] [Google Scholar]
- Lee D-K, Lee S-H. and Lee J-J. Surf. Coat. Technol. 2003;169–170:433. [Google Scholar]
- Hovsepian P E. and Münz W D. Vacuum. 2002;69:27. doi: 10.1016/S0042-207X(02)00305-6. [DOI] [Google Scholar]
- Imbeni V, Martini C, Lanzoni E, Poli G. and Hutchings I M. Wear. 2001;251:997. doi: 10.1016/S0043-1648(01)00706-2. [DOI] [Google Scholar]
- Altuncu E. and Ustel F. Mater. Manuf. Proc. 2009;24:796. doi: 10.1080/10426910902840995. [DOI] [Google Scholar]
- Yamamoto K, Kujime S. and Takahara K. Surf. Coat. Technol. 2005;200:435. doi: 10.1016/j.surfcoat.2005.02.175. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Yamamoto K, Veldhuis S C, Kovalev A I, Shuster L S. and Ning L. Surf. Coat. Technol. 2006;201:1852. doi: 10.1016/j.surfcoat.2006.03.010. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Yamamoto K, Kovalev A I, Veldhuis S C, Ning L, Shuster L S. and Elfizy A. Surf. Coat. Technol. 2008;202:2015. doi: 10.1016/j.surfcoat.2007.08.067. [DOI] [Google Scholar]
- Feynman R. J. Microeleciromech. Syst. 1992;1:60. doi: 10.1109/84.128057. [DOI] [Google Scholar]
- Chang C-L, Chen J-H, Tsai P-C, Ho W-Y. and Wang D-Y. Surf. Coat. Technol. 2008;203:619. doi: 10.1016/j.surfcoat.2008.04.096. [DOI] [Google Scholar]
- Jiang W, More A S, Brown W D. and Malshe A P. Surf. Coat. Technol. 2006;201:2443. doi: 10.1016/j.surfcoat.2006.04.026. [DOI] [Google Scholar]
- Stueber M, Albers U, Leiste H, Ulrich S, Holleck H, Barna P B, Kovacs A, Hovsepian P. and Gee I. Surf. Coat. Technol. 2006;200:6162. doi: 10.1016/j.surfcoat.2005.11.012. [DOI] [Google Scholar]
- Veprek S. and Reiprich S. Thin Solid Films. 1995;268:64. doi: 10.1016/0040-6090(95)06695-0. [DOI] [Google Scholar]
- Hauert R. and Patscheider J. Adv. Eng. Mater. 2000;2:247. doi: 10.1002/(SICI)1527-2648(200005)2:5%3C247::AID-ADEM247%3E3.0.CO;2-U. [DOI] [Google Scholar]
- Musil J. Surf. Coat. Technol. 2000;125:322. doi: 10.1016/S0257-8972(99)00586-1. [DOI] [Google Scholar]
- Veprek S, Veprek-Heijman M G J, Karvankova P. and Prochazka J. Thin Solid Films. 2005;476:1. doi: 10.1016/j.tsf.2004.10.053. [DOI] [Google Scholar]
- Patscheider J, Zehnder T. and Diserens M. Surf. Coat. Technol. 2001;146–147:201. doi: 10.1016/S0257-8972(01)01389-5. [DOI] [Google Scholar]
- Russell W C, Malshe A P, Yedave S N. and Brown W D. J. Manuf. Sci. Eng. 2003;125:431. doi: 10.1115/1.1581886. [DOI] [Google Scholar]
- Uhlmann E, Fuentes J. and Keunecke M. 7th Int. Conf.: The Coatings in Manufacturing Engineering; October 2008; Chalkidiki, Greece. 2008. [Google Scholar]
- Bushe N. and Gershman I. Compatibility of tribosystems. In: Fox-Rabinovich G, , Totten G, , editors. Self-Organization During Friction. Advanced Surface-Engineered Materials and Systems Design. Boca Raton, FL CRC Press; 2006. [Google Scholar]
- Davis J, editor. Tool Materials (ASM Specialty Handbook) Materials Park, OH ASM International; 1995. [Google Scholar]
- Fischer-Cripps A C, Karvánková P. and Veprek S. Surf. Coat. Technol. 2006;200:5645. doi: 10.1016/j.surfcoat.2005.07.096. [DOI] [Google Scholar]
- Mayrhofer P H, Fischer F D, Böhm H J, Mitterer C. and Schneider J M. Acta Mater. 2007;55:1441. doi: 10.1016/j.actamat.2006.09.045. [DOI] [Google Scholar]
- Rovere F, Music D, Ershov S, Baben M T, Fuss H-G, Mayrhofer P H. and Schneider J M. J. Phys. D: Appl. Phys. 2010;43:043001. doi: 10.1088/0022-3727/43/3/035302. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Endrino J L, Beake B D, Aguirre M H, Veldhuis S C, Quinto D T, Bauer C E, Kovalev A I. and Gray A. Surf. Coat. Technol. 2008;202:2985. doi: 10.1016/j.surfcoat.2007.10.034. [DOI] [Google Scholar]
- Endrino J L, Fox-Rabinovich G S, Escobar Galindo R, Kalss W, Veldhuis S, Soriano L, Andersson J. and Gutiérrez A. Surf. Coat. Technol. 2009;204:256. doi: 10.1016/j.surfcoat.2009.07.010. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Endrino J L, Beake B D, Kovalev A I, Veldhuis S C, Ning L, Fontaine F. and Gray A. Surf. Coat. Technol. 2006;201:3524. doi: 10.1016/j.surfcoat.2006.08.075. [DOI] [Google Scholar]
- Veprek S, Männling H D, Jilek M. and Holubar P. Mater. Sci. Eng. A. 2004;366:202. doi: 10.1016/j.msea.2003.08.052. [DOI] [Google Scholar]
- Chang Y-Y, Yang S-J, Wu W, Kuo Y-C, Lee J-W. and Wang C-J. Thin Solid Films. 2009;517:4934. doi: 10.1016/j.tsf.2009.03.036. [DOI] [Google Scholar]
- Vaz F, Rebouta L, Andritschky M, da Silva M F. and Soares J C. J. Mater. Proc. Technol. 1999;92–93:169. doi: 10.1016/S0924-0136(99)00159-4. [DOI] [Google Scholar]
- Ding X-Z, Zeng X T. and Liu Y C. Thin Solid Films. 2011;519:1894. doi: 10.1016/j.tsf.2010.10.022. [DOI] [Google Scholar]
- Tanaka Y, Ichimiya N, Onishi Y. and Yamada Y. Surf. Coat. Technol. 2001;146–147:215. doi: 10.1016/S0257-8972(01)01391-3. [DOI] [Google Scholar]
- Veprek S, Reiprich S. and Shizhi L. Appl. Phys. Lett. 1995;66:2640. doi: 10.1063/1.113110. [DOI] [Google Scholar]
- Chang C-L, Lee J-W. and Tseng M-D. Thin Solid Films. 2009;517:5231. doi: 10.1016/j.tsf.2009.03.082. [DOI] [Google Scholar]
- Carvalho S, Ribeiro E, Rebouta L, Pacaud J, Goudeau P, Renault P O, Rivière J P. and Tavares C J. Surf. Coat. Technol. 2003;172:109. doi: 10.1016/S0257-8972(03)00323-2. [DOI] [Google Scholar]
- Jiang W, Malshe A P. and Goforth R C. Surf. Coat. Technol. 2005;200:1849. doi: 10.1016/j.surfcoat.2005.08.103. [DOI] [Google Scholar]
- Hörling A, Hultman L, Odén M, Sjölén J. and Karlsson L. Surf. Coat. Technol. 2005;191:384. doi: 10.1016/j.surfcoat.2004.04.056. [DOI] [Google Scholar]
- Bull S J. and Jones A M. Surf. Coat. Technol. 1996;78:173. doi: 10.1016/0257-8972(94)02407-3. [DOI] [Google Scholar]
- Yoon S Y, Yoon S-Y, Chung W-S. and Kim K H. Surf. Coat. Technol. 2004;177–178:645. doi: 10.1016/j.surfcoat.2003.08.067. [DOI] [Google Scholar]
- Ma S, Procházka J, Karvánková P, Ma Q, Niu X, Wang X, Ma D, Xu K. and Veprek S. Surf. Coat. Technol. 2005;194:143. doi: 10.1016/j.surfcoat.2004.05.007. [DOI] [Google Scholar]
- Fuentes G G, Almandoz E, Pierrugues R, Martínez R, Rodríguez R J, Caro J. and Vilaseca M. Surf. Coat. Technol. 2010;205:1368. doi: 10.1016/j.surfcoat.2010.09.004. [DOI] [Google Scholar]
- Chen L, Wang S Q, Du Y, Zhou S Z, Gang T, Fen J C, Chang K K, Li Y W. and Xiong X. Surf. Coat. Technol. 2010;205:582. doi: 10.1016/j.surfcoat.2010.07.043. [DOI] [Google Scholar]
- Veprek S. and Jilek M. Vacuum. 2002;67:443. doi: 10.1016/S0042-207X(02)00229-4. [DOI] [Google Scholar]
- Chen L, Du Y, Wang A J, Wang S Q. and Zhou S Z. Int. J. Refract. Met. Hard Mater. 2009;27:718. doi: 10.1016/j.ijrmhm.2008.12.002. [DOI] [Google Scholar]
- Donnet C. and Erdemir A. Surf. Coat. Technol. 2004;180–181:76. doi: 10.1016/j.surfcoat.2003.10.022. [DOI] [Google Scholar]
- Voevodin A A, O'Neill J P. and Zabinski J S. Surf. Coat. Technol. 1999;116–119:36. doi: 10.1016/S0257-8972(99)00228-5. [DOI] [Google Scholar]
- John P J, Prasad S V, Voevodin A A. and Zabinski J S. Wear. 1998;219:155. doi: 10.1016/S0043-1648(98)00167-7. [DOI] [Google Scholar]
- Havey K S, Zabinski J S. and Walck S D. Thin Solid Films. 1997;303:238. doi: 10.1016/S0040-6090(96)09529-6. [DOI] [Google Scholar]
- Voevodin A A. and Zabinski J S. Compos. Sci. Technol. 2005;65:741. [Google Scholar]
- Ivanova V, Bushe N. and Gershman I. J. Frict. Wear. 1997;18:74. [Google Scholar]
- Kostetsky B. J. Frict. Wear. 1993;14:773. [Google Scholar]
- Nicholls J R, Simms N J, Chan W Y. and Evans H E. Surf. Coat. Technol. 2002;149:236. doi: 10.1016/S0257-8972(01)01499-2. [DOI] [Google Scholar]
- Brady M, Gleeson B. and Wright I. JOM. 2000;52:16. doi: 10.1007/s11837-000-0109-x. [DOI] [Google Scholar]
- Hardwicke C. JOM. 2003;55:15. doi: 10.1007/s11837-003-0004-3. [DOI] [Google Scholar]
- Wax S, Fischer G. and Sands R. JOM. 2003;55:17. doi: 10.1007/s11837-003-0005-2. [DOI] [Google Scholar]
- Derflinger V, Brändle H. and Zimmermann H. Surf. Coat. Technol. 1999;113:286. doi: 10.1016/S0257-8972(99)00004-3. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Bushe N A, Kovalev A I, Korshunov S N, Shuster L S. and Dosbaeva G K. Wear. 2001;249:1051. doi: 10.1016/S0043-1648(01)00829-8. [DOI] [Google Scholar]
- Fratzl P. and Barth F G. Nature. 2009;462:442. doi: 10.1038/nature08603. [DOI] [PubMed] [Google Scholar]
- Ortiz C. and Boyce M C. Science. 2008;319:1053. doi: 10.1126/science.1154295. [DOI] [PubMed] [Google Scholar]
- Launey M E, Munch E, Alsem D H, Saiz E, Tomsia A P. and Ritchie R O. J. R. Soc. Interface. 2010;7:741. doi: 10.1098/rsif.2009.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratzl P. J. R. Soc. Interface. 2007;4:637. doi: 10.1098/rsif.2007.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch E, Launey M E, Alsem D H, Saiz E, Tomsia A P. and Ritchie R O. Science. 2008;322:1516. doi: 10.1126/science.1164865. [DOI] [PubMed] [Google Scholar]
- Bonderer L J, Studart A R. and Gauckler L J. Science. 2008;319:1069. doi: 10.1126/science.1148726. [DOI] [PubMed] [Google Scholar]
- Zwaag S, editor. Self Healing Materials: An Alternative Approach to 20 Centuries of Materials Science. Dordrecht Springer; 2007. (Springer Series in Materials Science vol 100). [Google Scholar]
- Messersmith P B. Science. 2008;319:1767. doi: 10.1126/science.1155122. [DOI] [PubMed] [Google Scholar]
- Vaia R. and Baur J. Science. 2008;319:420. doi: 10.1126/science.1152931. [DOI] [PubMed] [Google Scholar]
- Prigogine I. Etude Thermodynamique des Processus Irreversible. Liege , Belgium Desoer; 1947. [Google Scholar]
- Glansdorff P. and Prigogine I. Thermodynamic Theory of Structure, Stability and Fluctuations. London Wiley-Interscience; 1970. [Google Scholar]
- Prigogine I. The End of Certainty. New York Free Press; 1997. [Google Scholar]
- Prigogine I. and Kondepudi D. Modern Thermodynamics. New York Wiley; 1999. [Google Scholar]
- Tabor E. Friction as dissipative process. In: Singer I, and Pollock H, , editors. Fundamentals of Friction. Macroscopic and Microscopic Processes. vol 220. Dordrecht Kluwer; 1992. p. p 3. [Google Scholar]
- Klamecki B E. Wear. 1980;58:325. doi: 10.1016/0043-1648(80)90161-1. [DOI] [Google Scholar]
- Klamecki B E. Wear. 1980;63:113. doi: 10.1016/0043-1648(80)90078-2. [DOI] [Google Scholar]
- Kostetsky B. Surface Strength of the Materials at Friction. Kiev Technica; 1976. [Google Scholar]
- Bryant M. Unification of friction and wear. In: Nikas G, editor. Recent Developments in Wear Prevention, Friction and Lubrication. Kerala, India Research Signpost; 2010. p. p 159. [Google Scholar]
- Amiri M. and M K M K. Entropy. 2010;12:1021. doi: 10.3390/e12051021. [DOI] [Google Scholar]
- Chen Q. and Li D Y. Wear. 2005;259:1382. doi: 10.1016/j.wear.2004.12.025. [DOI] [Google Scholar]
- Ebeling W, Engel A. and Feistel R. Physik der Evolutionsprozesse. Berlin Springer; 1990. [Google Scholar]
- Prigogine I. Futures. 1989;21:396. doi: 10.1016/S0016-3287(89)80009-6. [DOI] [Google Scholar]
- Bak P. How Nature Works: The Science of Self-organized Criticality. New York Springer; 1996. [Google Scholar]
- Zypman F, Ferrante J, Mark Jansen M, Scanlon K. and Abel P. J. Phys.: Condens. Matter. 2003;15:191. doi: 10.1088/0953-8984/15/12/101. [DOI] [Google Scholar]
- Nosonovsky M. Phil. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2010;368:4755. doi: 10.1098/rsta.2010.0179. [DOI] [PubMed] [Google Scholar]
- Chin J-H. and Chen C-C. Int. J. Mech. Sci. 1993;35:353. doi: 10.1016/0020-7403(93)90008-I. [DOI] [Google Scholar]
- Ezugwu E O, Wang Z M. and Machado A R. J. Mater. Proc. Technol. 1998;86:1. doi: 10.1016/S0924-0136(98)00314-8. [DOI] [Google Scholar]
- Ezugwu E O. Int. J. Mach. Tools Manuf. 2005;45:1353. doi: 10.1016/j.ijmachtools.2005.02.003. [DOI] [Google Scholar]
- Jacobson S. and Hogmark S. Tribofilms—On the crucial importance of tribologically induced surface modifications. In: Nikas G, editor. Recent Developments in Wear Prevention, Friction and Lubrication. Kerala, India Research Signpost; 2010. p. p 197. [Google Scholar]
- Kostetsky B. Friction, Lubrication and Wear in Machines. Kiev , Ukraine Technika; 1970. [Google Scholar]
- Mansson B. and Lindgren K. Thermodynamics, information and structure. In: Sieniutycz S, , Salamon P, , editors. Nonequilibrium Theory and Extremum Principles. New York Taylor and Francis; 1990. p. p 95. [Google Scholar]
- Kostetskaya N. PhD Thesis. 1985. [Google Scholar]
- Kostetskaya N. J. Frict. Wear. 16:108. [Google Scholar]
- Bershadsky L. J. Frict. Wear. 1992;8:1077. [Google Scholar]
- Gershman I. and Bushe N. J. Frict. Wear. 1989;10:24. [Google Scholar]
- Garbar I I. and Skorinin J V. Wear. 1978;51:327. doi: 10.1016/0043-1648(78)90271-5. [DOI] [Google Scholar]
- Holmberg K. and Matthews A. Tribology of engineered surfaces. In: Stachowiak G, editor. Wear: Materials Mechanisms and Practice. Chichester Wiley; 2005. p. p 123. [Google Scholar]
- Gershman J S. and Bushe N A. Surf. Coat. Technol. 2004;186:405. doi: 10.1016/j.surfcoat.2003.11.016. [DOI] [Google Scholar]
- Gershman I, Bushe N, Mironov A. and Nikiforov V. J. Frict. Wear. 2003;24:329. [Google Scholar]
- Hovsepian P, Ehiasarian A. and Petrov I. Surf. Eng. 2010;26:610. doi: 10.1179/026708408X336337. [DOI] [Google Scholar]
- Ajayi O O. and Ludema K C. Wear. 1990;140:191. doi: 10.1016/0043-1648(90)90083-M. [DOI] [Google Scholar]
- Cermets G W. In: Metals Handbook. 9th edn. Burdes B, editor. vol 16. Metals Park, OH American Society for Metals; 1989. p. p 90. [Google Scholar]
- Fox-Rabinovich G S, Kovalev A I. and Afanasyev S N. Wear. 1996;198:280. doi: 10.1016/0043-1648(96)07204-3. [DOI] [Google Scholar]
- Russell B. History of Western Phylosophy. London Routledge Classic; 1945. [Google Scholar]
- Fox-Rabinovich G S, Kovalev A I, Shuster L S, Bokiy Y F, Dosbayeva G K, Wainstein D L. and Mishina V P. Wear. 1998;214:279. doi: 10.1016/S0043-1648(97)00157-9. [DOI] [Google Scholar]
- Kovalev A I. and Wainstein D L. Surface analysis techniques for investigations of modified surfaces, nanocomposites, chemical and structure transformations. In: Fox-Rabinovich G, , Totten G, , editors. Self-Organizatin During Friction: Advanced Surface Engineered Materials and Systems Design. Boca Raton, FL Taylor and Francis; 2006. p. p 81. [Google Scholar]
- Wainstein D L. and Kovalev A I. Surf. Interface Anal. 2002;34:230. doi: 10.1002/sia.1289. [DOI] [Google Scholar]
- De Crescenzi M, Lozzi L, Picozzi P, Santucci S, Benfatto M. and Natoli C R. Phys. Rev. B. 1989;39:8409. doi: 10.1103/PhysRevB.39.8409. [DOI] [PubMed] [Google Scholar]
- Yoshizawa N, Yamada Y. and Shiraishi M. Carbon. 1993;31:1049. doi: 10.1016/0008-6223(93)90055-F. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Weatherly G C, Dodonov A I, Kovalev A I, Shuster L S, Veldhuis S C, Dosbaeva G K, Wainstein D L. and Migranov M S. Surf. Coat. Technol. 2004;177–178:800. doi: 10.1016/j.surfcoat.2003.05.004. [DOI] [Google Scholar]
- Schulz H, Dörr J, Rass I J, Schulze M, Leyendecker T. and Erkens G. Surf. Coat. Technol. 2001;146–147:480. doi: 10.1016/S0257-8972(01)01375-5. [DOI] [Google Scholar]
- Chan C F. and Ko Y C. J. Am. Ceram. Soc. 1996;79:2961. doi: 10.1111/j.1151-2916.1996.tb08733.x. [DOI] [Google Scholar]
- Kramer B M. and Judd P K. J.Vac. Sci. Technol. A: Vac., Surf. Films. 1985;3:2439. doi: 10.1116/1.572854. [DOI] [Google Scholar]
- Brookes K, editor. Hardmetals and Other Hardmaterials. 3rd edn. Hertfordshire East Barnet; 1998. [Google Scholar]
- Cotton F, , Wilkinson G and Murillo C, , editors. Advanced Inorganic Chemistry. 6th edn. New York Wiley; 1998. [Google Scholar]
- Magneli A. Acta Crystollagr. 1953;6:495. doi: 10.1107/S0365110X53001381. [DOI] [Google Scholar]
- Storz O, Gasthuber H. and Woydt M. Surf. Coat. Technol. 2001;140:76. doi: 10.1016/S0257-8972(01)01024-6. [DOI] [Google Scholar]
- Woydt M, Skopp A, Dörfel I. and Witke K. Wear. 1998;218:84. doi: 10.1016/S0043-1648(98)00181-1. [DOI] [Google Scholar]
- Lugscheider E, Knotek O, Bobzin K. and Bärwulf S. Surf. Coat. Technol. 2000;133–134:362. doi: 10.1016/S0257-8972(00)00963-4. [DOI] [Google Scholar]
- Erdemir A. Tribol. Lett. 2000;8:97. doi: 10.1023/A:1019183101329. [DOI] [Google Scholar]
- Crist B. XPS Handbook of the Elements and Native Oxides. New York Wiley; 2000. [Google Scholar]
- Gassner G, Mayrhofer P H, Kutschej K, Mitterer C. and Kathrein M. Surf. Coat. Technol. 2006;201:3335. doi: 10.1016/j.surfcoat.2006.07.067. [DOI] [Google Scholar]
- Lugscheider E, Knotek O, Barimani C, Leyendecker T, Lemmer O. and Wenke R. Surf. Coat. Technol. 1999;112:146. doi: 10.1016/S0257-8972(98)00775-0. [DOI] [Google Scholar]
- Gemmill G. and Smith C. Human Relat. 1985;38:751. doi: 10.1177/001872678503800804. [DOI] [Google Scholar]
- Wallach A, Eytan D, Marom S. and Meir R. PLoS Comput. Biol. 2008;4:e29. doi: 10.1371/journal.pcbi.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molins R, Normand B, Rannou G, Hannoyer B. and Liao H. Mater. Sci. Eng. A. 2003;351:325. doi: 10.1016/S0921-5093(02)00839-0. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Yamomoto K, Veldhuis S C, Kovalev A I. and Dosbaeva G K. Surf. Coat. Technol. 2005;200:1804. doi: 10.1016/j.surfcoat.2005.08.057. [DOI] [Google Scholar]
- Samsonov G, editor. Physicochemical Properties of Oxides Handbook. Moscow Metallurgy; 1976. [Google Scholar]
- Samsonov G. and Grebenkina V. Powder Metall. Met. Ceram. 1968;7:107. doi: 10.1007/BF00774301. [DOI] [Google Scholar]
- Holleck H. J. Vac. Sci. Technol. A: Vac. Surf. Films. 1986;4:2661. doi: 10.1116/1.573700. [DOI] [Google Scholar]
- Scheel J, and Fukuda T, , editors. Crystal Growth Technology. New York Wiley; 2003. [Google Scholar]
- Fox-Rabinovich G S. and Kovalev A I. Wear. 1995;189:25. doi: 10.1016/0043-1648(95)06619-5. [DOI] [Google Scholar]
- Mah T I. and Mazdiyasni K S. J. Am. Ceram. Soc. 1983;66:699. doi: 10.1111/j.1151-2916.1983.tb10532.x. [DOI] [Google Scholar]
- Hulse C O. and Pask J A. J. Am. Ceram. Soc. 1966;49:312. doi: 10.1111/j.1151-2916.1966.tb13271.x. [DOI] [Google Scholar]
- Lessing P A, Gordon R S. and Mazdiyasni K S. J. Am. Ceram. Soc. 1975;58:149. doi: 10.1111/j.1151-2916.1975.tb19585.x. [DOI] [Google Scholar]
- Osendi M I. and Baudín C. J. Europ. Ceram. Soc. 1996;16:217. doi: 10.1016/0955-2219(95)00133-6. [DOI] [Google Scholar]
- Aksay I A, Dabbs D M. and Sarikaya M. J. Am. Ceram. Soc. 1991;74:2343. doi: 10.1111/j.1151-2916.1991.tb06768.x. [DOI] [Google Scholar]
- Hynes A P. and Doremus R H. J. Am. Ceram. Soc. 1991;74:2469. doi: 10.1111/j.1151-2916.1991.tb06787.x. [DOI] [Google Scholar]
- Bertalanffy L. Biophysik des Fliessgleichgewichtes. Braunschweig Sammlung Viehweg; 1953. [Google Scholar]
- Zhu D, Jacobson N. and Miller R. NASA Report 1999 Thermal–Mechanical stability of single crystal oxide refractive concentrators for high-temperature solar thermal propulsion.
- Choi J B, Cho K, Lee M-H. and Kim K H. Thin Solid Films. 2004;447–448:365. doi: 10.1016/S0040-6090(03)01083-6. [DOI] [Google Scholar]
- Honda F. and Saito T. Appl. Surf. Sci. 1996;92:651. doi: 10.1016/0169-4332(95)00312-6. [DOI] [Google Scholar]
- Kovalev A, Wainstein D, Rashkovskiy A, Fox-Rabinovich G, Veldhuis S, Aguirre M. and Yamamoto K. Surf. Interface Anal. 2010;42:1368. doi: 10.1002/sia.3234. [DOI] [Google Scholar]
- Oh S H, Kauffmann Y, Scheu C, Kaplan W D. and Ruhle M. Science. 2005;310:661. doi: 10.1126/science.1118611. [DOI] [PubMed] [Google Scholar]
- Angel R. and Prewitt C. Am. Mineral. 1986;71:1476. [Google Scholar]
- Lanin A G, Muravin E L, Popov V P. and Turchin V N. J. Eur. Ceram. Soc. 2003;23:455. doi: 10.1016/S0955-2219(02)00093-6. [DOI] [Google Scholar]
- Shackelford J, and Alexander W, , editors. CRC Materials Science and Engineering Handbook. 3rd edn. Boca Raton , FL CRC Press; 2001. [Google Scholar]
- Schulz B. J. Nucl. Mater. 1988;155–157:348. doi: 10.1016/0022-3115(88)90269-3. [DOI] [Google Scholar]
- Endrino J, Palacín S, Gutiérrez A, Schäffers F. and Krzanowski J. JOM. 2007;42:7607. [Google Scholar]
- Endrino J L, Palacín S, Aguirre M H, Gutiérrez A. and Schäfers F. Acta Mater. 2007;55:2129. doi: 10.1016/j.actamat.2006.11.014. [DOI] [Google Scholar]
- Krzanowski J E, Palacín S, Gutiérrez A, Schäfers F, Mertin M, Endrino J L. and Soriano L. Scr. Mater. 2007;56:1011. doi: 10.1016/j.scriptamat.2007.03.001. [DOI] [Google Scholar]
- Li D, Bandcroft G, Kasrai M, Fleet M, Secco R, Feng X, Tan K. and Yang B. Am. Mineral. 1994;79:622. [Google Scholar]
- Fox-Rabinovich G S, Kovalev A I, Aguirre M H, Beake B D, Yamamoto K, Veldhuis S C, Endrino J L, Wainstein D L. and Rashkovskiy A Y. Surf. Coat. Technol. 2009;204:489. doi: 10.1016/j.surfcoat.2009.08.021. [DOI] [Google Scholar]
- Deacon T. The hierarchic logic of emergence: untangling the interdependence of evolution and self-organization. In: Weber B, and Depew D, , editors. Evolution and learning: The Baldwin Effect Reconsidered. Cambridge, MA MIT Press; 2003. p. p 35. [Google Scholar]
- Xingzhong Z, Jiajun L, Baoliang Z, Hezhou M. and Zhenbi L. Ceram. Int. 1999;25:309. doi: 10.1016/S0272-8842(98)00040-6. [DOI] [Google Scholar]
- Kabaldin Y, Kojevnikov N. and Kravchuk K. J. Frict. Wear. 1990;11:130. [Google Scholar]
- Kabaldin Y. J. Frict. Wear. 1989;10:800. [Google Scholar]
- Kabaldin Y. Structure, Strength and Wear Resistance of Composite Tool Materials. Vladivostok Dalnauka; 1996. [Google Scholar]
- König W, Fritsch R. and Kammermeier D. Annal. CIPR. 1992;41:49. [Google Scholar]
- Ditmars D, Ishihara S, Chang S, Bernstein G. and West E. J. Res. Natl. Bur. Stand. (USA) 1982;87:159. doi: 10.6028/jres.087.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert S, Litovsky E, Kleiman J I. and Heimann R B. Surf. Coat. Technol. 2006;200:3404. doi: 10.1016/j.surfcoat.2005.02.033. [DOI] [Google Scholar]
- Erkens G, Cremer R, Hamoudi T, Bouzakis K D, Mirisidis I, Hadjiyiannis S, Skordaris G, Asimakopoulos A, Kombogiannis S, Anastopoulos J. and Efstathiou K. Surf. Coat. Technol. 2004;177–178:727. doi: 10.1016/j.surfcoat.2003.08.013. [DOI] [Google Scholar]
- Biksa A, Yamamoto K, Dosbaeva G, Veldhuis S C, Fox-Rabinovich G S, Elfizy A, Wagg T. and Shuster L S. Tribol. Int. 2010;43:1491. doi: 10.1016/j.triboint.2010.02.008. [DOI] [Google Scholar]
- Fox-Rabinovich G. J. Appl. Phys. 2012;111:043001. doi: 10.1063/1.3693032. [DOI] [Google Scholar]
- Beake B, Fox-Rabinovich G, Losset Y, Yamamoto K, Aguirre M, Veldhuis S, Endrino J and Kovalev A. 2012. Faraday Discuss. 156 (doi: 10.1039/C2FD00131D) [DOI] [PubMed] [Google Scholar]
- Fox-Rabinovich G S, Beake B D, Yamamoto K, Aguirre M H, Veldhuis S C, Dosbaeva G, Elfizy A, Biksa A. and Shuster L S. Surf. Coat. Technol. 2010;204:3698. doi: 10.1016/j.surfcoat.2010.04.050. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Yamamoto K, Aguirre M H, Cahill D G, Veldhuis S C, Biksa A, Dosbaeva G. and Shuster L S. Surf. Coat. Technol. 2010;204:2465. doi: 10.1016/j.surfcoat.2010.01.024. [DOI] [Google Scholar]
- Horling A, Hultman L, Oden M, Sjolen J. and Karlsson L. J. Vac. Sci. Technol. A. 2002;20:1815. doi: 10.1116/1.1503784. [DOI] [Google Scholar]
- Chen L, Du Y, Mayrhofer P H, Wang S Q. and Li J. Surf. Coat. Technol. 2008;202:5158. doi: 10.1016/j.surfcoat.2008.05.036. [DOI] [Google Scholar]
- Kawate M, Kimura Hashimoto A. and Suzuki T. Surf. Coat. Technol. 2003;165:163. doi: 10.1016/S0257-8972(02)00473-5. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Beake B D, Endrino J L, Veldhuis S C, Parkinson R, Shuster L S. and Migranov M S. Surf. Coat. Technol. 2006;200:5738. doi: 10.1016/j.surfcoat.2005.08.132. [DOI] [Google Scholar]
- Hovsepian P E, Reinhard C. and Ehiasarian A P. Surf. Coat. Technol. 2006;201:4105. doi: 10.1016/j.surfcoat.2006.08.027. [DOI] [Google Scholar]
- Yashar P C. and Sproul W D. Vacuum. 1999;55:179. doi: 10.1016/S0042-207X(99)00148-7. [DOI] [Google Scholar]
- Arzt E. Acta Mater. 1998;46:5611. doi: 10.1016/S1359-6454(98)00231-6. [DOI] [Google Scholar]
- Holleck H. and Schier V. Surf. Coat. Technol. 1995;76–77:328. doi: 10.1016/0257-8972(95)02555-3. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Weatherly G C, Wilkinson D S, Kovalev A I. and Wainstein D L. Intermetallics. 2004;12:165. doi: 10.1016/j.intermet.2003.09.014. [DOI] [Google Scholar]
- Goward G W. Surf. Coat. Technol. 1998;108–109:73. doi: 10.1016/S0257-8972(98)00667-7. [DOI] [Google Scholar]
- Lewis D B, Donohue L A, Lembke M, Münz W D, Kuzel R, Jr, Valvoda V. and Blomfield C J. Surf. Coat. Technol. 1999;114:187. doi: 10.1016/S0257-8972(99)00047-X. [DOI] [Google Scholar]
- Rovere F. and Mayrhofer P H. J. Vac. Sci. Technol. A. 2008;26:29. doi: 10.1116/1.2806943. [DOI] [Google Scholar]
- Moser M. and Mayrhofer P H. Scr. Mater. 2007;57:357. doi: 10.1016/j.scriptamat.2007.04.019. [DOI] [Google Scholar]
- Kutschej K, Fateh N, Mayrhofer P H, Kathrein M, Polcik P. and Mitterer C. Surf. Coat. Technol. 2005;200:113. doi: 10.1016/j.surfcoat.2005.02.072. [DOI] [Google Scholar]
- Fox-Rabinovich G S, Wilkinson D S, Veldhuis S C, Dosbaeva G K. and Weatherly G C. Intermetallics. 2006;14:189. doi: 10.1016/j.intermet.2005.05.011. [DOI] [Google Scholar]
- Knotek O, Böhmer M, Leyendecker T. and Jungblut F. Mater. Sci. Eng. A. 1988;105–106:481. [Google Scholar]
- Martin P J, Bendavid A, Cairney J M. and Hoffman M. Surf. Coat. Technol. 2005;200:2228. doi: 10.1016/j.surfcoat.2004.06.012. [DOI] [Google Scholar]
- Diserens M, Patscheider J. and Lévy F. Surf. Coat. Technol. 1998;108–109:241. doi: 10.1016/S0257-8972(98)00560-X. [DOI] [Google Scholar]
- Hasegawa H, Ohashi K, Tsukamoto S, Sato T. and Suzuki T. Surf. Coat. Technol. 2007;202:786. doi: 10.1016/j.surfcoat.2007.05.050. [DOI] [Google Scholar]
- Ichimura H. and Kawana A. J. Mater. Res. 1993;8:1093. doi: 10.1557/JMR.1993.1093. [DOI] [Google Scholar]
- Rovere F, Music D, Schneider J M. and Mayrhofer P H. Acta Mater. 2010;58:2708. doi: 10.1016/j.actamat.2010.01.005. [DOI] [Google Scholar]
- Guo M H, Wang Q M, Gong J, Sun C. and Wen L S. Surf. Coat. Technol. 2006;201:1302. doi: 10.1016/j.surfcoat.2006.01.063. [DOI] [Google Scholar]
- Gao W, Liu Z, Li Z. and Gong H. Int. J. Mod. Phys. 2002;16:128. doi: 10.1142/S021797920200955X. [DOI] [Google Scholar]
- Gao W, Liu Z. and Li Z. Adv. Mater. 2001;13:1001. doi: 10.1002/1521-4095(200107)13:12/13%3C1001::AID-ADMA1001%3E3.0.CO;2-V. [DOI] [Google Scholar]
- Hatakeyama T. and Quinn F. Thermal Analysis. New York Wiley; 1999. [Google Scholar]
- Cao X Q, Vassen R, Tietz F. and Stoever D. J. Eur. Ceram. Soc. 2006;26:247. doi: 10.1016/j.jeurceramsoc.2004.11.007. [DOI] [Google Scholar]
- Shevela V. J. Frict. Wear. 1990;11:136. [Google Scholar]
- Hovsepian P E, Lewis D B. and Münz W D. Surf. Coat. Technol. 2000;133–134:166. doi: 10.1016/S0257-8972(00)00959-2. [DOI] [Google Scholar]
- Hassani S, Bielawski M, Beres W, Martinu L, Balazinski M. and Klemberg-Sapieha J E. Surf. Coat. Technol. 2008;203:204. doi: 10.1016/j.surfcoat.2008.08.050. [DOI] [Google Scholar]
- Beake B, Bell G, Goodes S, Pickford N. and Smith J. Surf. Eng. 2010;26:37. doi: 10.1179/174329409X451137. [DOI] [Google Scholar]
- Ning L, Veldhuis S. and Yamamoto K. Mach. Sci. Technol. 2007;11:45. doi: 10.1080/10910340601172230. [DOI] [Google Scholar]
- Dosbaeva G, Veldhuis S, Elfizy A, Fox-Rabinovich G. and Wagg T. J. Mater. Eng. Perform. 2010;19:1193. doi: 10.1007/s11665-009-9587-3. [DOI] [Google Scholar]


