Abstract
There has been unprecedented development in tissue engineering (TE) over the last few years owing to its potential applications, particularly in bone reconstruction or regeneration. In this article, we illustrate several advantages and disadvantages of different approaches to the design of electrospun TE scaffolds. We also review the major benefits of electrospun fibers for three-dimensional scaffolds in hard connective TE applications and identify the key strategies that can improve the mechanical properties of scaffolds for bone TE applications. A few interesting results of recent investigations have been explained for future trends in TE scaffold research.
Keywords: tissue engineering, hard tissue, electrospinning, nanofiber, polymer, scaffolding
1. Introduction
There has been enormous progress in tissue engineering (TE) in recent years. TE scaffolding materials have been extensively explored for the regeneration of various parts of the human body, including skin [1], muscle [2], teeth [3], bone [4,5], cartilage [4], ligament [6], spinal cord [7], nerve [8], genome [9] and artificial organs, particularly cardiac tissues or heart [10], lung [11], kidney [12], liver [13], urinary bladder [14], blood vessels [15], stomach [16], intestine [17], breast [18], ear [19], eye [20] and nose [21]. TE includes the combined growth of living cells, tissues or organs, as well as methods and materials that can restrain the shape of a particular tissue for better functions. When a group of cells (e.g. osteoblasts, osteocytes, osteoclasts and osteoprogenitor cells) perform the same function together, they are called tissue (e.g. bone tissue), which can relate to different organs. The essential human organs, including bone, with different engineering key factors of scaffolds for corresponding functional systems are illustrated in figure 1. In the skeletal system, the extracellular matrix becomes calcified in bone tissue unlike other connective tissues. The hard tissue or bone provides an internal support to the body via attached tendons and muscles and has several metabolic functions, especially in calcium homeostasis. The remodeling and reorganization of bone tissue have various causes, including mechanical stimuli, metabolic causes (i.e. lack of dietary calcium, illness and aging), endocrine changes and effects of drugs. Over the past two decades, many techniques have been developed to design suitable scaffolds for repairing and regenerating various tissues. However, no suitable scaffold has been designed or developed yet for the repair or regeneration of bone tissue because of its complex composition, peculiar structure and extraordinary mechanical and biological properties. Therefore, in this review, we aim to outline key strategies that can improve the mechanical properties of scaffolds for bone TE applications. We also focus on electrospun scaffolds made of nanofibers to emphasize their benefits for bone tissue repair or regeneration.
Figure 1.
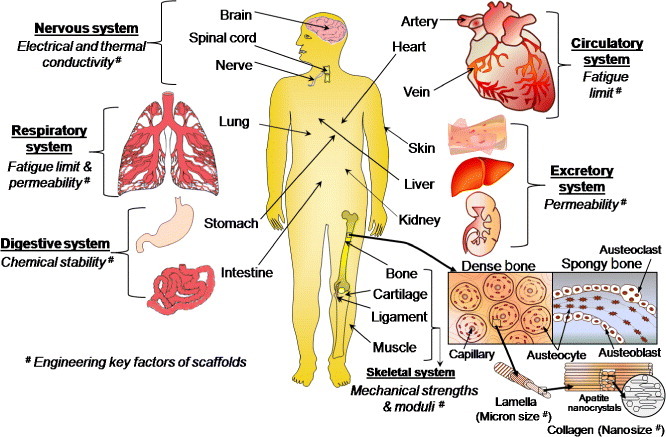
Schematic of essential human organs listing functionalities required from implanted scaffolds.
1.1. Key engineering properties of primary tissues
The five main primary tissues, which are generally prepared by electrospinning for implantation into the human body, are epithelial, connective, fluid, nerve and muscles. The basic desired properties of engineering scaffolds for different tissues and the effect of electrospun materials on the TE scaffolds are illustrated in table 1.
Table 1.
Properties of TE scaffolds desired for different tissues and effects of electrospun materials on TE scaffolds.
| Tissue | Biological description | Key engineering properties | Effect of electrospun materials on TE scaffolds | Use of scaffolds |
|---|---|---|---|---|
| Epithelial tissue e.g. skin | Joined together with same tissue; soft and elastic | Very low elastic moduli (0.1–0.2 MPa), optimum pore size (20–125 μm) [22] | Electrospun collagen nanofibers can improve the structural integrity and mechanical strength of skin tissues. The scaffolds made of electrospun nanofibers provide a high surface area-to-volume ratio, which promotes the cell–matrix interaction at the nanoscale [23] |  |
| Connective tissue e.g. bone, tendon, ligament, cartilage and fat | Joins different tissues; strong and tough | High tensile, compressive, and torsional strengths, high elastic moduli, optimum pore size for bone (100– 250 μm) [24], ideal porosity (>90%) [25] | Electrospun nanofibers with high surface porosity improve cell ingrowth and the mechanical properties of the scaffold [26,27] |  |
| Fluid tissue e.g. blood, fibrinogen (natural polymer present in blood plasma), thrombin | Transports food, nutrients and waste products; viscous | Specified viscosity, surface tension, mass transport property and pH | The high surface-to-volume ratio of the electrospun synthetic fibrinogen nanofibers can improve the blood clotting process in wound healing after reacting with thrombin by forming a network structure of a fibrous compound called fibrin [28] |  |
| Nerve tissue | Sensitive to various stimuli | Smart properties; high ionic, electrical and thermal conductivities, electrochemical and chemoelectrical transduction properties [29] | High aspect ratio of electrospun nanofibers enhances the conductivity of sensors via electron transport, which is extremely effective for nerve tissue scaffolds [29] |  |
| Muscle tissues: | ||||
| Voluntary muscle tissue e.g. arm, leg and skeletal muscles | Made of striated muscle fibers supported by connective tissues and stimulated by nerves | Medium elastic modulus and high fatigue endurance under cyclic load | Electrospun nanofibers of polyester urethane and poly(l-lactide-co-ε-caprolactone) have satisfactory mechanical properties and encouraging cellular response in terms of adhesion and differentiation; they can be used in scaffolds for skeletal [30] or smooth [31] muscles |  |
| Involuntary muscle tissue e.g. intestines, heart or cardiac muscles | Smooth and not human-controlled; soft | Low elastic modulus and high fatigue endurance limit under cyclic load | Small intestinal submucosa (SIS) composed of type-I and type-III collagens and various cytokines leads to superior initial cell attachment and proliferation compared with synthetic polymeric scaffolds in presence of growth factors. Electrospun SIS/poly(ε-caprolactone) hybrids have a stable micro/nanofibrous structure, which provide improved hydrophilicity, mechanical properties and cellular behavior to the scaffolds [32] |  |
Depending on the applications and functions, an electrospun TE scaffold can be temporary or permanent. Usually, a temporary scaffold is highly porous and fully biodegradable, without side effects of by-products, whereas a permanent scaffold is highly biocompatible, mechanically strong, nondegradable, and remains inside the body for a long time.
1.2. Different tissue engineering approaches
Depending on the source of scaffolds, raw materials and applications, TE can be approached by using four main types of material: premade porous scaffold [33], decellularized extracellular matrix (ECM) [34], cell-sheets with secreted ECM [35] and cell-encapsulated self-assembled hydrogels [36] (see figure 2).
Figure 2.
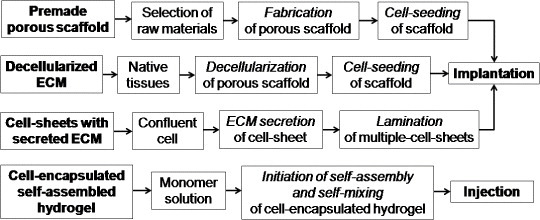
Different TE regeneration techniques.
1.2.1. Premade porous scaffolding.
In this approach, a porous scaffold is produced from different synthetic raw materials using various scaffold fabrication techniques. Then, living cells are seeded on the porous scaffold, and the resulting scaffold is implanted into the body for growing host tissues. The main advantages of this approach are low cost, availability of diverse raw materials, easy handling and simple technique. However, this strategy results in undesired responses from host tissues owing to the lack of biocompatibility, tissue adherence, surface chemistry, mechanochemical stability, and mismatch of degradation rate kinetics with the formation of new tissue.
1.2.2. Decellularized ECM scaffolding.
The decellularized ECM technique is similar to the premade porous scaffolding; only the source of the scaffold is different. Here, native tissues are collected from decellularized ECM of similar tissue from another part of the body. The ECM includes the structural components of the niche such as soluble cue, matrix cue, stem cells, basement membrane and niche cells. It provides a physical platform for cell attachment, migration and division. The ECM acts as a reservoir of growth factors and potentiates their actions. It also sends biochemical signals to the cells that are modulated via molecular interactions with ECM biomolecules, such as heparin sulfate proteoglycans (HSPGs), or through adjacent cells. Here, the decellularized ECM exhibits several attractive characteristics as a TE scaffold that favors the ECM tissues for long-term in vivo applications. Some of the main advantages of this technique over premade scaffolding are lower toxicity, cacogenicity and bioincompatibility. However, this approach has failed in many cases owing to the immunogenicity of the used biomaterials and the cell necrosis at the bulk scaffold related to oxygen deficiency and diffusion of nutrients. Another demerit of this approach is that the donor tissue is likely to elicit immunogenic responses and contain large variation over different batches [37].
1.2.3. Cell-sheets with secreted ECM.
In this method, the confluent cells are collected from its own ECM secretion, which is secreted from different glands or organs, to prepare a cell-sheet layer. The secreted cells are harvested only by cell culture (without using enzymatic treatment), on a culture dish coated with a thermoresponsive polymer (e.g. poly(N-isopropylacrylamide)), until confluence. The confluent cell-sheet is then isolated using thermally regulated hydrophobic polymer coatings. Such approach can be repeated to obtain a thicker matrix of multiple-cell-sheet in a thermoresponsive culture dish by laminating a few single-cell sheets. After that, the cell-sheets are recovered from the dish using a low-temperature treatment. Finally, the multiple-cell-sheets are transferred and implanted into the body to observe the ingrowth properties of the host tissues. In some cases, this method is more advantageous than the decellularized ECM scaffolding due to the lower immunogenicity of the biomaterials used to form new tissues.
1.2.4. Cell-encapsulated self-assembled hydrogel.
Cell-encapsulation is a method of entrapping living cells within a homogenous solid mass or a semipermeable membrane. The biomaterials used for encapsulation are usually natural and synthetic hydrogels, which are made by covalent or ionic crosslinking of water-soluble polymers. Here, usually, one monomer solution of a completely biodegradable polymer is prepared by a self-mixing technique to make a cell-encapsulated hydrogel. The hydrogels can be formed through several gelation mechanisms, where polymer chains are crosslinked via covalent, ionic or physical bonds. Finally, the encapsulated hydrogel material is injected into the body to regenerate the host tissue. This technique is generally used for tissue regeneration via drug delivery or for soft tissue regeneration because of the high biodegradability and reduced mechanical properties of the hydrogel. Recently, this technique has also been tried for cartilage TE scaffolds by increasing the mechanical fracture strength through developing double networks in the hydrogel polymer chains. Interestingly, it has been found that the toughness and strength of the hydrogels are increased with void formation up to a certain void size or optimum void fraction (e.g. 1–3 vol% for polyacrylamide, PAAm). Below the critical size, the polymer (i.e. PAAm) chains can bridge the void gap and create additional stress on the void. Above the critical void size, the chains are too small to bridge the gap and the void can form a true hollow stress-free structure [38].
Over the past decades, many scaffold materials have been tried for TE applications. However, no material was fully suitable for long-term implantations of bone TE scaffolds despite the specific advantages of each material. The failure might have occurred owing to the lack of consistency in material properties including mechanical stability, biodegradability, biocompatibility, toxicity, thermal or electrical sensitivity, permeability, surface adhesivity, hydrophilicity or hydrophobicity and fluidity in terms of viscosity. The consistency in material properties can be improved by the proper selection of materials, design and fabrication procedure. In this context, electrospun scaffolds performed better than other scaffolds.
2. Basic criteria for design and development of TE scaffolds
The essential criteria for designing and developing an ideal three-dimensional (3D) TE scaffold are summarized in table 2.
Table 2.
Criteria for designing electrospun fibers and TE scaffolds.
| Function | Design criteria |
|---|---|
| Strong, uniform and bead-free fibers | High-molecular-weight polymers having high ionic conductivity, concentration, or viscosity; high operating voltage in electrospinning process [39] |
| Thin fibers, needle-like tip design | Electrospinning at high voltages and low flow rates [40] |
| Aligned electrospun fibers | High rotating target speed [40]; collector geometry |
| Structural stability to retain tissue shape | Maintaining mechanical properties throughout the 3D scaffold by strong electrospun nanofibers |
| Transport of nutrients and waste in and out of the electrospun scaffold | High porosity and interconnectivity between pores formed by maintaining preferred orientation of fibers [41] |
| Degradation integrity of electrospun scaffold to leave host tissue | Balancing degradation and formation of tissue without toxic by-products [42] |
| Elimination of inflammatory response or toxicity from the electrospun scaffold | Materials must be biocompatible, nontoxic and noncarcinogenic |
| High cell seeding density and cell migration leading to tissue growth throughout the scaffold | Large pore size, high porosity and high interconnectivity between pores using preferred unit cell geometry of the electrospun scaffolds [43] |
| Better cell attachment and proliferation | Optimized surface chemistry/topography and high surface-to-volume ratio |
| New cell or ECM growth in preferred direction | Proper fiber orientation within the scaffold [22] |
| Growth of 3D tissues and organs | Specific 3D shape of electrospun scaffolds using preferred unit cell geometry [43] |
2.1. Scaffold design
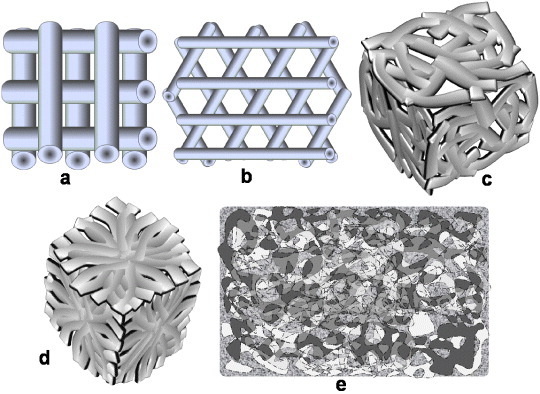
Since the design of scaffold architecture is crucial for improving its performance, the selection of a unit cell for obtaining an ideal 3D scaffold must be included. The unit cell, which is a building block of a 3D scaffold material, is similar to a cell in a tissue. The different geometrical shapes of unit cells such as cubic, diamond, gyroid [44], honeycomb [45] and accordion-like honeycomb [46] provide different 3D pores to scaffolds (figure 3). It has also been found that the tissue growth depends on the geometrical shape of the unit cells of 3D pores in a scaffold [47]. The shape of the unit cells depends on the fabrication technique. For electrospun fibrous scaffolds, the unit cell geometry of a 3D scaffold is defined by the arrangement of fibers, which in turn controls the interconnecting porosity in the scaffold [2, 37, 48]. However, controlling the uniformity in 3D structure generation via electrospinning is very difficult without a deep involvement of the whole architecture of the scaffold, including the unit cell geometry and fiber orientation. To better understand the basic use of fibers in 3D TE scaffold development, different fiber arrangements and the surface morphology of a regenerative scaffold are illustrated in figure 4.
Figure 3.
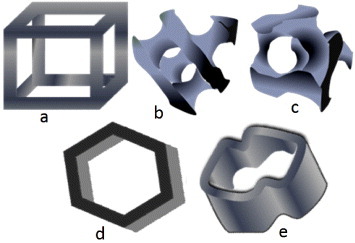
Unit cells for 3D scaffolds: (a) cubic, (b) diamond, (c) gyroid, (d) honeycomb and (e) accordion-like honeycomb.
Figure 4.
Different fiber arrangements in 3D scaffolds: (a) 0°/90°, (b) 0°/60°/120°, (c) randomly oriented and (d) converging oriented fibers. (e) Surface morphology of a regenerative scaffold.
2.2. Scaffold material development
Many scaffold materials have been exploited recently for a wide range of TE applications. Among the three classes of scaffolding materials, namely, ceramics, polymers and composites only the last two can be easily electrospun into nanofibers, as ceramic materials have not been electrospun thus far without adding an organic functional material or polymer. Table 3 presents a summary of attempts [49–53] to fabricate porous 3D scaffolds for TE applications by electrospinning.
Table 3.
Materials, techniques and applications of porous 3D TE scaffolds.
| Scaffold material | Application | Technique | Electrospinning as a possible alternative Technique |
|---|---|---|---|
| Polymers | |||
| Porous biodegradable poly (dl-lactide-co-glycolide) (PLGA) copolymers | Tissue regeneration or reconstruction | Emulsion freeze-drying [22] | Yes |
| Porous poly(l-lactic acid) (PLLA), PLGA, chitosan and alginate | Skin tissue scaffolding using ROS 17/2.8 osteoblast-like cells (rat osteosarcoma) | Freeze-extraction and freeze-gelation [54] | Yes |
| Porous polyethylene glycol terephthalate/polybutylene terephthalate (PEGT/PBT) | Scaffold for cartilage TE applications using chondrocytes | Compression molding and particle leaching [55] | Yes |
| Poly(ethylene oxide) and poly(ethyleneglycol)dimethacrylate photopolymerizable hydrogels | Scaffolds for soft tissues in terms of elasticity | Stereolithography [50] | Yes |
| Polycaprolactone | Bone scaffolds for bone morphogenetic protein-7 (BMP-7)-transduced fibroblasts | Selective laser sintering [56] | Yes |
| Chitosan | Electrobiological | Electrochemical process [57] | Yes |
| DNA ‘square-U’-based structure | Single-strand DNA origami for biological nanoelectronics | Polymerase chain reaction [9] | Yes |
| Biodegradable polyurethane (PU) | Skin tissue scaffolding using human fetal foreskin fibroblast cells | Melt electrospinning [58] | – |
| Ceramics | |||
| Porous hydroxyapatite (HAp) | Load-bearing bone scaffold | Combination of gel casting and polymer sponge [59] | No |
| Biomorphic silicon carbide ceramics, uncoated or coated with bioactive glass | Bone implants, e.g. load-bearing prostheses using MG-63 human osteoblast-like cells | Biotemplating [60] | No |
| High-strength HAp | Load-bearing bone scaffold | Solid-state reaction [5] | No |
| Bioactive, degradable and cytocompatible bredigite (Ca7MgSi4O16) | Bone tissue scaffold using osteoblast-like cells | Sol–gel [61] | No |
| Biomorphic HAp | Bone tissue scaffold and implant | Combination of novel biotemplating and sol–gel methods [62] | No |
| Nanostructure HAp | Low-strength TE including drug delivery and cell loading | Gel casting [63] | No |
| Composites | |||
| Polyvinyl alcohol (PVA)/HAp | Scaffolds for craniofacial and joint defects | Selective laser sintering [64] | Yes |
| PLGA/HAp composite and PLGA-dichloromethane-HAp-DNA/nanoparticles | DNA and PLGA/HAp composite scaffold for bone TE | Electrospinning [65] | – |
| Chitosan/calcium phosphates | TE | Membrane diffusion followed by effective freeze-drying [66] | Yes |
| Polyether etherketone (PEEK)/HAp | Human trabecular bone TE scaffold | Unconfined uniaxial compression [67, 68] | Yes |
| Thermoplastic PU/ collagen | TE scaffold using pig iliac endothelial cell (PIEC) proliferation | Coaxial electrospinning [69] | – |
| Polycaprolactone with 0–50 wt% ceramic (20 wt% HAp/ 80 wt% β-tricalcium) | Scaffold for bone TE | Electrospinning [70] | – |
In the previous decade, conventional techniques such as solvent casting, particle leaching, salt fusion, gas foaming, fused deposition modeling and so on were exploited for TE applications. More recently, the rapid prototype (RP) process, also known as solid free-form fabrication (SFF) process, has achieved great success owing to its automatic or semiautomatic rapid manufacturing procedures and the ability to design any complex geometry layer by layer [53]. The most important RP techniques are stereolithography [50], photopolymerizing, fused deposition modeling, selective laser sintering, 3D printing and bioplotting. Although most materials processed in commercial RP or SFF machines have been optimized for several technical applications, they could not be used for medical applications owing to their toxicity [53]. The scaffolds made by electrospinning exhibit better cellular attachment, growth and differentiation compared with those made by other techniques. These properties probably originate from the large specific surface area (i.e. surface area-to-volume ratio) provided by a low-dimension fibrous structure, which facilitates cell adhesivity to the electrospun fibers. Thus, in this review article, we present the advantages of electrospinning techniques and the scaffolds made from electrospun fibers for TE applications. In this context, the connective TE is the most promising field of research that provides a potential clinical application to repair or regenerate injured tissues. After the pioneering discovery of mesenchymal stem cells (MSCs) by Friedenstein in the late 1970s [71], the number of studies on bone and cartilage TE has grown dramatically. Recently, bone-marrow-derived mesenchymal stem cells (BM-MSCs) [72], umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) [73], muscle-derived stem cells (MDSCs) [74], embryonic stem cells (ESCs) [75], adipose tissue-derived stem cells (ADSCs) [76] and dental pulp stem cells (DPSCs) [77] have been extensively explored in the field of connective TE owing to their distinct biological capability to differentiate into osteogenic lineages [78].
3. Progress in bone scaffold development
More than 50 polymers were employed during the last decade [24, 67, 68, 79] for scaffolding hard tissues, more specifically bone tissue. However, no single-phase polymer has shown the desired properties for bone TE applications. Regeneration or reconstruction of bone tissue involves three key factors namely, osteogenic progenitor cells, osteoinductive growth factors, and osteoconductive matrices [53]. Also, the selection of both materials and fabrication techniques is crucial for an ideal scaffold. The bone is such a structural composite material that it has a very high specific strength (i.e. strength-to-weight ratio). The tubular shape of bone can resist a bending force that causes compression on one side and tension on the reverse side.
3.1. Strategy for selection of scaffold materials to improve mechanical properties
Selection of materials for hard TE scaffolds depends on the properties required for real applications. For bone tissue, one of the major criteria is high strength and toughness. The ultimate strength should be such that the scaffold does not fracture before the complete growth of new tissues. The essential mechanical properties of different materials for bone TE scaffolds can be found in [68, 79]. The mechanical properties of polymers can be improved by several methods as discussed below.
3.1.1. Crystallinity.
The mechanical properties of most polymers depend on crystallinity, which generally increases with the number of polar groups in the polymer chain. However, an opposite tendency is observed for some functionalized polymers and attributed to the asymmetric (i.e. atactic or syndiotactic) stereographic position of the pendant functional groups. This asymmetric structure helps to increase the mobility of polymer chains, and therefore reduces the crystallinity and mechanical properties of the polymer. A more detailed explanation of this new finding is given in the original report [80].
3.1.2. Copolymerization.
Copolymerization is one of the best techniques to increase the mechanical properties of polymers. The mechanical properties of copolymers can be improved by increasing the size of crystalline domains [81], molecular bond strength [82], tacticity or stereographic position [80], hydrophilicity [83] and so on. In copolymerization, the molecular bond strength is increased by grafting between different polymers, which in turn increases the mechanical properties of bulk copolymer. Polymerization may also affect the mechanical properties. For example, the mechanical strength increases with time and temperature of corona discharge polymerization, whereas ultraviolet irradiation decreases the mechanical strength of polymer materials [82].
3.1.3. Hydrophilicity.
The mechanical and biological properties of polymeric materials can be improved by increasing their hydrophilicity [84]. Normally, their mechanical properties vary for the dry and hydrated states, whist the last condition is mostly employed in TE scaffolds or implant devices [84]. In 2009, Li et al conducted a tensile test on poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] (PHBV) and its blends with poly[(R)-3-hydroxybutyrate]-alt-poly(ethylene oxide) (HE) after immersion in phosphate buffer solution for 24 h at 37 °C. Their results showed that the strain and hydrophilicity of the PHBV/HE blends increased gradually with the HE content, and the ultimate tensile strength (UTS) also increased (by 110%) up to a certain weight percentage, i.e. 5 wt% HE. Conversely, above 5 wt% HE, the PHBV/HE hybrids had higher elastic strain and hydrophilicity, but lower UTS. The results indicate that HE improves the hydrophilicity of PHBV/HE polymers through surface modification, and the hydrophilicity further increases the mechanical properties by increasing the crosslinking between the poly[(R)-3-hydroxybutyrate] (PHB) segments of HE and PHBV matrix up to a certain level (i.e. 5 wt% HE for this PHBV/HE system). Above a critical level, the water absorption increases rapidly, and this deteriorates the mechanical strength [84]. Lu et al [85] reported that the tensile and tear strengths of addition silicone, which is generally hydrophobic and is used as an impression material, can be improved by making it hydrophilic after the addition of surfactants. It has also been found that the moderately hydrophilic polymers support the attachment of a high fraction of fibroblast cells [83]. Moreover, the cell adhesion via adsorbed protein can be improved by surfaces with intermediate wettability. On the other hand, hydrophobic polymers such as polytetrafluoroethylene polypropylene and polyethyleneterapthalate, polystyrene provide a limited support for cell attachment. The poor cell attachment to nonpolar polymers (e.g. polystyrene) is possibly related to the lower degree of crystallinity in amorphous materials or hydrophobic polymers [83]. The different crystallinities of hydrophobic polymers further alter the mechanical properties. However, a technical problem associated with hydrophilic polymers, which is frequently faced when changing the medium during in vitro cell culture experiments, is that smaller hydrophilic electrospun fibers are lifted off the bulk scaffold owing to higher wetting in aqueous medium [40]. This problem can be solved by optimizing the hydrophilicity of polymers using proper surface modifiers.
3.1.4. Surface treatment.
A few recent studies have shown that the mechanical properties of the bulk polymers or fibrous polymers can be improved by several surface treatment techniques [80, 86]. The polymer surface is effectively modified with polar groups containing ester (–COOR), ether (–O–), ketone (>CO), epoxy, carboxylic acid (–COOH), hydroxyl (–OH), acetyl (–COCH3), amide (–CONH2), amine (–NH2) or other moieties by a suitable surface treatment. The treatment improves the surface activity, polarity, hydrophilicity and mechanical properties of the electrospun polymers. The resulting surface-modified electrospun fiber may be advantageous for TE scaffolds used in connective tissues, including bone, cartilage, ligament and tendon reconstructions or replacements.
3.1.5. Composite fabrication or hybridization.
Most of the recent investigations on tissue scaffoldings follow this technique because it is inexpensive and allows easy and precise control of the physical, chemical and biological properties. The organic part of bone tissue, such as protein, gives tensile strength and the inorganic part, calcium and phosphorus salts, provides compressive strength to the bone. The inorganic part of the natural bone is mostly similar to synthetic hydroxyapatite (HAp, [Ca5(PO4)3OH]) in structure and chemical composition. The structure of bone is a highly organized hierarchy of the inorganic (HAp) and organic (e.g. Type-I collagen) phases [60], and in situ growth of inorganic phosphate compounds such as nanohydroxyapatite on the nanoparticles or nanofibers of an organic polymer may enhance the interfacial bonding between inorganic ceramics and organic polymers [60, 87, 88]. This innovative technique also improves the cell attachment and cell proliferation on the scaffold surfaces.
3.1.6. Solubility.
Many materials with low solubility have good mechanical properties, biocompatibility and low immune response. However, their limited solubility (e.g. for chitosan, polyether ether ketone) hinders electrospinning [80, 89]. Their good mechanical properties originate from the network structure and strong hydrogen bonding. The mechanical properties of electrospun composite scaffolds also depend on the solubility of second-phase materials used with the polymer matrix in the body plasma or simulated body fluids. If the materials start to biodegrade or dissolve during in vivo use, the mechanical properties of the scaffold can deteriorate in an uncontrollable manner. This is often observed in composites of polymers and calcium phosphates. Several types of calcium phosphates have been used in various biomedical fields, and their stability in water under physiological conditions (pH~7.4, at 37 °C) increases in the following sequence: monocalcium phosphate [MCP, Ca(H2PO4)2·H2O], tetracalcium phosphate [TTCP, Ca4P2O9], α-tricalcium phosphate [α-TCP, α-Ca3(PO4)2], dicalcium phosphate dihydrate [DCPD, CaHPO4·2H2O], dicalcium phosphate [DCP, CaHPO4], octacalcium phosphate [OCP, Ca8(HPO4)2(PO4)4·5H2O], β-tricalcium phosphate [β-TCP, β-Ca3(PO4)2], calcium-deficient hydroxyapatite [CDHAp, Ca9(HPO4)(PO4)5OH], HAp [90]. Therefore, depending on the mode of application, different ceramics and biopolymers should be combined in composite or hybrid scaffolds.
3.2. Selection of electrospinning technique
Synthetic polymer-based electrospun fibers offer adjustable mechanical properties and surface functionalization via biomolecular coatings or chemical conjugation of specific signaling molecules. The vital properties of electrospun fibers that can be tuned via electrospinning are nanofiber diameter, surface morphology, mechanical properties, porosity and pore-size distribution [91]. The basic principle of the electrospinning process is that an electrical voltage, which is sufficient to overcome the surface tension of the polymeric solution, causes the polymer droplets to elongate and eject as a fine fiber [70]. The electrospun fibers are used to form non-woven mats that are assembled into scaffolds. In this process, better fibers are produced from polymers with higher molecular weights. One of the major advantages of electrospun fibers is that they have better molecular binding, which in turn improves their mechanical properties. Electrospinning can be performed in various modes including solution, melt and coaxial electrospinning. In solution electrospinning, the polymer is dissolved in a suitable solvent, whereas in melt electrospinning, the polymer is directly electrospun from the melt. The former method produces a wide range of fiber diameters, which are limited to microns in the latter technique. Melt electrospinning does not require toxic organic solvents and is ideal for scaled-up processes. However, it requires elevated temperatures for the melt, whereas stable solutions can be electrospun at room temperature. The third variety, coaxial electrospinning, is advantageous in the sense that it produces hollow fibers that can transport nutrients and waste in and out of scaffolds as elsewhere [2]. The morphology or microstructure of such scaffolds is similar to the bone architecture. The crucial electrospinning parameters that can alter the fiber properties are applied voltage, spinneret flow rate, target speed [40], collection distance, target properties, polymer molecular weight and polymer solution properties such as solvent type, concentration, viscosity, conductivity and surface tension [91, 92]. In general, the fiber diameter decreases and the fiber quality increases as the voltage is increased; the fiber diameter also decreases at lower flow rates [40]. Fiber alignment in a particular direction can be improved by increasing the speed of the target in an electrospinning system. The electrostatic forces exerted by interfiber interactions mainly control the motion of fibers ejected from the polymer jet during electrospinning; these forces depend on the external field, collector type and charges. As these forces have no preferential direction, randomly oriented nanofibers are formed on a stationary target. However, more aligned and parallel fibers are deposited on a rotating target or a drum [93]. The bead density in fibers decreases with increasing polymer concentration or viscosity [39], and the uniformity of bead-free fibers can be improved by increasing the ionic conductivity of the source polymer. Conducting polymer solutions carry more electric charge and generate stronger repulsive forces on the polymer jet. Therefore, polymer solutions containing ionic salts form more uniform fibers than pure polymers [94]. The properties and process parameters required for aligned fibers are summarized in figure 5.
Figure 5.
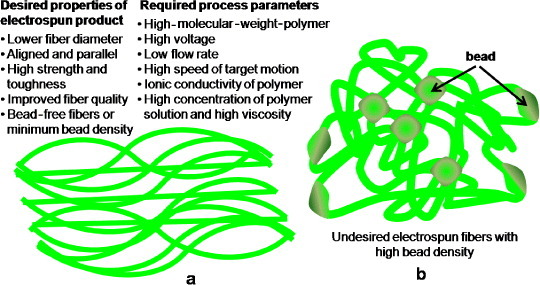
Schematic of electrospun fibers with (a) desired properties and required process parameters in aligned morphology and (b) high bead density and randomly oriented (undesired) morphology.
3.2.1. Electrospun scaffolds in premade porous scaffolding approach.
The research on electrospun scaffolds has recently been invigorated by introducing the premade porous scaffolding approach for skeletal system or connective tissues, including bone repair and/or regeneration. The cell growth property depends on the morphology and fiber orientations in scaffolds [57]. The space between fibers and the position of the fibers in a scaffold should allow the easy growth of cells [95, 96] as illustrated in figure 6.
Figure 6.

(a) Optimum interfiber space (supporting cell growth) and (b) large interfiber space (hindering cell growth).
Recently, Dalton et al have obtained a peculiar result (figure 7) on coiled electrospun fibers [40]. Fibroblast cells have been found to grow nicely inside and outside coil or ring-like fibers (figures 7(c) and (d)), whereas the cells could not attach properly to randomly oriented, entangled fibers [40] (figure 7(e)). This behavior can be attributed to the amorphous and hydrophobic nature of highly entangled long-chain polymeric fibers. Therefore, better cell attachment and tissue ingrowth are expected for scaffolds made of coiled electrospun fibers compared with randomly oriented, entangled fibers.
Figure 7.
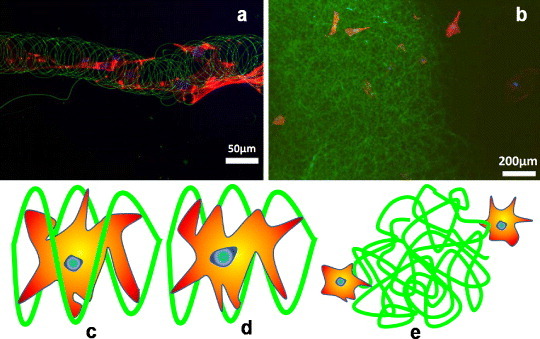
Redrawn fluorescent images of electrospun fibers (a) with cells grown inside and outside the coil or ring-type fibers and (b) cells outside the randomly oriented entangled structure (adopted from [40] with permission). Schematic of cell attachment to electrospun fibers: (c) cell inside the coil, (d) cell outside the coil and (e) cells outside randomly oriented, long entangled fibers.
Besides in vitro attempts, many in vivo investigations have been carried out to repair bone defects using TE scaffolds made of various electrospun nanofibers. A schematic of an ideal electrospinning technique (coaxial method) for bone reconstruction is illustrated in figure 8(A). The treatment of complicated fractures and large osseous defects is a challenging problem for orthopedic surgeons. In 2011, Kolambkar et al [97] made a formidable effort to introduce electrospun nanofiber mesh tubes (effective pore size < 5 μm, porosity 80–90%) as a guide for rat bone regeneration in a segmental bone defect. The nanofibers were made of polycaprolactone and had diameters ranging from 51 to 974 nm, with 82% of the values lying between 50 and 150 nm. The nanofiber mesh combined with peptide-modified alginate hydrogel (i.e., recombinant bone morphogenetic protein-2 (rhBMP-2)) was injected inside the tube for sustaining the growth factor release. A typical in vivo use of the perforated scaffold or mesh made of electrospun nanofibers, combined with growth factor (rhBMP-2), for bone defects is illustrated in figures 8(B)–(F). Figure 8(G) shows the performance of this model up to 21 days, revealing that most alginate release (98.6%) took place within the first 7 days. This novel electrospun hybrid system allowed complete bony bridging of challenging segmental bone defects in rat.
Figure 8.
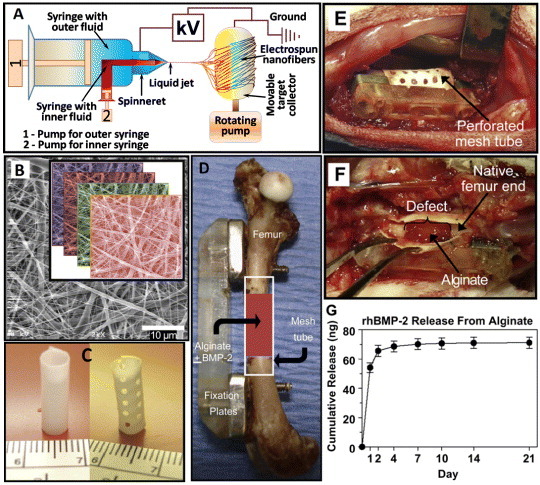
(A) Schematic of a coaxial electrospinning setup. (B) Scanning electron microscopy image of electrospun nanofiber mesh illustrating the smooth and bead-free fibers with nanosized diameters; the inset shows the layers of fibers in a scaffold. (C) Hollow tubular implant made from nanofiber meshes without and with perforations. (D) In vivo application of the nanofiber mesh tubes as implants placed around an 8 mm segmental femoral rat bone defect (in some groups, alginate hydrogel, with or without rhBMP-2, is injected inside the hollow tube). (E) Defect after implantation of a perforated mesh tube; the alginate inside the tube can be seen through the perforations. (F) After 1 week, the mesh tube was cut open, and the alginate was still present inside the defect with hematoma at the bone ends. (G) In vitro alginate release kinetics: sustained release of the rhBMP-2 was observed during the first week (images B-G are taken from [97] with permission).
3.2.2. Electrospun scaffolds in decellularized ECM approach.
Recently, bone regeneration has been attempted with natural materials fabricated into scaffolds, such as demineralized bone as a nanoscale-bone-matrix (NBM) powder, for improving the mechanical and osteoinductive properties [98]. Interactions between cells and ECM are very important for isolating cells and modulating or redirecting their functions [99]. The major advantages of electrospun fibers in the engineered scaffolds are that they provide micro- to nanoscale topography and high porosity similar to the natural ECM [92]. The high surface-to-volume ratio of nanoscale electrospun scaffolds can enhance the cell attachment, drug loading, and mass transfer properties [100]. The porosities resulting from electrospinning help transport nutrients and waste within the decellularized ECM approach. On the other hand, the nonwoven fibrous mats consisting of nanofibers provide a high surface area to interact with and attach to cells [101]. In this context, electrospun fibers of poly(l-lactide) (PLA) with 20% NBM have shown a higher Young's modulus than pure PLA. These electrospun NBM/PLA composite nanofibers exhibit the properties similar to those of the native collagen-rich mineralized bone matrix and therefore can be used as a temporary substrate for facilitating the isolation and mineralization of bone-forming cells [1].
3.2.3. Electrospun scaffolds in cell-sheets with secreted ECM approach.
The uniform aligned electrospun collagen fibers may be an ideal candidate to mimic complex structures in regular connective tissues such as tendons, ligaments and bones. The aligned electrospun fibers will also allow the fibroblasts and collagens to organize in the same orientation. Recently, the cell-sheet approach has been exploited to establish a novel method of tissue reconstruction in bone tissue regenerative medicine [103]. In this approach, there is no need for the scaffold to construct a cell-dense tissue, and it can be transplanted to various damaged tissues without any other treatment (e.g. suturing). The cell sheets can also be controlled with a polymer-coated plunger for transferring and stacking onto other cell sheets. Importantly, the stacking method allows two cell sheets to contact each other physically, as well as to communicate biologically. The control of cell alignment may be a key factor in the next-generation cell-sheet-based tissue reconstruction technology [103].
3.2.4. Electrospun scaffolds in cell-encapsulated self-assembled hydrogel approach.
Several hydrogel materials have been scaffolded for connective TE applications using the cell-encapsulated self-assembled hydrogel approach [104]. The porous hydrogel scaffolds can be used for microcarrier suspension culture of cells and for injection of the cell/microsphere constructs into a tissue defect. The injected porous scaffolds permit infiltration of cells and ingrowth of tissue from the host, and facilitate the regeneration process [105]. A variety of electrospun hydrogels fibers have also been developed for advanced applications [106], and different composites or hybrids of hydrogel materials have been studied for tissue engineering applications [107]. Self-assembling biomolecules (e.g. peptides) have been used for in vitro 3D culture of cells, in vivo tissue regeneration or repair of bones and optical nerves, as well as drug delivery and other applications [108]. Many important properties (e.g. swelling, mechanical properties, diffusion and degradation) of hydrogel in scaffolds can be tuned by controlling the molecular structure of the hydrogels. The molecular structure depends on both degree and density of crosslinking—the mechanical properties of the hydrogel improve as the crosslinking density increases [36]. However, a higher crosslinking density results in inferior swelling properties and mesh sizes, i.e. the distance between the crosslinks, which influences diffusion through the hydrogel. Thus, while the mechanical strength, which is desirable for in situ placement in articular cartilage, is improved, the swelling and diffusion properties are sacrificed [36]. More research is needed on the cell-encapsulated self-assembled hydrogel approach to develop new strategies in bone tissue regeneration [95, 96].
4. Concluding remarks
Selection of the raw material for bone TE applications is crucial for the development of an ideal scaffold—the materials and fabrication technique are to be chosen in accordance with the specific tissue application. Selection of the fabrication technique is also important for developing a proper morphology in the electrospun scaffold. Melt electrospinning can produce finer fibers as compared with other methods. However, hollow tubular fibers produced by coaxial electrospinning are preferred for the transport of nutrients and waste in and out of the bone scaffold. All the physicochemical properties including porosity, pore geometry, surface roughness, surface chemistry and biological and mechanical properties should be considered when designing a scaffold. Therefore, a combination of solid and hollow fibers may be optimal for bone applications. After developing a scaffold, it is essential to evaluate its safety for long-term implantations. Therefore, more in vitro experiments on electrospun scaffolds are needed for developing a novel strategy of bone tissue repair and/or regeneration before actual in vivo implementations [95, 96]. In this context, the development of new TE scaffolds that could assist in the regeneration of large bone defects must be considered. Additionally, the scaffolds should promote not only the biomineralization, but also the maintenance of biological and mechanical properties for tissue regeneration.
Acknowledgments
The authors gratefully acknowledge the Ministry of Higher Education (MOHE), Malaysia, for the UM/MOHE/HIR grant (Project number: D000010-16001).
References
- Renner R, Harth W. and Simon J C. Int. Wound J. 2009;6:226. doi: 10.1111/j.1742-481X.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao I-C. and Leong K W. Biomaterials. 2011;32:1669. doi: 10.1016/j.biomaterials.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K-T, Suh J D, Kim J, Choung P-H. and Chung J H. J. Biomed. Mater. Res. B: Appl. Biomater. 2011;99B:399. doi: 10.1002/jbm.b.31912. [DOI] [PubMed] [Google Scholar]
- Reddi A H. Tissue Eng. 2000;6:351. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- Pramanik S, Agarwal A K, Rai K N. and Garg A. Ceram. Int. 2007;33:419. doi: 10.1016/j.ceramint.2005.10.025. [DOI] [Google Scholar]
- Liu H, Ge Z, Wang Y, Toh S L, Sutthikhum V. and Goh J C H. J. Biomed. Mater. Res. B: Appl. Biomater. 2007;82:129. doi: 10.1002/jbm.b.30714. [DOI] [PubMed] [Google Scholar]
- Hofstetter C P, Holmstrom N A V, Lilja J A, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad S N, Frisén J. and Olson L. Nature. Neurosci. 2005;8:346. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Chen W. and Tong Y W. Acta Biomater. 2011;8:540. doi: 10.1016/j.actbio.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Pound E, Ashton J R, Becerril H A. and Woolley A T. Nano Lett. 2009;9:4302. doi: 10.1021/nl902535q. [DOI] [PubMed] [Google Scholar]
- Butcher J T, Mahler G J. and Hockaday L A. Adv. Drug Deliv. Rev. 2011;63:242. doi: 10.1016/j.addr.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Nichols J E, Niles J A. and Cortiella J. Organogenesis. 2009;5:57. doi: 10.4161/org.5.2.8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer D L. and Nigam S K. Am. J. Physiol. Renal. Physiol. 2004;286:F1. doi: 10.1152/ajprenal.00167.2003. [DOI] [PubMed] [Google Scholar]
- Chamuleau R A F M. World J. Gastrointest. Surg. 2009;1:21. doi: 10.4240/wjgs.v1.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic V, Stankovic J. and Stefanovic V. Sci. World J. 2011;11:1479. doi: 10.1100/tsw.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C Y, Inai R. and Kotaki M. Biomaterials. 2004;25:877. doi: 10.1016/S0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- Maemura T, Ogawa K, Shin M, Mochizuki H. and Vacanti J P. Transpl. Proc. 2004;36:1595. doi: 10.1016/j.transproceed.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Day R M. Therapy. 2006;1:113. doi: 10.2174/157488806775269124. [DOI] [PubMed] [Google Scholar]
- Patrick C W., Jr. Annu. Rev. Biomed. Eng. 2004;6:109. doi: 10.1146/annurev.bioeng.6.040803.140032. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Isogai N, Enjo M, Otani H, Ikada Y, Jacquet R, Lowder E. and Landis W J. Wound Repair Regen. 2009;17:136. doi: 10.1111/j.1524-475X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- Ge J. and Liu J. Front. Med. China. 2007;1:6. doi: 10.1007/s11684-007-0002-x. [DOI] [PubMed] [Google Scholar]
- Lee J-L, Bai C-Y, Shen Y-K. and Hung J-H. IPCBEE. vol 2. Singapore IACSIT Press; 2011. p. p 62. [Google Scholar]
- Whang K, Thomas C H, Healy K E. and Nuber G. Polymer. 1995;36:837. doi: 10.1016/0032-3861(95)93115-3. [DOI] [Google Scholar]
- Zhong S P, Zhang Y Z. and Lim C T. WIREs Nanomed. Nanobiotechnol. 2010;2:510. doi: 10.1002/wnan.100. [DOI] [PubMed] [Google Scholar]
- Spector M, Michno M J, Smarook W H. and Kwiatkowski G T. J. Biomed. Mater. Res. 1978;12:665. doi: 10.1002/jbm.820120508. [DOI] [PubMed] [Google Scholar]
- Agrawal C M. and Ray R. J. Biomed. Mater. Res. 2001;55:141. doi: 10.1002/1097-4636(200105)55:2%3C141::AID-JBM1000%3E3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rainer A. Ann. Biomed. Eng. 2011;39:1882. doi: 10.1007/s10439-011-0289-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Venugopal J R, El-Turki A, Ramakrishna S, Su B. and Lim C T. Biomaterials. 2008;29:4314. doi: 10.1016/j.biomaterials.2008.07.038. [DOI] [PubMed] [Google Scholar]
- Wnek G E, Carr M E, Simpson D G. and Bowlin G L. Nano Lett. 2002;3:213. doi: 10.1021/nl025866c. [DOI] [Google Scholar]
- Bellamkonda R. and Aebischer P. Biotechnol. Bioeng. 1994;43:543. doi: 10.1002/bit.260430703. [DOI] [PubMed] [Google Scholar]
- Riboldi S A, Sampaolesi M, Neuenschwander P, Cossu G. and Mantero S. Biomaterials. 2005;26:4606. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Mo X M, Xu C Y, Kotaki M. and Ramakrishna S. Biomaterials. 2004;25:1883. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Yoon H. and Kim G. J. Biomater. Sci. Polym. Ed. 2010;21:553. doi: 10.1163/156856209X429166. [DOI] [PubMed] [Google Scholar]
- Wang H, Leeuwenburgh S C, Li Y. and Jansen J A. Tissue Eng. B. 2012;18:24. doi: 10.1089/ten.teb.2011.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badylak S F, Taylor D. and Uygun K. Annu. Rev. Biomed. Eng. 2011;13:27. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirraco R P, Obokata H, Iwata T, Marques A P, Tsuneda S, Yamato M, Reis R L. and Okano T. Tissue Eng. A. 2011;17:1507. doi: 10.1089/ten.tea.2010.0470. [DOI] [PubMed] [Google Scholar]
- Nicodemus G D. and Bryant S J. Tissue Eng. B. 2008;14:149. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S H. and Mao H-Q. Adv. Drug Deliv. Rev. 2009;61:1084. doi: 10.1016/j.addr.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Furukawa H, Tanaka Y, Kurokawa T. and Gong J P. J. Polym. Sci. B: Polym. Phys. 2011;49:1246. doi: 10.1002/polb.22293. [DOI] [Google Scholar]
- Katti D S, Robinson K W, Ko F K. and Laurencin C T. J. Biomed. Mater. Res. B: Appl. Biomater. 2004;70:286. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- Dalton P D, Joergensen N T, Groll J. and Moeller M. Biomed. Mater. 2008;3:043002. doi: 10.1088/1748-6041/3/3/034109. [DOI] [PubMed] [Google Scholar]
- Mikes A G, Bao Y, Coma L G, Ingher D E, Vacanti J P. and Langer R. J. Biomed. Mater. Res. 1992;27:183. doi: 10.1002/jbm.820270207. [DOI] [PubMed] [Google Scholar]
- Cutright D E, Perez D, Beasley J D, Larson W J. and Posey W R. Oral Surg. 1974;37:142. doi: 10.1016/0030-4220(74)90171-6. [DOI] [PubMed] [Google Scholar]
- Coma L G, Vacanti J P, Vacanti C, Ingber D, Moonev D. and Langer R J. J. Biomech. Eng. 1991;113:143. doi: 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- Melchels F P W, Bertoldi K, Gabbrielli R, Velders A H, Feijen J. and Grijpma D W. Biomaterials. 2010;31:6909. doi: 10.1016/j.biomaterials.2010.05.068. [DOI] [PubMed] [Google Scholar]
- Bettinger C J. Pure Appl. Chem. 2009;81:2183. doi: 10.1351/PAC-CON-09-07-10. [DOI] [Google Scholar]
- Engelmayr G C, Cheng M, Bettinger C J, Borenstein J T, Langer R. and Freed L E. Nature. Mater. 2008;7:1003. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpler M, Woesz A, Dunlop J W C, van Dongen J T. and Fratzl P. J. R. Soc. Interface. 2008;5:1173. doi: 10.1098/rsif.2008.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid Q, Snyder J, Wang C, Timmer M, Hammer J, Guceri S. and Sun W. Biofabrication. 2011;3:043002. doi: 10.1088/1758-5082/3/3/034109. [DOI] [PubMed] [Google Scholar]
- Nam Y S, Yoon J J. and Park T G. J. Biomed. Mater. Res. A: Appl. Biomater. 2000;53:1. doi: 10.1002/(SICI)1097-4636(2000)53:1%3C1::AID-JBM1%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Dhariwala B, Hunt E. and Boland T. Tissue Eng. 2004;10:1316. doi: 10.1089/ten.2004.10.1316. [DOI] [PubMed] [Google Scholar]
- Leukers B, Gulkan H, Irsen S H, Milz S, Tille C, Schieker M. and Seitz H. J. Mater. Sci.: Mater. Med. 2005;16:1121. doi: 10.1007/s10856-005-4716-5. [DOI] [PubMed] [Google Scholar]
- Hollister S J. Nature Mater. 2005;4:518. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- Schieker M, Seitz H, Drosse I, Seitz S. and Mutschler W. Eur. J. Trauma. 2006;32:113. [Google Scholar]
- Ho M-H, Kuo P-Y, Hsieh H-J, Hsien T-Y, Hou L-T, Lai J-Y. and Wang D-M. Biomaterials. 2004;25:129. doi: 10.1016/S0142-9612(03)00483-6. [DOI] [PubMed] [Google Scholar]
- Malda J, Woodfield T B F, van der Vloodt F, Kooy F K, Martens D E, Tramper J, van Blitterswijk C A. and Riesle J. Biomaterials. 2004;25:5773. doi: 10.1016/j.biomaterials.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Williams J M, Adewunmi A, Schek R M, Flanagan C L, Krebsbach P H, Feinberg S E, Hollister S J. and Das S. Biomaterials. 2005;26:4817. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- Twu Y-K, Chang I-T. and Ping C-C. Carbohydr. Polym. 2005;62:113. doi: 10.1016/j.carbpol.2005.07.006. [DOI] [Google Scholar]
- Karchin A, Simonovsky F I, Ratner B D. and Sanders J E. Acta Biomater. 2011;7:3277. doi: 10.1016/j.actbio.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramay H R. and Zhang M. Biomaterials. 2003;24:3293. doi: 10.1016/S0142-9612(03)00171-6. [DOI] [PubMed] [Google Scholar]
- de Carlos A, Borrajo J P, Serra J, González P. and León B. J. Mater. Sci.: Mater. Med. 2006;17:523. doi: 10.1007/s10856-006-8935-1. [DOI] [PubMed] [Google Scholar]
- Wu C, Chang J, Zhai W. and Ni S. J. Mater. Sci.: Mater. Med. 2007;18:857. doi: 10.1007/s10856-006-0083-0. [DOI] [PubMed] [Google Scholar]
- Qian J, Kang Y, Zhang W. and Li Z. J. Mater. Sci.: Mater. Med. 2008;19:3373. doi: 10.1007/s10856-008-3475-5. [DOI] [PubMed] [Google Scholar]
- Ghomi H, Fathi M H. and Edris H. J. Sol–Gel. Sci. Technol. 2011;58:642. doi: 10.1007/s10971-011-2439-2. [DOI] [Google Scholar]
- Chua C K, Leong K F, Tan K H, Wiria F H. and Chea C M. J. Mater. Sci.: Mater. Med. 2004;15:1113. doi: 10.1023/B:JMSM.0000046393.81449.a5. [DOI] [PubMed] [Google Scholar]
- Nie H. and Wang C-H. J. Control. Release. 2007;120:111. doi: 10.1016/j.jconrel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Thanaphat P, Thunyakitpisal P. and Tachaboonyakit W. J. Met. Mater. Min. 2008;18:67. [Google Scholar]
- Converse G L, Conrad T L. and Roeder R K. J. Mech. Behav. Biomed. Mater. 2009;2:627. doi: 10.1016/j.jmbbm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Pramanik S. PhD Thesis. 2011. p. p 392. [Google Scholar]
- Chen R, Huang C, Ke Q, He C, Wang H. and Mo X. Colloids. Surf. B. 2010;79:315. doi: 10.1016/j.colsurfb.2010.03.043. [DOI] [PubMed] [Google Scholar]
- Patlolla A, Collins G. and Arinzeh T L. Acta Biomater. 2010;6:90. doi: 10.1016/j.actbio.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Friedenstein A J. Int. Rev. Cytol. 1976;47:327. doi: 10.1016/j.actbio.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Wu M-Y, Chen N, Liu L-K, Yuan H, Li Q-L. and Chen S-H. Polymers. 2009;24:301. [Google Scholar]
- Kim J-Y, Jeon H B, Yang Y S, Oh W. and Chang J W. World J. Stem Cells. 2010;2:34. doi: 10.4252/wjsc.v2.i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usas A. and Huard J. Biomaterials. 2007;28:5401. doi: 10.1016/j.biomaterials.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes J M, Both S K, Leusink A, Sterk L M T, van Blitterswijk C A. and de Boer J. Proc. Natl Acad. Sci. USA. 2008;105:6840. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterodimas A, de Faria J, Nicaretta B. and Pitanguy I. J. Plast. Reconstr. Aesthetic Surg. 2010;63:1886. doi: 10.1016/j.bjps.2009.10.028. [DOI] [PubMed] [Google Scholar]
- Cordeiro M M, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith A J. and Nör J E. J. Endod. 2008;34:962. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Seong J M, Kim B-C, Park J-H, Kwon I K, Mantalaris A. and Hwang Y-S. Biomed. Mater. 2010;5:043002. doi: 10.1088/1748-6041/5/6/062001. [DOI] [PubMed] [Google Scholar]
- Pramanik S. and Kar K K. In: Development in Nanocomposites. Kar K K, , Hodzic A, , editors. Singapore RPS Publisher; 2012. (at press) [Google Scholar]
- Pramanik S. and Kar K K. J. Appl. Polym. Sci. 2012;123:1100. doi: 10.1002/app.34582. [DOI] [Google Scholar]
- Grijpma D W. and Pennings A J. Macromol. Chem. Phys. 1994;195:1649. doi: 10.1002/macp.1994.021950516. [DOI] [Google Scholar]
- Lei J. and Liao X. Eur. Polym. J. 2001;37:771. doi: 10.1016/S0014-3057(00)00177-4. [DOI] [Google Scholar]
- Lydon M, Minett T W. and Tighe B J. Biomaterials. 1985;6:396. doi: 10.1016/0142-9612(85)90100-0. [DOI] [PubMed] [Google Scholar]
- Li X, Liu K L, Wang M, Wong S Y, Tjiu W C, He C B, Goh S H. and Li J. Acta Biomater. 2009;5:2002. doi: 10.1016/j.actbio.2009.01.035. [DOI] [PubMed] [Google Scholar]
- Lu H, Nguyen B. and Powers J M. J. Prosthet. Dent. 2004;92:151. doi: 10.1016/j.prosdent.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Abdolahifard M, Bahrami S H. and Malek R M A. ISRN Org. Chem. 2011;2011:043002. doi: 10.5402/2011/265415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H W. and Wang M. Bioceram. Dev. Appl. 2011;1:043002 [Google Scholar]
- Kar K K. and Pramanik S. 20120107612 A1. US Patent. 2012 Published on May 3, 2012 (World Patent. WO 2012/004637 Date: 12 January 2012)
- Park H N, Lee J B, Moon H-J, Yang D H. and Kwon I K. In: Nanofibers—Production, Properties and Functional Applications. Lin T, editor. Rijeka, Croatia InTech Publisher; 2011. p. p 327. [Google Scholar]
- Horvath L, Smit I, Sikiric M. and Filipovic-Vincekovic N. J. Cryst. Growth. 2000;219:91. doi: 10.1016/S0022-0248(00)00597-2. [DOI] [Google Scholar]
- Kumbar S G, James R, Nukavarapu S P. and Laurencin C T. Biomed. Mater. 2008;3:043002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- Sill T J. and von Recum H A. Biomaterials. 2008;29:1989. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Chang Z, Zhu M, Mo X. and Chen D. Nanotechnology. 2007;18:043002. doi: 10.1088/0957-4484/18/11/115611. [DOI] [Google Scholar]
- Kim S J, Lee C K. and Kim S I. J. Appl. Polym. Sci. 2005;96:1388. doi: 10.1002/app.21567. [DOI] [Google Scholar]
- Stevens M M. and George J H. Science. 2005;310:1135. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- Mager M D, LaPointe V. and Stevens M M. Nature. Chem. 2011;3:582. doi: 10.1038/nchem.1090. [DOI] [PubMed] [Google Scholar]
- Kolambkar Y M, Dupont K M, Boerckel J D, Huebsch N, Mooney D J, Hutmacher D W. and Guldberg R E. Biomaterials. 2011;32:65. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traphagen S. and Yelick P C. Regen. Med. 2009;4:747. doi: 10.2217/rme.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Mondrinos M J, Gandhi M R, Ko F K, Weiss A S. and Lelkes P I. Biomaterials. 2005;26:5999. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Flemming R G, Murphy C J, Abrams G A, Goodman S L. and Nealey P F. Biomaterials. 1999;20:573. doi: 10.1016/S0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- Sharma B. and Elisseeff J H. Ann. Biomed. Eng. 2004;32:148. doi: 10.1023/B:ABME.0000007799.60142.78. [DOI] [PubMed] [Google Scholar]
- Ko E K, Jeong S I, Lee J H. and Shin H. Macromol. Biosci. 2008;8:1098. doi: 10.1002/mabi.200800089. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Nakayama M, Shimizu T, Yamato M. and Okano T. Biomaterials. 2011;32:8830. doi: 10.1016/j.biomaterials.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S. and Grodzinsky A J. Proc. Natl. Acad. Sci. USA. 2002;99:9996. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H J. and Park T G. Adv. Drug Deliv. Rev. 2007;59:249. doi: 10.1016/j.addr.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Burdick J A. and Prestwich G D. Adv. Mater. 2011;23:H41. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A K, Yang W, Kirn-Safran C B, Farach-Carson M C. and Jia X. Biomaterials. 2009;30:6964. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semino C E. J. Dent. Res. 2008;87:606. doi: 10.1177/154405910808700710. [DOI] [PubMed] [Google Scholar]


