Abstract
The biological activities of long and short forms of the prolactin receptor have been compared. These two receptors expressed in mammalian cells were shown to bind prolactin with equal high affinity. The ability of these different forms to transduce the hormonal message was estimated by their capacity to stimulate transcription by using the promoter of a milk protein gene fused to the chloramphenicol acetyltransferase (CAT) coding sequence. Experiments were performed in serum-free conditions to avoid the effect of lactogenic factors present in serum. An approximately 17-fold induction of CAT activity was obtained in the presence of prolactin when the long form of the prolactin receptor was expressed, whereas no induction was observed when the short form was expressed. The present results clearly establish that only the long form of the prolactin receptor is involved in milk protein gene transcription.
Full text
PDF


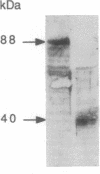
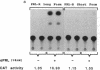
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989 Oct 31;164(2):788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Boutin J. M., Edery M., Shirota M., Jolicoeur C., Lesueur L., Ali S., Gould D., Djiane J., Kelly P. A. Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol Endocrinol. 1989 Sep;3(9):1455–1461. doi: 10.1210/mend-3-9-1455. [DOI] [PubMed] [Google Scholar]
- Boutin J. M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P. A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988 Apr 8;53(1):69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- Damante G., Rapoport B. TSH stimulates the activity of the c-fos promoter in FRTL5 rat thyroid cells. Mol Cell Endocrinol. 1988 Aug;58(2-3):279–282. doi: 10.1016/0303-7207(88)90165-7. [DOI] [PubMed] [Google Scholar]
- Davis J. A., Linzer D. I. Expression of multiple forms of the prolactin receptor in mouse liver. Mol Endocrinol. 1989 Apr;3(4):674–680. doi: 10.1210/mend-3-4-674. [DOI] [PubMed] [Google Scholar]
- Djiane J., Durand P., Kelly P. A. Evolution of prolactin receptors in rabbit mammary gland during pregnancy and lactation. Endocrinology. 1977 May;100(5):1348–1356. doi: 10.1210/endo-100-5-1348. [DOI] [PubMed] [Google Scholar]
- Edery M., Jolicoeur C., Levi-Meyrueis C., Dusanter-Fourt I., Pétridou B., Boutin J. M., Lesueur L., Kelly P. A., Djiane J. Identification and sequence analysis of a second form of prolactin receptor by molecular cloning of complementary DNA from rabbit mammary gland. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2112–2116. doi: 10.1073/pnas.86.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Seto Y., Nagata S. Expression cloning of a receptor for murine granulocyte colony-stimulating factor. Cell. 1990 Apr 20;61(2):341–350. doi: 10.1016/0092-8674(90)90814-u. [DOI] [PubMed] [Google Scholar]
- Gasser F., Mulsant P., Gillois M. Long-term multiplication of the Chinese hamster ovary (CHO) cell line in a serum-free medium. In Vitro Cell Dev Biol. 1985 Oct;21(10):588–592. doi: 10.1007/BF02620890. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. G., Friend D., Ziegler S. F., Jerzy R., Falk B. A., Gimpel S., Cosman D., Dower S. K., March C. J., Namen A. E. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990 Mar 23;60(6):941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Djiane J., Leblanc G. Preferential establishment of a slowly dissociable component in plasma membrane compared to intracellular prolactin receptors. Proc Soc Exp Biol Med. 1983 Feb;172(2):219–224. doi: 10.3181/00379727-172-41549. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Heber A., Schmid W., Danesch U., Posseckert G., Beato M., Schütz G. Glucocorticoid responsiveness of the transcriptional enhancer of Moloney murine sarcoma virus. Cell. 1986 Jul 18;46(2):283–290. doi: 10.1016/0092-8674(86)90745-2. [DOI] [PubMed] [Google Scholar]
- Mohun T. J., Garrett N., Gurdon J. B. Upstream sequences required for tissue-specific activation of the cardiac actin gene in Xenopus laevis embryos. EMBO J. 1986 Dec 1;5(12):3185–3193. doi: 10.1002/j.1460-2075.1986.tb04628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B., Beckmann M. P., March C. J., Idzerda R. L., Gimpel S. D., VandenBos T., Friend D., Alpert A., Anderson D., Jackson J. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989 Oct 20;59(2):335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Friesen H. G. Induction of a lactogenic receptor in rat liver: influence of estrogen and the pituitary. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2407–2410. doi: 10.1073/pnas.71.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Shiu R. P., Friesen H. G. Studies of insulin, growth hormone and prolactin binding: tissue distribution, species variation and characterization. Endocrinology. 1974 Aug;95(2):521–531. doi: 10.1210/endo-95-2-521. [DOI] [PubMed] [Google Scholar]
- Richards J. F., Beer C. T., Bourgeault C., Chen K., Gout P. W. Biochemical response of lymphoma cells to mitogenic stimulation by prolactin. Mol Cell Endocrinol. 1982 Apr;26(1-2):41–49. doi: 10.1016/0303-7207(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Shirota M., Banville D., Ali S., Jolicoeur C., Boutin J. M., Edery M., Djiane J., Kelly P. A. Expression of two forms of prolactin receptor in rat ovary and liver. Mol Endocrinol. 1990 Aug;4(8):1136–1143. doi: 10.1210/mend-4-8-1136. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Taga T., Hibi M., Hirata Y., Yamasaki K., Yasukawa K., Matsuda T., Hirano T., Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989 Aug 11;58(3):573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- Yamasaki K., Taga T., Hirata Y., Yawata H., Kawanishi Y., Seed B., Taniguchi T., Hirano T., Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988 Aug 12;241(4867):825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]