Abstract
Porous ceramics with unidirectionally oriented pores have been prepared by various methods such as anodic oxidation, templating using wood, unidirectional solidification, extrusion, etc. The templating method directly replicates the porous microstructure of wood to prepare porous ceramics, whereas the extrusion method mimics the microstructures of tracheids and xylems in trees. These two methods are therefore the main focus of this review as they provide good examples of the preparation of functional porous ceramics with properties replicating nature. The well-oriented cylindrical through-hole pores prepared by the extrusion method using fibers as the pore formers provide excellent permeability together with high mechanical strength. Examples of applications of these porous ceramics are given, including their excellent capillary lift of over 1 m height which could be used to counteract urban heat island phenomena, and other interesting properties arising from anisotropic unidirectional porous structures.
Keywords: porous ceramics, unidirectional porous microstructure, anodic oxidation, templating, unidirectional solidification, extrusion process
Introduction
Porous ceramics have been widely exploited for their high specific surface area, permeability, sound absorption, thermal insulating and other properties. Since the required properties depend on the applications, a variety of porous microstructures have been developed using a number of preparation methods. The typical porous properties of various porous materials are summarized in figure 1 [1]. Porous ceramics with small pore sizes are generally gels, zeolites, mesoporous silicas, activated carbons, etc. Their pore sizes are in the micropore range (<2 nm) and/or the mesopore range (2–50 nm) [2]. By contrast, macroporous ceramics with pores larger than 50 nm are fabricated using special processes. Zeolites are relatively abundant natural minerals [3] and many have been synthesized for industrial use as catalysts by mimicking the natural hydrothermal processes by which zeolites are formed in nature. Zeolites are therefore good examples of porous ceramics produced by copying nature.
Figure 1.

Pore size and porosity of typical porous substances and ceramics.
Porous structures are grouped into three types: those with three-dimensionally connected and distributed open pores (3D structures), those with two-dimensionally extended slit-shaped open pores (2D structures) and those with unidirectionally oriented continuous open pores (1D structures). Porous ceramics with 3D structures are useful as catalysts, catalyst supports, filters, scaffolds, adsorbents, etc because of the high accessibility of their pores. One interesting porous ceramic with a well-ordered 3D porous structure is an inverse opal structure mimicking the microstructure of opal, which consists of ordered packing of fine uniform submicron amorphous SiO2 particles [4]. This unique structure gives it a beautiful rainbow color, making it a well-known gemstone [5]. The National Institute for Research in Inorganic Materials (NIRIM), the predecessor of the present National Institute for Materials Science (NIMS), pioneered research on the synthesis of artificial opal [6], opening the way to the development of various opal structures for applications as photonic crystals [7] and inverse opal structures for functional porous materials [8].
Porous ceramics with 2D structures are typically observed in activated carbons [9] and pillared clays [10, 11]. Activated carbons are prepared by reaction at elevated temperatures of various carbonaceous precursors with oxidizing gases (physical activation) or oxidizing chemicals (chemical activation). They have the highest specific surface areas of all the porous materials (3000–4000 m2 g−1) [12]. Pillared clays are synthesized by a unique process in which isopoly ions, heteropoly ions or sol particles are intercalated in the interlayers of host compounds where they are heat-treated to form pillars. Some of these pillared clays show high adsorption properties and solid acidity with moderate acid strength [13, 14]. Since most of the host compounds are natural clay minerals, pillared clays are a good example of functional porous ceramics produced from natural resources by utilizing nature effectively.
One important application of porous ceramics is as filters and membranes for use in severe environments at high temperatures and in reactive and corrosive solutions. The ideal porous microstructures for high permeability and separation are well-ordered and unidirectionally oriented cylindrical through-holes (1D structures). Many attempts have been reported to achieve such ideal porous microstructures by anodic oxidation, templating using wood, unidirectional solidification of green compacts and melts, extrusion of precursor ceramic pastes, etc. Some examples of 1D porous ceramics synthesized by these methods are discussed in the following sections.
We have examined papers dealing with technologies related to ‘nature’ for the preparation of porous ceramics using the Web of Science database [15]. The numbers of papers with combinations of the keywords ‘porous ceramics’ and ‘biomimetic’, ‘bioinspired’, ‘biomineralization’, ‘natural technology’, ‘nature technology’ and ‘wood template’ are plotted in figure 2 for the past 20 years. A steep increase in numbers occurs from 2000 to 2005, with these high numbers of papers continuing to the present. Thus, there is considerable interest in nature-inspired technology to develop functional porous ceramics. This review focuses on the preparation and properties of unidirectional porous ceramics with wood-like porous microstructures mimicking ‘nature’.
Figure 2.
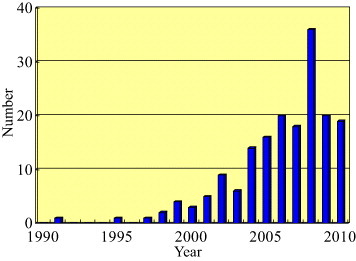
The number of papers related to nature mimetic methods for the preparation of porous ceramics included to the Web of Science database over the past two decades.
Processes for preparation of unidirectional porous ceramics
In this section we discuss methods for preparing unidirectional porous ceramics by anodic oxidation, templating, unidirectional solidification, extrusion and other methods. We divide unidirectional solidification into two groups: methods based on freeze casting and unidirectional solidification of molten melts. We also classify extrusion into multi-pass extrusion methods and extrusion using fibers. Other methods to be discussed include bubbling, the use of magnetic fields and filament winding methods. The characteristics of these different preparation methods are listed in table 1.
Anodic oxidation
Metal surfaces are more or less oxidized in ambient atmosphere, but some metals such as aluminum can be covered by a passive oxide layer by electrochemical treatment, producing an anodic coating called ‘almite’ or ‘alumite’. This process was invented at RIKEN (Japan) in 1924. Although the formation of surface-oxidized layers has been known for some time, their unique porous microstructures have been seen only relatively recently and are attracting great interest because of the well-ordered hexagonal pore arrays formed anodically by alumina, titania, silica, etc [16–18].
Anodic oxidation is generally performed in a solution of concentrated acid such as oxalic, sulfuric or phosphoric acid, by applying dc voltages of typically 10–200 V. The resulting microstructures consist of cylindrical nanopores in a hexagonal array running perpendicular to the metal substrate with open tops and closed bottoms. The sizes and wall thicknesses of the pores and the ordering of the hexagonal pore arrays depend on the experimental conditions such as the type and concentration of the acid, the chemical additives in the solution, the applied voltage and current, the mode of applying the voltage and current and the reaction temperature and duration. The size of the arrays was reported to increase with applied voltage [19, 20], e.g., as the applied voltage was increased from 25 to 160 V the interpore distance (pore diameter + wall thickness) increased from 60 to 420 nm [19]. Self-organized hexagonal pore arrays are formed under certain conditions; Masuda and Fukuda [21] reported that a longer anodization time (≽ 160 h) and low temperature (0 °C) is very effective in enhancing the degree of ordering of the array. Li et al [19] found that the highest ordering was obtained when the volume expansion, occurring when aluminum metal oxidizes to form small γ-alumina grains, is about 1.4. They therefore considered that the formation mechanism can be explained by a mechanical stress model in which the repulsive forces between neighboring pores at the oxide/metal interface promote the arrangement by the anodic oxidation.
The anodic oxidation process has been most intensively investigated for aluminum, but the numbers of studies on silicon and particularly titanium have increased steeply in the past few years. Most studies of silicon have been focused on the photoluminescence properties generated by the nanostructures [22]. By contrast, the major motivation for the work on titanium is the formation of titania nanotubes with hexagonal arrays showing potentially interesting photocatalytic properties [20]. Much of the work on aluminum is directed towards the development functionality exploiting these unique nanoporous microstructures. For example, Masuda and Fukuda [21] reported the fabrication of highly ordered precious metal nanohole arrays by two-step replication of the well-ordered hexagonal pore array of anodic alumina. The resulting microstructure, consisting of ≈70 nm diameter pores with 1–3 nm wall thickness, showed a notable color change compared with the bulk metal. Itoh et al [23] fabricated an interesting hierarchical porous composite material consisting of 200 nm diameter anodic mesoporous alumina infiltrated by 8 nm diameter mesoporous silica containing encapsulated enzymes. The parallel alignment of the two different sized pore structures in the composites was confirmed by transmission electron microscopy and permeativity measurements. The enzymes encapsulated in the mesopores were found not to lose their activity, by contrast with enzymes exposed to the ambient atmosphere, which progressively lose their activity after extraction from living bodies. This important result is thought to be due to the limited space of the pores in the mesoporous silica. One interesting application is as a high-selectivity sensor for organophosphorous pesticides [24].
Thus, anodic oxidation is useful for preparing highly unidirectional nano-sized hexagonal pore arrays. Scanning electron microscopy (SEM) images of the surfaces of these porous anodic aluminas, titanias and silicas show a honeycomb bees-nest structure that is also similar to a natural landscape of sinkhole arrays (dolines) in karst [25].
Templating
Where the target is to produce porous microstructures mimicking wood-like unidirectionally aligned cylindrical pores, a direct approach is to use wood as a template.
Carbonization is a simple method for obtaining porous materials from wood [26], but this is a direct use of the natural material rather than mimicking its structure. Activated carbon is also prepared by chemical activation of wood at relatively low heating temperatures to preserve the original microtexture [27]. The resulting specific surface areas are about 1800–2000 m2 g−1 [28]. A similar technique can be used with other carbonaceous precursors to prepare carbons and activated carbons that preserve the original microstructures.
The approaches for replicating wood microstructures by porous ceramics have been summarized by Greil [29] as (1) pyrolytic decomposition resulting in a porous carbon replica (template) which may either be subsequently reacted to form carbide phases or infiltrated with non-reacting sols or salts that are then further processed to yield oxide reaction products, and (2) infiltration of chemically preprocessed native or technical lignocellulosic products with gaseous or liquid organometallic and metalorganic precursors, followed by oxidation to remove the free carbon. Many papers have reported the use of these replicating techniques to prepare a variety of biomorphic porous ceramics. These process routes are shown schematically in figure 3 and the related data collated in table 2.
Figure 3.
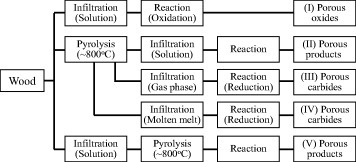
The five different routes for preparing unidirectional porous ceramics by the wood templating method.
Table 2 shows that many varieties of wood have been used as starting materials for the preparation of porous ceramics. The morphology and arrangement of the pores in these different woods varies widely, especially between hardwoods and softwoods. Large pores (vessel cells) present in hardwoods are characteristic of broad-leaved angiosperm trees, whereas small but numerous tracheids and xylems, which are tubes conducting water upwards in softwoods, are present in coniferous trees. These differences in the porous microstructures of the wood are a major factor affecting the porous properties of the resulting products.
The three processes generally used in the wood templating method are infiltration, pyrolysis and reaction (oxidation, reduction, etc figure 3). Route I has been widely used to prepare porous oxides by infiltrating ceramic precursors using salts and alkoxides at near-ambient temperatures (20–80 °C), followed by reaction (oxidation) at high temperatures to form oxides. Unidirectional porous oxides with high porosity prepared in this way include Al2O3 [30, 31], Fe2O3 [32], TiO2 [31], ZrO2 [31], Cr2O3 [33], NiO [34], ZnO [35, 36], Mn2O3 [37], Mn3O4 [37] and Ni−Zn ferrites [38]. The pores in these products had a bimodal or multimodal size distribution consisting of larger pores originating from the wood microstructure, and smaller pores generated by agglomeration of the introduced oxide particles. Routes II, III and IV are similar to each other and are used mainly to prepare non-oxide ceramics and composites. In these cases, the wood was first pyrolyzed at about 800 °C and then infiltrated with ceramic precursors. In the case of route II, the infiltration was performed using a solution at near-ambient temperatures, followed by drying and annealing at high temperatures to obtain unidirectional porous ceramics. Porous hydroxyapatite [39] as well as SiO2/SiC/C [40], SiC/C [41], TiC/C [41] and ZrC/C composites [41] have been prepared in this way. In route III, infiltration is performed using hot gaseous ceramic precursors, whereas route IV involves infiltration with the molten compound. A further reaction step is required to form pores after infiltration by route IV. Porous SiC [42] has been prepared by both routes III [42] and IV [43], while the latter route has also been used to prepare Si3N4/SiC [44] and Si/SiC/zeolite composites [45]. Route V, which involves infiltration of a pre-ceramic polymer in solution, pyrolysis at 800 °C, then reaction at high temperatures, has been used to prepare porous YSZ [46] and TiN/C composites [47].
Thus, various porous ceramics with biomorphic microstructures have been prepared by replicating wood textures, but have the disadvantages of generally poor mechanical strength or low porosity, and the need for multi-step processing that increases the difficulty of scaling up. These disadvantages limit the applications of biomorphic porous products prepared by wood templating.
Unidirectional solidification
Weathered volcanic ash soil such as Kanto loam is relatively porous and has a considerable water content. These soils grow frost pillars on their surfaces on cold mornings, and these pillars are ascribed to the unidirectional thermal flux arising from the temperature difference between the soil and the night atmosphere [48]; this is an example of natural unidirectional solidification. There are two ways to fabricate the target products by this method, namely, unidirectional freeze casting of a slurry at low temperatures and solidification of a molten melt at high temperatures.
Unidirectional freezing
The phenomenon underlying the formation of the above-mentioned frost pillars has been used to prepare porous ceramics by freeze drying the solvent from a ceramic slurry [49]. The aqueous slurry was molded in a cylindrical plastic vessel and set on a cold metal substrate [49]. The temperature gradient between the cold bottom of the mold and the top brings about the formation of ice pillars perpendicular to the substrate. A unidirectional temperature difference was also successfully achieved by slowly dipping the slurry-containing vessel into a cold solvent [50]. Uniform unidirectionally oriented porous microstructures requite a well-dispersed and stable slurry. The important factors to be considered are pre-treatment to disaggregate the ceramic particles, suitable solids concentration and good dispersion of the ceramic particles in the slurry by the use of appropriate organic dispersants. On the other hand, a pore size gradient may intentionally be introduced by accelerating the evaporation of the solvent from the open upper surface of the slurry using a highly volatile organic solvent such as an alcohol [51]. Tert-butyl alcohol (TBA) generates unique unidirectional pores with hexagonal cross sections depending on the structure of the hexamers in the frozen TBA [51]. The porosity of the products can be enhanced by the use of organic pore formers such as polyvinyl alcohol (PVA) [52], polymethyl methacrylate (PMMA) [53], gelatin [54] or polyurethane sponge [55]. In these cases, the ceramic matrix tends to be porous and form a three-dimensional pore network as well as a unidirectionally connected pore structure.
There are reports of the preparation of porous ceramics by a combination of unidirectional freeze-casting and other processes to develop unique porous microstructures. Zhang et al [56] fabricated a dense/porous bilayer microstructure with unidirectional pores by alternatively switching an external electric field on and off. Freezing the slurry under an applied electric field is similar to electrophoretic deposition (EPD), a process used to promote denser packing of the deposit [57]. In this way, products consisting of dense/porous bilayers can be produced by combining the unidirectional freeze-casting and EPD processes. Another example is the combination of unidirectional freeze-casting with tape-casting. In this method, the slurry is first formed into a tape on a casting bed by a doctor blade, then and successively moved to a freezing bed to form acicular pores unidirectionally aligned perpendicular to the moving film [58]. This interesting process is different from other unidirectional solidification methods because the resulting acicular pores are unidirectionally aligned perpendicular rather than parallel to the lengthwise direction of the products.
Unidirectional solidification of molten melts
Well-ordered microstructures consisting of a unidirectionally separated cylindrical phase in a surrounding matrix can be obtained by unidirectional solidification of eutectic melts in the ZrO2/MgO and MgAl2O4/Al2O3 systems [59]. The possibility of obtaining a wood-like porous microstructure from such a unidirectionally separated microstructure by selectively leaching one phase was suggested by Suzuki and Morgan [59] but the realization of such a process has not yet been reported.
On the other hand, Nakajima [60] produced porous metals with unidirectional porous structures by unidirectional solidification of molten melts; these materials were described as lotus-type because of the similarity of their pores with those of lotus roots. In this method, the metals were melted under a pressurized H2 atmosphere to dissolve the H2 into the melt which was then unidirectionally cooled and solidified. The dissolved H2 formed bubbles during solidification due to the lower solubility of the gas in the solid phase than in the melt, thus forming unidirectional cylindrical pores in the solidified products. A floating zone method based on this technique was used to prepare unidirectional porous Al2O3 [61], mullite [62] and MgAl2O4 [63]. The resulting pore sizes ranged from 100 μm to millimeters but it was difficult to prepare the smaller pores. The porosities were limited to ⩽40% by the limited solubility of H2 in the melts. The compressive strength of the porous Al2O3 (512 MPa at a porosity of 25.9%) was greater than reported for porous ceramics prepared by unidirectional freeze-casting.
Thus, unidirectional solidification is an interesting method for fabricating a variety of ceramics with unidirectionally aligned through-hole pores which do not contact the neighboring pores. The products should therefore show anisotropic properties in the axial and radial directions. The disadvantages of this method are the need for multi-step processing and the difficulty in controlling the porous microstructure and fabricating products up to several centimeters in size.
Extrusion
Extrusion is a widely used process in the ceramics industry that allows a relatively easy fabrication of large-sized products. Two different approaches have been reported to fabricate wood-like unidirectional porous ceramics. One is a multi-pass extrusion method proposed by Li et al [64–68]. The other is an extrusion method using flammable fibers as the pore formers, reported by Isobe et al [69–71].
Multi-pass extrusion
The multi-pass process proposed by Li et al [64] to prepare unidirectional porous ceramics is as follows: two mixtures are made, one containing carbon and ethylene vinyl acetate (EVA) and the other containing alumina and EVA. These are used with a rod and tube mold to fabricate a core/shell rod structure. The EVA mixed in both the core rod and shell tube acts as the binder and pore former. The core/shell rod is loaded into an extrusion die warmed to 120 °C and extruded to form a filament. These filaments are then stacked and re-loaded into the extrusion die at an extrusion ratio of 60–70:1. The second extrusion produces bundles of elongated and thinned core/shell filaments with microstructures that can contain greater numbers of thinner filaments if more extrusion passes are used. Firing at high temperatures produces unidirectionally aligned cylindrical pores in the product. The porous properties and mechanical properties of ceramics prepared by the multi-pass extrusion method are shown in table 3. The porosities are typically 20–40% but can be increased up to about 70%, depending on the core/shell ratio of the filaments. Pore sizes can be produced in the range of 35–470 μm, depending on the number of extrusion passes and the extrusion ratio. The products show good mechanical strengths because of their well-controlled unidirectional porous microstructures and densification of the matrix during the repeated extrusion passes.
Although extrusion processes are popular in the ceramics industry for forming large-scale products, the multi-pass extrusion method for fabricating well-controlled unidirectional porous ceramics suffers from the disadvantage of needing repeated extrusions, making the process unduly lengthy.
Extrusion using fibers as the pore former (EF method)
If the particles incorporated in an extrusion paste have a high aspect ratio, they tend to orient in such as way as to reduce the flow resistance. Such anisotropy in the green product is generally unfavorable because it causes inhomogeneous shrinkage in the drying and firing stages, but it can be used intentionally to fabricate unidirectionally porous ceramics using flammable fibers as the pore formers [69–71].
Figure 4 shows the microstructures of the cross sections perpendicular and parallel to the extruded directions of porous alumina ceramics prepared using carbon fibers and nylon fibers as the pore formers. The microstructures of both products contain cylindrical pores unidirectionally aligned parallel to the extruded direction, as expected. The degree of ordering was higher in the products prepared using carbon fibers (figures 4(a) and (b)) than in those using nylon fibers (figures 4(c) and (d)). This difference is associated with mechanical properties of the two fibers, the carbon fibers being hard but brittle and the nylon fibers being soft but tough. Carbon fibers have the disadvantage of breaking during kneading to incorporate them in the ceramic paste, and they are also too expensive for many large-scale products. This type of porous ceramic can be fabricated using any other flammable fiber, but cellulose-based compositions such as rayon are preferable because of the lower environmental impact of their thermal decomposition.
Figure 4.
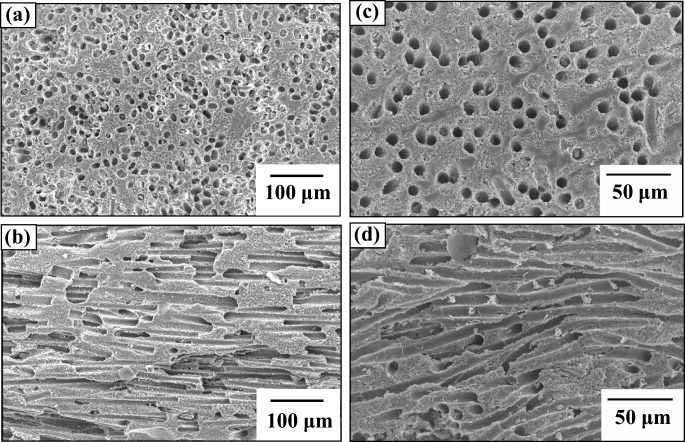
SEM micrographs of unidirectional porous alumina ceramics prepared by the extrusion method using carbon fibers(a) perpendicular and (b) parallel to the extrusion direction, and nylon fibers (c) perpendicular and (d) parallel to the extrusion direction.
The porous ceramics discussed here were fabricated using fibers that were not continuous, but chopped to lengths of several hundred μm. These chopped fibers made contact with each other during extrusion due to their high aspect ratio, forming characteristic through-hole pores. These pores are therefore not completely separated from each other but rather form three-dimensional networks by incomplete unidirectional orientation of continuous cylindrical pores. This is a unique structure compared with the other unidirectional porous materials discussed in this review and it results in unique properties of these ceramics.
Other methods
Bubbling method
Porous ceramics prepared using bubbles as the pore formers were introduced in section 2.3.2 [60–63]. Nakahira et al prepared unidirectional porous Al2O3 [73] and ZrO2 [74] ceramics using an aqueous EPD process in which Al2O3 particles suspended in water at pH 2.5–4 were deposited on a carbon cathode by applying a dc voltage of 5–500 V [73]. Electrolysis of the water produces H2 bubbles at the cathode which form unidirectional pores perpendicular to the deposit. The green product was freeze-dried and fired at 1100 °C, producing a ceramic with pore sizes ranging from 60 to 170 μm that could be controlled by adjusting the electrolysis current and voltage and the pH of the solution. The resulting porosity was typically <50% [74].
Song et al [75] used ethanol bubbles to fabricate unidirectional porous hydroxyapatite (HAp) by a simple process in which a suspension of HAp particles with a binder in an ethanol–water solution was warmed at 70–80 °C for 2 h to evaporate the ethanol and water, producing bubbles in the suspension. After complete evaporation of the solution, the green deposit was dried and fired at 1200 °C to obtain porous HAp ceramic. The unidirectionally aligned pores were 120–130 μm in diameter in a matrix containing small pores of 1–30 μm and <1 μm in size, i.e. the system contained a trimodal pore size distribution. The total porosity was about 70% and the compressive strength was about 10 MPa. Although pore control could not readily be achieved, this may be possible by simply changing the solvents and their ratio in the evaporative bubbling solution.
Use of magnetic fields
Many recent studies attempted to control the microstructure in ceramics using magnetic fields [76]. Miyagawa and Shinohara [77] prepared unidirectional porous Al2O3 ceramics from an Al2O3 slurry containing Ni wires cast in a plaster mold. The Ni wires were settled and unidirectionally aligned in the cast product by applying an external magnetic field and were converted to continuous pores by acid-leaching. An interesting possibility of this technique is its ability to control the pore separation by coating the Ni wires with Al2O3 prior to their alignment in the magnetic field, thereby changing the magnetic repulsion between the neighboring fibers.
Filament winding
The filament winding method was originally developed to fabricate continuous fiber-reinforced dense composites [78]. If the reinforcing fibers are flammable, they can be converted to pores by firing in air. Zhang et al [79] have reported the use of this method to fabricate porous alumina ceramics with unidirectionally aligned 165 μm continuous pores in a material with 35% porosity and a bending strength of 155 MPa. These properties compare well with those of conventional porous ceramics [70, 71].
Properties of unidirectional porous ceramics
Mechanical properties
The pores in conventional porous ceramics are randomly but homogeneously distributed, but in the materials discussed in this review, the continuous through-hole pores are unidirectionally aligned. Thus, the pore distribution and their arrangement in conventional porous ceramics are isotropic whereas the pores in unidirectional porous ceramics are anisotropic. A consequence of this may be that the properties of unidirectional porous ceramics are anisotropic. Chen et al [80] reported that the compressive strengths in the axial and radial directions of unidirectional porous alumina fabricated by the freeze casting method were 153±30 and 54±14 MPa, respectively, confirming the presence of a high degree of anisotropy in the strength of this unidirectional porous microstructure.
Figure 5 compares the bending strength as a function of porosity of unidirectional porous alumina prepared by the extrusion method using fibers (EF) [71] with porous alumina prepared by conventional sintering [71, 81], hot pressing (HP) [82], hot isostatic pressing (HIP) [81] and pulsed electric current sintering (PECS) [82]. The relationships between bending strength (σ) and porosity (P) of all these data can be described by the Knudsen equation [83]
where σ0 is the fracture strength at P=0 and b is an empirical constant obtained from the slope of the semi-log plot. Although the σ0 values are higher in samples sintered by HIP (590 MPa) and PECS (490 MPa), their decreasing slope ratios are high, especially compared with the EF sample of unidirectional porous alumina, as seen by comparison of the b parameters; this value for the EF sample (1.9) is clearly smaller than those of the other samples (3.9–5.9). These differences in the b values have a greater effect on the bending strength in the high porosity region and thus reflect the anisotropic behavior of the unidirectional porous alumina.
Figure 5.
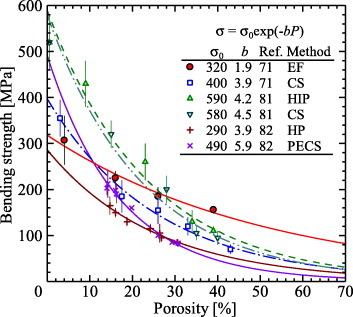
Bending strengths of porous alumina ceramics prepared by the EF method and conventional methods as a function of porosity.
Permeability
Permeability is a very important property in unidirectional porous ceramics, one of the target applications of which is for filters that require high permeability in addition to mechanical strength. Maximum permeability is obtained in an ideal model (the capillary permeability model) in which uniform capillary tubes are aligned parallel to the gas flow direction. The resulting permeability (μ) is given by
where d and P are the pore size and porosity, respectively. The resulting values of d and P allow calculating the μ value, which can then be compared with the experimental permeability. The observed and calculated permeability ratios μU/μC (%) of unidirectional porous ceramics [54, 70, 83] and conventional porous ceramics [70, 84, 85] are shown in the porosity-pore size plot of figure 6. These porosity and pore size data show a wide distribution range. Compared with the very low μU/μC ratios of the conventional porous ceramics, the μU/μC ratios of the unidirectional porous ceramics are much higher, especially at higher porosities. These high permeabilities are close to the maximum values, probably due to their well-controlled unidirectional porous structures.
Figure 6.

Porosity, pore size and observed and calculated permeability ratio (%) for unidirectional porous ceramics prepared by freeze casting ( [83],
[83],  : [54], EF,
: [54], EF,  : [70]), and conventional porous ceramics (▵: [84], ▿: [70],
: [70]), and conventional porous ceramics (▵: [84], ▿: [70],  : [85]). The solid lines represent the permeability calculated using equation (2).
: [85]). The solid lines represent the permeability calculated using equation (2).
Figure 5 shows that the mechanical strengths are greater for the unidirectional porous ceramics than conventional porous ceramics. The permeability is also higher in the unidirectional than in conventional porous ceramics. Comparison of the bending strengths and permeabilities (figure 7) shows that the data for three of the conventional porous ceramics lie on a smooth line, but the data for the EF samples fall in a different region with several times higher bending strength at a permeability of 10−14 m2 and about 104 times higher permeability at bending strength of 150 MPa. These are very promising results for a number of applications.
Figure 7.

Bending strength and permeability of unidirectional porous ceramics prepared by the EF method and conventional porous ceramics (▵: [84], ▿: [70],  : [85]). The numbers (%) represent the porosities of the samples.
: [85]). The numbers (%) represent the porosities of the samples.
The high permeability of unidirectional porous ceramics prepared by the EF method makes them ideal for filters and membranes, and also for a possible application as a ceramic bubble [86]. The bubbles obtained from this ceramic ranged in diameter from 100 to 200 μm, much smaller than the mm-sized bubbles generated by a commercial bubbler. These bubble sizes are also much smaller than ∼1 mm diameter predicted by the Fritz equation, but closer to the size calculated by the nanobubble equation developed for porous glass by Kukizaki and Goto [87]. The high permeability of these ceramics allows a minimum applied pressure for bubble generation to be as low as 0.02 MPa, such as produced by a domestic air pump; this gives these ceramic bubblers a considerable advantage over other bubblers which require much higher power and/or pressure. The effectiveness of these unidirectional porous ceramics as bubblers is confirmed by their 3–4 times faster dissolution rate constant for bubbling gas into the solution, compared with a commercial bubbler [86].
Capillary rise
The excellent ability of green trees to draw water up to their tops is due to three main mechanisms, namely, osmotic pressure in the roots, capillary forces in the body and branches and evaporation from the leaves. Unidirectional porous ceramics should therefore posses similarly good ability to lift water by capillary action that mimics the microstructure of xylems and tracheids in the body and branches of trees. The equilibrium capillary rise height (heq) is given as
where γ is the surface tension of water, θ is the contact angle between water and the pore wall, ρ is the density of water, g is the gravitational acceleration constant and r is the pore radius. The relationship between the capillary rise height and the pore radius for water is written as heq=1.49×10−5 cos θ/r, assuming γ=73 mN m−1, ρ=1000 kg m−3 and g=9.8 m s−2. Thus, the capillary rise height increases with decreasing contact angle and pore size of the porous ceramic. For applications of this material, its capillary rise rate is also important, and can be calculated using the Fries and Dreyer's equation
Here t is the time, h(t) is the capillary rise height at time t, reff is the effective pore radius and η is the viscosity of water. We have fabricated unidirectional porous mullite ceramics by the EF method and investigated their capillary rise characteristics [88]. The capillary rise curve of this ceramic is shown in figure 8, which was calculated by fitting the two parameters θ and reff. The value of heq for this ceramic (1.29 m) was determined experimentally after a prolonged time and was related to θ and reff by equation (3). Thus, the number of required parameters is reduced to only one which can readily be determined by trial and error. The solid line shown in figure 8 was obtained by setting heq=1.29 m, reff=4 μm and θ=69.5°. This value of heq is much greater than the reported value of 40 cm [89] because of the well-controlled unidirectional pore structure of this ceramic.
Figure 8.
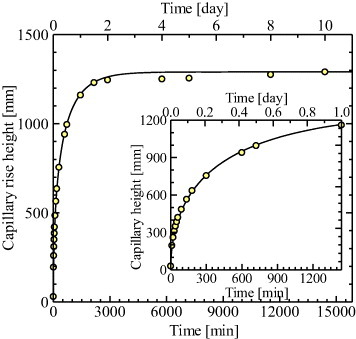
Observed and calculated capillary rise height of unidirectional porous ceramics prepared by the EF method.
The excellent capillary rise ability of this ceramic suggests its application as a means of counteracting urban heat island effects. Heating of the ceramic surfaces by strong sunshine on fine summer days can be offset by utilizing the capillary action of the ceramic to draw up water which then evaporates, cooling the surface from >40 °C in the dry material to about 30 °C when wetted [90]. A passive cooling system using these ceramics has been proposed as an effective method for counteracting urban heat island phenomena [91].
Other properties
Other interesting potential applications of porous ceramics exploit their sound absorption or thermal conductivity properties, the latter being of interest for heat exchangers.
Commercial materials for sound absorption and noise reducing applications are generally based on glass wool, although there have been reports on the sound absorptivity of porous ceramics. Fuji et al [92] and Zhang et al [93] prepared porous ceramics using a gel casting method and demonstrated the effectiveness of their sound absorptivity over a frequency range of several 100 to several 1000 Hz, concluding that the open porosity is an important factor in absorbing sound energy by the viscosity and thermal conduction within the limited pore spaces. Although there have been no reports of sound absorption by unidirectional porous ceramics, Xie et al [94] investigated this property in unidirectionally solidified porous metals with a lotus-like structure containing 43–62% porosity and 660–460 μm pores. In the frequency range 125–4000 Hz a higher coefficient of sound absorptivity was found along the pore channels with higher porosity and smaller pore size. These materials performed better than a commercial glass wool sound absorption material in the frequency range 1000–2000 Hz, suggesting that unidirectional porous ceramics prepared by extrusion methods are good sound absorption candidates because of their unique structure.
Another conventional application of porous materials exploits their thermally insulating properties for use as burners, thermal barrier coatings and insulating layers. Many studies have been made of the relationship between the thermal conductivity (k) and porosity, which can be expressed by the widely used empirical equation [95]
where kr is the relative thermal conductivity, k0 is the thermal conductivity of the dense material and P is the porosity. Figure 9 shows the relative thermal conductivities and porosities reported for various porous ceramics [51, 92, 93, 96]. The agreement with the empirical equation (5) is due to the variation of k0 value with the grain size and discrepancies arising from the variation of pore sizes.
Figure 9.
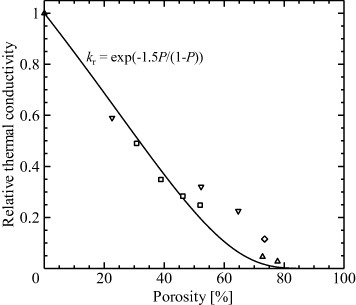
Relationship between relative thermal conductivity and porosity for various porous ceramics (□: [96], ▵: [51], ▿: [92],  : [93]).
: [93]).
Hu et al [51] reported the k values of unidirectional porous YSZ prepared by a freeze casting method in directions both perpendicular and parallel to the pore channels. The k values in the parallel direction ranged from 0.2 to 0.54 W (mK)−1 whereas those in the perpendicular direction ranged from 0.06 to 0.1 W (mK)−1. This distinctly anisotropic thermal conductivity is clearly due to the anisotropic unidirectional porous structure, and suggests that these materials are potentially good thermal insulators.
In addition to their thermal insulating applications, another potential application of these ceramics is as heat exchange materials for rapid thermal equilibration between the porous material and a fluid passing through it; the unidirectionally aligned parallel channels would be advantageous for this purpose. Volumetric solar receivers based on such porous ceramics have been proposed by Fend et al [97]. Noudem et al [98] have proposed the use of thermoelectric Ca3Co4O9 and Ca0.95Sm0.05MnO3 containing open pores for thermoelectric modules, to take the advantage of their reduced thermal conductivity due to the presence of the small pores (figure 9). The particularly low thermal conductivity of anisotropic unidirectional porous structures should provide the further advantage of their unique thermal properties [51]. Thermal management using porous materials has been reviewed by Clyne et al [99].
Summary
This review is concerned with the preparation and properties of materials mimicking nature, particularly unidirectional porous ceramics. Five methods for preparing these porous ceramics were discussed, namely, (1) anodic oxidation, (2) templating using wood, (3) unidirectional solidification, (4) extrusion and (5) other methods, with conclusions as follows:
Anodic oxidation is suitable for fabricating highly ordered hexagonal arrays of unidirectionally oriented mesoporous microstructures which provide good substrates and precursors for the growth of nanomembranes, nanowires, nanodots and other complex composites.
Templating is capable of readily fabricating highly porous ceramics replicating various wood microstructures.
Unidirectional solidification provides a means of fabricating microstructures containing unidirectional pores aligned independently, by either freeze casting or solidification of molten melts to give lotus-like structures. Freeze casting provides a wider range of porosities whereas higher mechanical strengths are achieved by melt solidification.
Multi-pass extrusion and extrusion of green bodies containing flammable fibers as the pore formers (the EF method) have been reported. Multi-pass extrusion produces porous microstructures with the lotus-like structure whereas the EF method produces unique unidirectionally oriented porous microstructures with three-dimensional pore networks capable of raising water to a height of >1 m by capillary action. The excellent permeability of these materials also makes them good generators of microbubbles.
Preparation of porous ceramics using bubbles as the pore formers and alignment of fibrous metallic pore formers in a magnetic field was presented as alternative methods.
This review also discussed the effect of the unique anisotropic properties of the unidirectional porous microstructures on their properties, including mechanical strength, permeability, capillary rise, sound absorption and thermal conduction, and compared these with the properties of conventional isotropic porous ceramics.
References
- Tomita T, Kawasaki S. and Okada K. J. Porous Mater. 2004;11:107. doi: 10.1023/B:JOPO.0000027366.90408.1f. [DOI] [Google Scholar]
- Sing K S W, Everett D H, Moscou L, Pierotti R A, Rouquerol J. and Siemieniewska T. Pure Appl. Chem. 1985;57:603. doi: 10.1351/pac198557040603. [DOI] [Google Scholar]
- Karge H G. and Weitkamp J. Springer Berlin; 1998. (Molecular Sieves vol I–IV) [Google Scholar]
- Gates B, Yin Y. and Xia Y. Chem. Mater. 1999;11:2827. doi: 10.1021/cm990195d. [DOI] [Google Scholar]
- Roberts W L, Campbell T J, Rapp G R., Jr . 2nd Edn. Princeton, NJ Van Nostrand Reinhold, ISBN-13: 978-0442276812; 1990. Encyclopedia of Minerals. [Google Scholar]
- Shimohira T, Mori T. and Tsutsumi M. J. Ceram. Soc. Japan. 1984;92:55. [Google Scholar]
- Blanco A.et al Nature. 2000;405:437. doi: 10.1038/35013024. [DOI] [PubMed] [Google Scholar]
- Stein A, Li F. and Denny N R. Chem. Mater. 2008;20:649. doi: 10.1021/cm702107n. [DOI] [Google Scholar]
- Marsh H. and Rodriguez-Reinoso F. (ISBN-13: 978-0-08-044463-5) Amsterdam Elsevier; 2005. Activated Carbon. [Google Scholar]
- Brindley G W. and Sempels R E. Clay Miner. 1977;12:229. doi: 10.1180/claymin. [DOI] [Google Scholar]
- Yamanaka S, Inoue Y, Hattori M, Okumura F. and Yoshikawa M. Bull. Chem. Soc. Japan. 1992;65:2494. doi: 10.1246/bcsj.65.2494. [DOI] [Google Scholar]
- Kyotani T. Bull. Chem. Soc. Japan. 2006;79:1322. doi: 10.1246/bcsj.79.1322. [DOI] [Google Scholar]
- Kameshima Y, Tamura Y, Nakajima A. and Okada K. Appl. Clay Sci. 2009;45:20. doi: 10.1016/j.clay.2009.03.005. [DOI] [Google Scholar]
- Kameshima Y, Yoshizawa Y, Nakajima A. and Okada K. Appl. Clay Sci. 2009;46:181. doi: 10.1016/j.clay.2009.08.001. [DOI] [Google Scholar]
- Thomson Reuters. Web of Science. http://wokinfo.com/ [Google Scholar]
- Therese G H A. and Kamath P V. Chem. Mater. 2000;12:1195. doi: 10.1021/cm990447a. [DOI] [Google Scholar]
- Ghicov A. and Schmuki P. Chem. Commun. 2009;20:2791. doi: 10.1039/b822726h. [DOI] [PubMed] [Google Scholar]
- Cullis A G, Canham L T. and Calcott P D J. J. Appl. Phys. 1997;82:909. doi: 10.1063/1.366536. [DOI] [Google Scholar]
- Li A P, Mueller F, Bimer A, Nielsch K. and Goesele U. J. Appl. Phys. 1998;84:6023. doi: 10.1063/1.368911. [DOI] [Google Scholar]
- Gong D, Grimes C A, Varghese O K, Hu W C, Singh R S, Chen Z. and Dickey E C. J. Mater. Res. 2001;16:3331. doi: 10.1557/JMR.2001.0457. [DOI] [Google Scholar]
- Masuda H. and Fukuda K. Science. 1995;268:1466. doi: 10.1126/science.268.5216.1466. [DOI] [PubMed] [Google Scholar]
- Brandt M S, Fuchs H D, Stutzmann M, Weber J. and Cardona M. Solid State Commun. 1992;81:307. doi: 10.1016/0038-1098(92)90815-Q. [DOI] [Google Scholar]
- Itoh T.et al J. Mol. Catal. 2009;57(B):183. doi: 10.1016/j.molcatb.2008.08.014. [DOI] [Google Scholar]
- Shinomura T, Itoh T, Sumiya T, Mizukami F. and Ono M. Enzyme Microb. Technol. 2009;45:443. doi: 10.1016/j.enzmictec.2009.08.007. [DOI] [Google Scholar]
- Khorsandi A. and Miyata T. Acta Carsologica. 2007;36:203. [Google Scholar]
- Gao P Z, Hu P F, Wang W X. and Gong W W. J. Ceram. Proc. Res. 2010;11:297. [Google Scholar]
- Barton T J. et al Chem. Mater. 1999;11:2633. doi: 10.1021/cm9805929. [DOI] [Google Scholar]
- Jagtoyen M. and Derbyshire F. Carbon. 1993;31:1185. doi: 10.1016/0008-6223(93)90071-H. [DOI] [Google Scholar]
- Greil P. J. Eur. Ceram. Soc. 2001;21:105. doi: 10.1016/S0955-2219(00)00179-5. [DOI] [Google Scholar]
- Cao J, Rambo C R. and Sieber H. J. Porous Mater. 2004;11:163. doi: 10.1023/B:JOPO.0000038012.58705.c9. [DOI] [Google Scholar]
- Mizutani M, Takase H, Adachi N, Ota T, Daimon K. and Hikichi Y. Sci. Technol. Adv. Mater. 2005;6:76. doi: 10.1016/j.stam.2004.08.004. [DOI] [Google Scholar]
- Liu Z, Fan T, Gu J, Zhang D, Gong X, Gu Q. and Xu J. Mater. Trans. 2007;48:878. doi: 10.2320/matertrans.48.878. [DOI] [Google Scholar]
- Fan T X, Li X F, Liu Z T, Gu J J, Zhang D. and Guo Q X. J. Am. Ceram. Soc. 2006;89:3511. doi: 10.1111/jace.2006.89.issue-11. [DOI] [Google Scholar]
- Liu Z T, Fan T X. and Zhang D. J. Am. Ceram. Soc. 2006;89:662. doi: 10.1111/jace.2006.89.issue-2. [DOI] [Google Scholar]
- Liu Z T, Fan T X, Ding J, Zhang D, Guo Q X. and Ogawa H. Ceram. Int. 2008;34:69. doi: 10.1016/j.ceramint.2006.08.006. [DOI] [Google Scholar]
- Liu Z T, Fan T X, Zhang D, Gong X L. and Xu J Q. Sensors Actuators. 2009;136(B):499. doi: 10.1016/j.snb.2008.10.043. [DOI] [Google Scholar]
- Li X F, Fan T X, Liu Z T, Ding J, Guo Q X. and Zhang D. J. Eur. Ceram. Soc. 2006;26:3657. doi: 10.1016/j.jeurceramsoc.2005.10.015. [DOI] [Google Scholar]
- Sia C K, Sasaki Y, Adachi N. and Ota T. J. Ceram. Soc. Japan. 2009;117:958. doi: 10.2109/jcersj2.117.958. [DOI] [Google Scholar]
- Tampieri A, Spiro S, Ruffini A, Celotti G, Lesci I G. and Roveri N. J. Mater. Chem. 2009;19:4973. doi: 10.1039/b900333a. [DOI] [Google Scholar]
- Ghosh S S, Mandal P K. and Majumdar R. Ceram. Int. 2010;36:2063. doi: 10.1016/j.ceramint.2010.04.003. [DOI] [Google Scholar]
- Rambo C R, Cao J, Rusina O. and Sieber H. Carbon. 2005;43:1174. doi: 10.1016/j.carbon.2004.12.009. [DOI] [Google Scholar]
- Streitwieser D A, Popovska N. and Gerhard H. J. Eur. Ceram. Soc. 2006;26:2381. doi: 10.1016/j.jeurceramsoc.2005.03.259. [DOI] [Google Scholar]
- Greil P, Lifka T. and Kaindl A. J. Eur. Ceram. Soc. 1998;18:1961. doi: 10.1016/S0955-2219(98)00156-3. [DOI] [Google Scholar]
- Luo M, Hou G Y, Yang J F, Fang J Z, Gao J Q, Zhao L. and Li X. Mater. Sci. Eng. 2009;29(C):1422. doi: 10.1016/j.msec.2008.11.012. [DOI] [Google Scholar]
- Zampieri A, Kullmann S, Selvam T, Bauer J, Schwieger W, Sieber H, Fey T. and Greil P. Microp. Mesop. Mater. 2006;90:162. doi: 10.1016/j.micromeso.2005.10.049. [DOI] [Google Scholar]
- Rambo C R, Cao J. and Sieber H. Mater. Chem. Phys. 2004;87:345. doi: 10.1016/j.matchemphys.2004.05.031. [DOI] [Google Scholar]
- Luo M, Gao J Q, Zhang X, Hou G Y, Yang J F, Ouyang D, Wang H J. and Jin Z H. J. Mater. Sci. 2007;42:3761. doi: 10.1007/s10853-006-0425-9. [DOI] [Google Scholar]
- Kim M, Yabashi S. and Ameriya Y. J. Japan Inst. Landsc. Arch. 1993;56:133. [Google Scholar]
- Fu Q, Rahaman M N, Dogan F. and Bal B S. J. Biomed. Mater. Res. 2008;86(B):125. doi: 10.1002/jbm.b.30997. [DOI] [PubMed] [Google Scholar]
- Mukai S R, Nishihara H. and Tamon H. Microp. Mesop. Mater. 2003;63:43. doi: 10.1016/S1387-1811(03)00430-X. [DOI] [Google Scholar]
- Hu L, Wang C A, Huang Y, Sun C, Lu S. and Hu Z. J. Eur. Ceram. Soc. 2010;30:3389. doi: 10.1016/j.jeurceramsoc.2010.07.032. [DOI] [Google Scholar]
- Zuo K H, Zeng Y P. and Jiang D L. Mater. Des. 2010;31:3090. doi: 10.1016/j.matdes.2009.12.044. [DOI] [Google Scholar]
- Zuo K H, Zhang Y, Zeng Y P. and Jiang D. Ceram. Int. 2011;37:407. doi: 10.1016/j.ceramint.2010.08.015. [DOI] [Google Scholar]
- Fukushima M, Nakata M, Zhou Y, Ohji T. and Yoshizawa Y. J. Eur. Ceram. Soc. 2010;30:2889. doi: 10.1016/j.jeurceramsoc.2010.03.018. [DOI] [Google Scholar]
- Han J, Hu L, Zhang Y. and Zhou Y. J. Am. Ceram. Soc. 2009;92:2165. doi: 10.1111/jace.2009.92.issue-9. [DOI] [Google Scholar]
- Zhang Y, Hu L. and Han J. J. Am. Ceram. Soc. 2009;92:1874. doi: 10.1111/jace.2009.92.issue-8. [DOI] [Google Scholar]
- Corni I, Ryan M P. and Boccaccini A R. J. Eur. Ceram. Soc. 2008;28:1353. [Google Scholar]
- Sofie S W. J. Am. Ceram. Soc. 2007;90:2024. doi: 10.1111/jace.2007.90.issue-7. [DOI] [Google Scholar]
- Suzuki Y. and Morgan P E D. MRS Bull. 2009;34:587. doi: 10.1557/mrs2009.158. [DOI] [Google Scholar]
- Nakajima H. New Front. Proc. Eng. Adv. Mater. 2005;502:367. [Google Scholar]
- Ueno S, Lin L M, Nakajima H. and Yasuda E. J. Ceram. Soc. Japan. 2008;116:137. doi: 10.2109/jcersj2.116.137. [DOI] [Google Scholar]
- Yang T Y, Ji H B, Yoon S Y, Kim B K. and Park H C. Res. Cons. Recyles. 2010;54:816. doi: 10.1016/j.resconrec.2009.12.012. [DOI] [Google Scholar]
- Ueno S, Akatsu T. and Nakajima H. Ceram. Int. 2009;35:2469. doi: 10.1016/j.ceramint.2009.02.021. [DOI] [Google Scholar]
- Lee B T, Kang I C, Cho S H. and Song H Y. J. Am. Ceram. Soc. 2005;88:2262. doi: 10.1111/j.1551-2916.2005.00364.x. [DOI] [Google Scholar]
- Gain A K. and Lee B T. Mater. Sci. Eng. 2006;419(A):269. doi: 10.1016/j.msea.2005.12.033. [DOI] [Google Scholar]
- Gain A K, Han J K, Jang H D. and Lee B T. J. Eur. Ceram. Soc. 2006;26:2467. doi: 10.1016/j.jeurceramsoc.2005.06.038. [DOI] [Google Scholar]
- Lee B T, Paul R K, Lee C W. and Kim H D. Mater. Lett. 2007;61:2182. doi: 10.1016/j.matlet.2006.08.043. [DOI] [Google Scholar]
- Lee B T. and Sarkar S K. Scr. Mater. 2009;61:686. doi: 10.1016/j.scriptamat.2009.05.047. [DOI] [Google Scholar]
- Isobe T, Tomita T, Kameshima Y, Nakajima A. and Okada K. J. Eur. Ceram. Soc. 2006;26:957. doi: 10.1016/j.jeurceramsoc.2004.11.015. [DOI] [Google Scholar]
- Isobe T, Kameshima Y, Nakajima A, Okada K. and Hotta Y. J. Eur. Ceram. Soc. 2006;26:2213. doi: 10.1016/j.jeurceramsoc.2005.04.014. [DOI] [Google Scholar]
- Isobe T, Kameshima Y, Nakajima A, Okada K. and Hotta Y. J. Eur. Ceram. Soc. 2007;27:53. doi: 10.1016/j.jeurceramsoc.2006.02.030. [DOI] [Google Scholar]
- Bae C J, Kim H W, Koh Y H. and Kim H E. J. Mater. Sci.: Mater. Med. 2006;17:517. doi: 10.1007/s10856-006-8934-2. [DOI] [PubMed] [Google Scholar]
- Nakahira A, Nishimura F, Kato S, Iwata M. and Takeda S. J. Am. Ceram. Soc. 2003;86:1230. doi: 10.1111/jace.2003.86.issue-7. [DOI] [Google Scholar]
- Nakahira A, Ishihara S. and Nakamura S. J. Ceram. Soc. Japan. 2007;115:383. doi: 10.2109/jcersj.115.383. [DOI] [Google Scholar]
- Song H Y, Islam S. and Lee B T. J. Am. Ceram. Soc. 2008;91:3125. doi: 10.1111/jace.2008.91.issue-9. [DOI] [Google Scholar]
- Suzuki T S, Uchikoshi T. and Sakka Y. Sci. Technol. Adv. Mater. 2006;7:356. doi: 10.1016/j.stam.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa N. and Shinohara N. J. Ceram. Soc. Japan. 1999;107:673. [Google Scholar]
- Coblenz W S. Fibrous monolithic ceramic and method for production US Patent No. 4 772 524 1988 [Google Scholar]
- Zhang G J, Yang J F. and Ohji T. J. Am. Ceram. Soc. 2001;84:1395. doi: 10.1111/j.1151-2916.2001.tb00849.x. [DOI] [Google Scholar]
- Chen R, Wang C A, Huang Y. and Zhou Y. J. Am. Ceram. Soc. 2007;90:3478. doi: 10.1111/jace.2007.90.issue-11. [DOI] [Google Scholar]
- Kinemuchi Y, Fusamune N, Takata A. and Ishizaki K. J. Ceram. Soc. Japan. 1998;106:435. [Google Scholar]
- Oh S T, Tajima K, Ando M. and Ohji T. J. Am. Ceram. Soc. 2000;83:1314. doi: 10.1111/j.1151-2916.2000.tb01380.x. [DOI] [Google Scholar]
- Pekor C, Groth B. and Nettleship I. J. Am. Ceram. Soc. 2010;93:115. doi: 10.1111/jace.2010.93.issue-1. [DOI] [Google Scholar]
- Latella B L, Henkeel L. and Mehrtens E G. J. Mater. Sci. 2006;41:423. doi: 10.1007/s10853-005-2654-8. [DOI] [Google Scholar]
- Tomita T, Kawasaki S. and Okada K. J. Porous Mater. 2005;12:123. doi: 10.1007/s10934-005-6769-8. [DOI] [Google Scholar]
- Okada K, Shimizu M, Isobe T, Kameshima Y, Sakai M, Nakajima A. and Kurata T. J. Eur. Ceram. Soc. 2010;30:1245. doi: 10.1016/j.jeurceramsoc.2009.11.003. [DOI] [Google Scholar]
- Kukizaki M. and Goto M. J. Membr. Sci. 2006;281:386. doi: 10.1016/j.memsci.2006.04.007. [DOI] [Google Scholar]
- Okada K, Uchiyama S, Isobe T, Kameshima Y, Nakajima A. and Kurata T. J. Eur. Ceram. Soc. 2009;29:2491. doi: 10.1016/j.jeurceramsoc.2009.03.012. [DOI] [Google Scholar]
- Kubota T, Sugimoto H. and Komiya H. Rep. Obayashi Tech. Inst. 2003;67:1. [Google Scholar]
- Okada K, Kameshima Y, Nakajima A. and Madhusoodana C D. J. Heat Island Inst. Int. 2007;2:1. [Google Scholar]
- He J. and Hoyano A. Build Environ. 2011;46:98. doi: 10.1016/j.buildenv.2010.07.004. [DOI] [Google Scholar]
- Fuji M, Kato T, Zhang F Z. and Takahashi M. Ceram. Int. 2006;32:797. doi: 10.1016/j.ceramint.2005.06.003. [DOI] [Google Scholar]
- Zhang F Z, Kato T, Fuji M. and Takahashi M. J. Eur. Ceram. Soc. 2006;26:667. doi: 10.1016/j.jeurceramsoc.2005.07.021. [DOI] [Google Scholar]
- Xie Z, Ikeda T, Okuda Y. and Nakajima H. Mater. Sci. Eng. 2004;386(A):390. [Google Scholar]
- Zivcova Z, Gregorova E, Pabst W, Smith D S, Michot A. and Poulier C. J. Eur. Ceram. Soc. 2009;29:347. doi: 10.1016/j.jeurceramsoc.2008.06.018. [DOI] [Google Scholar]
- Sutcu M. and Akkurt S. Ceram. Int. 2009;35:2625. doi: 10.1016/j.ceramint.2009.02.027. [DOI] [Google Scholar]
- Fend T, Hoffschmidt B, Pitz-Paal R, Reutter O. and Rietbrock P. Energy. 2004;29:823. doi: 10.1016/S0360-5442(03)00188-9. [DOI] [Google Scholar]
- Noudem J G, Lemonnier S, Prevel M, Reddy E S, Guilmeau E. and Goupil C. J. Eur. Ceram. Soc. 2008;28:41. doi: 10.1016/j.jeurceramsoc.2007.05.012. [DOI] [Google Scholar]
- Clyne T W, Golosnoy I O, Tan J C. and Markaki A E. Phil. Trans. R. Soc. 2006;364(A):125. doi: 10.1098/rsta.2005.1682. [DOI] [PubMed] [Google Scholar]


