Abstract
In this paper, the key topics of tunable structural color in biology and material science are overviewed. Color in biology is considered for selected groups of tropical fish, octopus, squid and beetle. It is caused by nanoplates in iridophores and varies with their spacing, tilting angle and refractive index. These examples may provide valuable hints for the bioinspired design of photonic materials. 1D multilayer films and 3D colloidal crystals with tunable structural color are overviewed from the viewpoint of advanced materials. The tunability of structural color by swelling and strain is demonstrated on an example of opal composites.
Keywords: structural color, Bragg’s diffraction, multi-layer films, colloidal crystals, tunable interspace
Introduction
Iridescence is a structural color formed without using pigments, dye or luminescence [1, 2]. It originates from spectrally selective reflection of visible light from a periodic modulation of refractive index. We can observe the structural color in natural life forms, for example, in peacock feathers, outer shells of jewel beetles, wings of Morpho butterflies and many other insects. A previous review summarized the multilayer interference of light in aquatic organisms, particularly in fish scales [3]. Multilayer interference is also the major topic of this paper. Structural color in nature is used in camouflage, intimidation (warning), display and communication, and there have been recent discoveries in this area from the viewpoint of photonic crystals [4, 5]. However, the structural color of life forms cannot be expressed using a simple interference model, and its origin, particularly in butterflies, remains an active research topic in biology and physics [6–9].
The scales of some fishes and epidermises of insects can change their structural color [10, 11]. The blue color of Morpho butterfly wings is caused by their periodic nanostructure. It can be changed by varying the refractive index n, for example, from blue to green by soaking in acetone (n=1.362) [12]. After the wings are dried, their color returns to original. This is an example of passive color change, and in this review , we focus on active structural color in organisms, that is, voluntary color changes in some groups of tropical fish, octopus, squid and beetle in response to external stimuli. Revealing their mechanisms may provide hints for the fabrication of new photonic materials with tunable structural color. Such bioinspired or biomimetic materials are a new trend and an emerging technology [13–20]. This review is organized as follows. We first consider 1D photonic crystals consisting of multilayer films and block copolymers as well as 3D photonic crystals based on colloids. The tunable structural color of opal photonic crystals is described next [21], followed by an outlook of future developments.
Tunable structural color in nature
Various species of damselfish are known for their attractive bright-blue coloration as shown in figure 1(A), and this color can change to green depending on the environment. Kasukawa et al proposed a model for this color change [22]. Figure 1(B) shows a schematic of an iridophore and the mechanism of color tuning by a stack of guanine nanoplates. The refractive index of guanine crystals is 1.83 and it is 1.37 for the surrounding cytoplasm [23]. The color is reversibly changed by the movement of reflecting guanine plates, from blue at d1 to green at d2. Mäthger et al reported a rapid color change in multilayer reflection from paradise whiptail [24]. The color switched from blue to red within approximately 0.25 s, and the corresponding reflection peak shifted between 465 and 650 nm. The mechanism behind this phenomenon should be useful for designing color-tunable materials.
Figure 1.
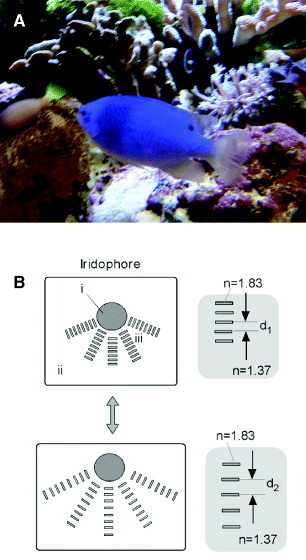
(A) Photo of a blue damselfish taken at an aquarium at Fukushima, Japan; this fish can reversibly change its color. (B) Illustration of the mechanism behind the reversible color change by motile iridophore [23]. Guanine crystal nanoplates are stacked on the nucleus (i) in the iridophore cell and their spacing is varied.
Figure 2 shows three mechanisms of tuning structural color in nature wherein the key factors are refractive index, periodic nanostructure and incident light angle. Tao et al reported a mechanism of rainbow color of squid iridophores as shown in figure 2(A) [25]. In the iridophore cell, membrane-enclosed protein nanoplatelets form a multilayer structure. The iridophore is chemically tuned by exposure to acetylcholine. The color is reversibly tuned by the thickness change of the protein platelets between d1 and d2. This mechanism is slightly different from that of the cobalt blue shown in figure 1(B). According to a recent review [26], squid, octopus and cuttlefish use this mechanism for camouflage and communication.
Figure 2.
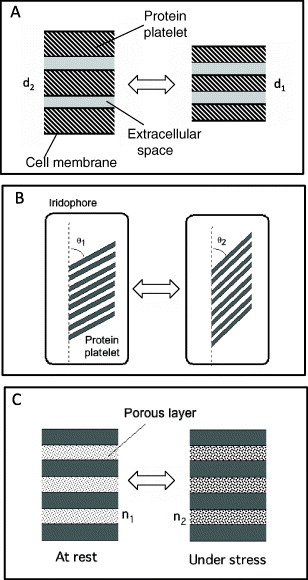
Color tuning mechanisms in living creatures: (A) expansion and compression of extracellular space between protein platelets in cephalopods, (B) tilting protein platelet in the iridophore of neon tetra fish, (C) changing refractive index of a porous layer by absorbing liquid onto the beetle shell.
The South American tropical fish neon tetra changes its color from green in daytime to violet–blue at night [27]. Figure 2(B) shows a schematic of the iridophore of neon tetra, which is attributed to the venetian blind type [23]. The structural color changes occur by tilting the platelets in the iridophore [28, 29]. A similar color tuning mechanism is observed in jellyfish. The comb jellyfish has a deformable and transparent body that iridesces across the whole visible spectrum as a result of the combs beating. This phenomenon originates from the angle-dependent reflection from a two-dimensional photonic crystal formed in the combs [30].
Some beetles can change color by varying the refractive index of epidermis. For example, shield bugs change their color using moisture [31]. Figure 2(C) shows the associated color tuning mechanism described in Berthier’s book [12]. At rest, the cuticle shell of a porous laminate layer is dry. When stressed, the porous layer absorbs water and its refractive index increases from n1 to n2. The Hoplia coerulea beetle changes its color from bright blue to emerald green [32]. Other beetles can combine structural color and pigment color. The tortoise beetle can reversibly change its shell color from metallic yellow to a more diffusive red within 90 s when disturbed [33]. Its shell contains a double layer bearing the structural color in the outer part and red pigment in the inner part. Discoloration of the outer layer reveals the red pigmentation. The Hercules beetle changes its color from khaki-green when dry to black at high humidity levels. When the 3D nanoporous structure of the cuticle absorbs water, it becomes less reflective and reveals the black color of the underlying melanin layer.
Bioinspired materials for tunable structural materials
Scientists have been working on realizing the color-tuning mechanism found in nature in synthetic materials [34]. For example, one commercially available bioinspired product is a structurally colored lamellar ridge fiber made of polyester (n=1.63) and polyamide (n=1.53) [35]. Here, we consider two representative architectures: 1D multilayer films and 3D colloidal crystals.
Multilayer interference
Figure 3(A) shows a model of 1D multilayer interference. Land mentioned that the multilayer interference plays an important role in the structural color of animals [3]. Guanine, chitin or protein acts as a high-refractive-index material in nature. Considering a stack of nonabsorbing layers with the thicknesses and refractive indices of (d1,n1) and (d2,n2) in air (refractive index n0), the peak reflectance wavelength λ at an angle θ can be expressed as
where m is a positive integer.
Figure 3.
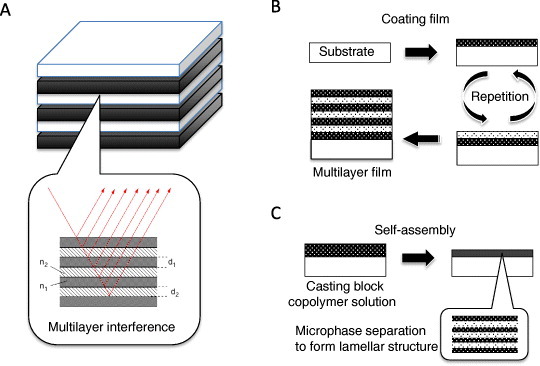
One-dimensional photonic crystals using interference in a multilayer structure: (A) a stack of polymer composite layers having refractive indices of n1 and n2 and thicknesses d1 and d2, (B) stacking procedure, (C) self-assembly by casting copolymer solution.
Multilayer interference structures include dielectric mirrors, Bragg mirrors, dichroic filters and distributed Bragg reflectors, and are widely used in optical devices. These devices are usually made from rigid inorganic materials such as magnesium fluoride, silicon oxide, tantalum oxide, zinc sulfide and titanium oxide. The multilayer films are fabricated by physical vapor deposition, chemical vapor deposition, ion beam deposition, molecular beam epitaxy or sputtering. Here, we focus on multilayer interference structures made of soft materials using a solution process. The hybrid and flexible composite films are 1D photonic crystals with a tunable photonic stop band.
Multilayer interference structures with tunable structural color are mainly fabricated via two approaches. Figure 3(B) shows the cyclic coating of two materials having different refractive indices and the same layer thickness. The layers are usually deposited by spin coating, dip coating or layer-by-layer deposition, and Hammond reviewed a new technique of layer-by-layer electrostatic assembly [36]. The resulting polymer-based Bragg mirror films can be used to detect acetone vapors [37, 38]. Calvo et al demonstrated that multilayer films of high- and low-refractive-index soft materials can exhibit a tunable structural color [39]. Wang et al produced water–vapor-responsive organic/inorganic multilayer films by alternated spin coatings of a titania precursor sol and a polymer solution [40]. The color tunability is achieved by changing the interlayer distance, as in the mechanism shown in figure 2(A). The color can also be tuned through the refractive index, as in the beetle example shown in figure 2(C). Inorganic porous films using this principle can be used in a color-tunable sensor. Their mesoporous SiO2 and TiO2 multilayers can be fabricated by depositing precursor solutions by spin coating [41], dip coating [42] and layer-by-layer assembly [43].
In a different approach, a multilayer film can be fabricated by casting solution blends of the block copolymer. The deposited thin liquid films are then annealed in chloroform vapor to stabilize them thermally. At an appropriate volume fraction of the block copolymer, a stable lamellar structure is formed by micro-phase separation [44, 45]. Figure 3(C) shows a well-ordered lamellar structure deposited on a solid substrate via self-assembly. Using this approach, Kang et al have grown chemically tunable block-copolymer multilayer films with a reflectivity range from 364 to 1627 nm [46], where the reflectivity from an ordered lamellar structure is reversibly tuned by swelling and deswelling. They also reported a block copolymer, polystyrene-polyvinyl pyridine, whose color is electrochemically tunable between red and green by applying a voltage of 10 V [18]. Valkama et al achieved a color change in block-copolymer solid films from green at room temperature (peak position, 530 nm) to colorless (UV region, 370 nm) at 134 °C due to the shrinking of a lamellar periodic structure [47]. Lu et al reversibly changed the color of a swollen lamellar block-copolymer film from violet to red by applying a voltage of 2.5 V [48]. Haque et al used a different approach, namely, unidirectional alignment of a lamellar bilayer in a polyacrylamide hydrogel [49]. The membrane like lamellar bilayer structure changed the color of the hydrogel between red and blue upon swelling or application of stress/strain.
Crystalline diffraction
Opal is a colloidal crystal and a typical 3D photonic crystal. The crystalline diffraction from opal has been studied for photonic applications [50, 51], and one of the milestone achievements was the design of an inverse silicone opal structure with a full band gap by the North American NEC group [52]. In addition to 3D photonic crystals, the structural color of colloidal crystals has been attracting increasing attention. From the viewpoint of bottom-up nanotechnology, nanostructured colloidal crystals can be fabricated using a simple and low cost process. A large number of research papers on opal photonic crystals were published in the past decade, as reviewed in references [53–60].
Natural and synthetic opals show a rainbow color due to Bragg diffraction at random orientation of the plane of the colloidal particle arrays [61]. Figure 4 shows a natural opal stone containing water inside (A) [62] and a synthetic opal plate consisting of sedimentation silica colloids (B) [63]. These opal materials show iridescent rainbow color from colorless silica colloids. On the other hand, crystal planes oriented on the substrate induce monochromatic structural color in colloidal crystal films. Simple preparation techniques based on convective self-assembly have been developed and are widely used in this research field [64–66]. Figure 4(C) shows a scanning electron microscopy (SEM) image of close-packed polystyrene (PS) colloids on a silicon wafer. Here, monodispersed 0.2 μm PS particles form a periodic 3D nanostructure with cubic close packing (ccp). The ccp (111) planes are parallel to the substrate and these (111)-oriented colloidal crystals are formed. The vertical stacking of ccp (111) planes on the substrate resulted in the monochromatic structural color of the opal thin film. This color depends on the refractive index, tilting angle and distance between the ccp (111) planes. The reflected wavelength λ is expressed by the combining of the Bragg equation with the Snell law [67]:
Here, m is a positive integer, d111 is the distance between the ccp (111) planes, neff is the average refractive index and θ is the angle of incidence.
Figure 4.
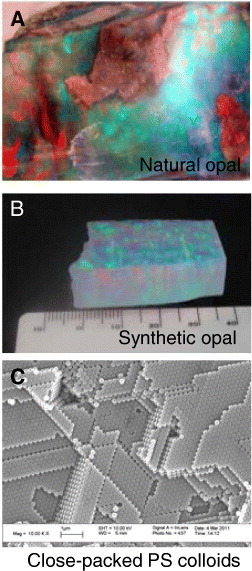
Diffraction of light from 3D structures: (A) natural opal gemstone displayed at the Ibaraki Nature Museum, Japan, (B) synthetic opal made by Kyocera—the iridescence originates from the random orientation of particle arrays, (C) SEM image of the opal film surface shows closely packed PS spheres.
Figures 1 and 2 provide inspiration for designing colloidal crystals with tunable structural color. Figure 5 shows a classification of colloidal crystals into three types: opal composite (A), 3D close-packed (B) and non close-packed (C) inverse opal. The elastomer in the opal composite plays an important role in the reversible tuning of the colloidal crystal lattice as shown in figure 5(A). From equation (2), the structural color can be changed by controlling three parameters: d111, neff and θ. Some research groups have developed opal composites focusing on tuning d111 [68–72], and an example of color tuning in the elastic opal composite is discussed in the next section. Using opal as a template, an inverse opal structure can be produced, which exhibits porous morphology and tunable color as shown in figure 5(B). Inverse opal hydrogels have potential applications in chemical sensors [73, 74], and elastomer inverse opal films can be used for mechanical fingerprinting [75]. Tunable structural color in inverse opal photonic crystals was overviewed in [53, 56, 57].
Figure 5.
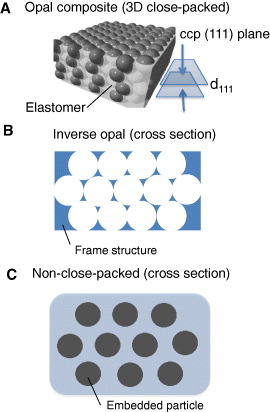
Three structure types of colloid-based soft materials, which exhibit tunable structural color by diffracting visible light: (A) opal composite made of 3D close-packed colloidal spheres bonded by an elastomer, (B) inverse opal made of a soft-material frame structure—here opal acts as a template for the soft material, (C) non close-packed colloidal crystal embedded in a soft material, typically a hydrogel.
Non close-packed colloidal crystals are formed in deionized water and exhibit iridescence, i.e. structural color. Diffraction of light by ordered suspensions is well known [76]. However, this type of colloidal crystal is unstable and can be destroyed even by a weak disturbance. Weissman et al have proposed a new soft material shown in figure 5(C), which is a colloidal crystal embedded in a poly(N-isopropylacrylamide) hydrogel [77]. The lattice spacing in this colloidal crystal is tuned by the temperature-induced phase transition in the hydrogel. As a result, the diffracted wavelength from the colloidal crystal is thermally tunable across the entire visible spectrum. The color of the hydrogel colloidal crystals is also sensitive to pH and ion concentration [78, 79], and colloidal crystals embedded in hydrogels can serve as mechanical sensors for measuring strains due to uniaxial stretching or compression [80]. Xia et al reported electrically tunable structural color [81], tunable laser emission [82] and structural color printing [83] using hydrogel colloidal crystals. A recently discovered topic is tuning the color of hydrogels by a magnetic field [84, 85].
Tunable structural color in opal composites
As illustrated in figure 1, damselfish can reversibly change its color by changing the spacing between guanine nanoplates. This mechanism of motile iridophores inspired the design of opal composites [21]. These soft materials consist of closely packed PS, colloids and a polydimethylsiloxane (PDMS) elastomer (see figure 5(A)). Expansion or compression, e.g. due to swelling or mechanical strain, reversibly tunes the spacing between the ccp (111) planes, d111, in opal composite films. Figure 6 shows how an opal composite film changes its color after dipping in a hydrophobic liquid, octane [86]. The dry film has the lattice spacing d1 and violet–blue color. In the liquid, PDMS swells without dissolving and the lattice spacing expands to d2 . According to equation (2), this expansion shifts the Bragg diffraction peak to longer wavelengths, which explains the red color of the wet part of the film. Evaporating the octane recovers the initial violet–blue color. This wavelength shift depends on the swelling liquid, and can be changed continuously, e.g. by using silicone oils. The shift amount decreases with the molecular weight of silicone that can be used in a new type of colorimetric detection.
Figure 6.
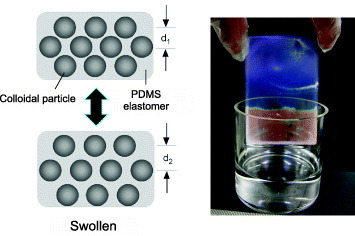
Reversible color tuning by swelling. A glass substrate coated with a soft opal film was dipped in a hydrophobic liquid, octane. In the photograph (right) , the dry area has the initial blue color and the wet area is red. The color is reverted from red to blue by evaporating the octane. The model (left) shows how the spacing between colloidal particles is changed by the swollen PDMS elastomer from d1 to d2.
Figure 7 shows that the structural color can be changed by stretching an array of colloidal particles deposited on a rubber sheet [87]. The color was reversed by releasing the stress. Reflectance measurements revealed that the Bragg diffraction peak shifted from 630 (red light) to 580 nm (green) upon stretching. The stress in the horizontal direction is expressed as σx=Eεx=E(ΔL/L0), where E is the Young’s modulus, L0 is initial length and ΔL=L−L0 is elongation. The horizontal stretching induces strain in the vertical direction, which can be expressed using Poisson’s ratio ν as εz=−ν(σx/E)=−ν(ΔL/L0). Lattice spacing d is proportional to εz; therefore, the Bragg diffraction peak shifts with the elongation of the rubber sheet. Such opal composite films can be used in practical devices such as a color indicator, tension meter or elongation strain sensor.
Figure 7.
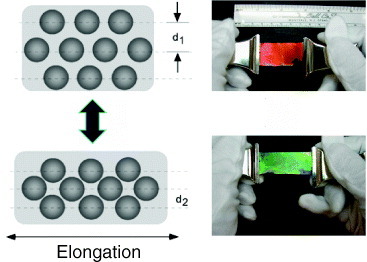
Tuning structural color by mechanical stretching. The model (left) shows the reduction of the spacing between the ccp (111) planes from d1 to d2. The photographs (right) show a soft opal film on a PDMS rubber sheet changing color from red to green upon stretching. The color is restored by releasing the strain.
Outlook
As outlined in this review, bioinspired approaches are useful for the design of photonic materials. A humidity sensor based on 3D opal photonic crystals was fabricated on the basis of the nanoporous structure of the Hercules beetle [88]. This sensor changes its color from blue to red at high humidity; however, it is yet unselective to the carrier gas. Potyrailo et al discovered that the nanostructure of Morpho butterfly wings indicates a high-performance optical gas sensor with a highly selective response [89]. The biomimetic approach is difficult, yet rather interesting for industrial applications, and the nanostructured organization of living creatures such as butterflies and beetles may open new approaches to the design of complex photonic functions [16, 90].
Another aspect of bio-inspired materials design is the discovery of new, more complex nanostructures in organisms. For example, gyroid and related phases have been reported recently [91, 92]. Galusha et al have grown diamond-structured 3D photonic crystals by replicating the beetle shell using the sol–gel technique [93]. Hajduk et al discovered the gyroid phase in a block copolymer [94] and synthesized 3D ceramic nanostructured films from a self-assembling double gyroid copolymer [95]. The group of Thomas has recently proposed a bioinspired material design from a self-assembled block copolymer, lamella, for an electrically tunable full color device [18]. These self-assembled nanostructures made of block copolymers are expected to bring a new design approach to photonic crystals with tunable color.
Acknowledgments
This work is part of the collaborations with Professor Younan Xia, Washington University in St Louis, and Dr Tsutomu Sawada, NIMS. The author thanks AMATA Metal Works Foundation, IKETANI Science Foundation, KUMAGAI Foundation for Science and Technology and Grants-in-Aid for Scientific Research, Grants-in-Aid for Scientific Research (C18560682 and B23360313) for their financial support.
References
- Berthier S. Berlin Springer; 2007. Iridescences—The physical colors of insects. [Google Scholar]
- Kinoshita S. and Yoshioka S. Osaka Osaka University Press; 2005. Structural Colors in Biological Systems—Principles and Applications. [Google Scholar]
- Land M F. Prog. Biophys. Mol. Biol. 1972;24:75. doi: 10.1016/0079-6107(72)90004-1. [DOI] [PubMed] [Google Scholar]
- Parker A R, Welch V L, Driver D. and Martini N. Nature. 2003;426:786. doi: 10.1038/426786a. [DOI] [PubMed] [Google Scholar]
- Vukusic P. and Sambles J R. Nature. 2003;424:852. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]
- Sharma V, Crne M, Park J O. and Srinivasarao M. Science. 2009;325:449. doi: 10.1126/science.1172051. [DOI] [PubMed] [Google Scholar]
- Vukusic P, Sambles J R. and Lawrence C R. Nature. 2000;404:457. doi: 10.1038/35006561. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Yoshioka S. and Kawagoe K. Proc. R. Soc. Lond. 2002;269(B):1417. doi: 10.1098/rspb.2002.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolle M, Salgard-Cunha P M, Scherer M R J, Huang F, Vukusic P, Mahajan S, Baumberg J J. and Steiner U. Nat. Nanotechnol. 2010;5:511. doi: 10.1038/nnano.2010.101. [DOI] [PubMed] [Google Scholar]
- Lythgoe J N, Shand J. and Foster R G. Nature. 1984;308:83. doi: 10.1038/308083a0. [DOI] [Google Scholar]
- Hinton H E. and Jarman G M. Nature. 1972;238:160. doi: 10.1038/238160a0. [DOI] [Google Scholar]
- Berthier S. Changing colors: structures or pigments? Berlin Springer; 2007. Iridescences—The Physical Colors of Insects; p. p 49. [Google Scholar]
- Lee L P. and Szema R. Science. 2005;310:1148. doi: 10.1126/science.1115248. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Arribart H. and Guille M M G. Nat. Mater. 2005;4:277. doi: 10.1038/nmat1339. [DOI] [PubMed] [Google Scholar]
- Parker A R. Phil. Trans. R. Soc. 2009;367(A):1759. doi: 10.1098/rsta.2009.0016. [DOI] [PubMed] [Google Scholar]
- Biro L P. and Vigneron J P. Laser Photonics Rev. 2010;5:27. doi: 10.1002/lpor.v5.1. [DOI] [Google Scholar]
- Sato O, Kubo S. and Gu Z Z. Acc. Chem. Res. 2009;42:1. doi: 10.1021/ar700197v. [DOI] [PubMed] [Google Scholar]
- Walish J J, Kang Y, Mickiewicz R A. and Thomas E L. Adv. Mater. 2009;21:3078. doi: 10.1002/adma.v21:30. [DOI] [Google Scholar]
- Wang J, Zhang Y, Wang S, Song Y. and Jiang L. Acc. Chem. Res. 2011;44:405. doi: 10.1021/ar1001236. [DOI] [PubMed] [Google Scholar]
- Kolle M. Berlin Springer; 2011. Photonic Structures Inspired by Nature. [Google Scholar]
- Fudouzi H. Adv. Powder Technol. 2009;20:502. doi: 10.1016/j.apt.2009.08.002. [DOI] [Google Scholar]
- Kasukawa H, Oshima N. and Fujii R. Zool. Sci. 1987;4:243. [Google Scholar]
- Oshima N. Structural Colors in Biological Systems—Principles and Applications. In: Kinoshita S, , Yoshioka S, , editors. Light reflection in motile iridophores of Fish. Osaka Osaka University Press; 2005. p. p 211. [Google Scholar]
- Mäthger L M, Land M F, Siebeck U E. and Marshall N J. J. Exp. Biol. 2003;206:3607. doi: 10.1242/jeb.00599. [DOI] [PubMed] [Google Scholar]
- Tao A R, DeMartini D G, Izumi M, Sweeney A M, Holt A L. and Morse D E. Biomaterials. 2010;31:793. doi: 10.1016/j.biomaterials.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Mäthger L M, Denton E J, Marshall N J. and Hanlon R T. J. R. Soc. Interface. 2009;6:S149. doi: 10.1098/rsif.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lythgoe J N. and Shand J. J. Physiol. 1982;325:23. doi: 10.1113/jphysiol.1982.sp014132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaishi H, Oshima N. and Fujii R. Comp. Biochem. Physiol. A: Physiol. 1990;95:337. doi: 10.1016/0300-9629(90)90229-L. [DOI] [Google Scholar]
- Yoshioka S, Matsuhana B, Tanaka S, Inouye Y, Oshima N. and Kinoshita S. J. R. Soc. Interface. 2011;8:56. doi: 10.1098/rsif.2010.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch V, Vigneron J P, Lousse V. and Parker A. Phys. Rev. 2006;73(E):041916. doi: 10.1103/PhysRevE.73.041916. [DOI] [PubMed] [Google Scholar]
- Miyamoto K. and Kosaki A. Structural Colors in Biological Systems—Principles and Applications. In: Kinoshita S, , Yoshioka S, , editors. Structural color of shield bugs in Japan. Osaka Osaka University Press; 2005. p. p 177. [Google Scholar]
- Rassart M, Simonis P, Bay A, Deparis O. and Vigneron J P. Phys. Rev. 2009;80(E):031910. doi: 10.1103/PhysRevE.80.031910. [DOI] [PubMed] [Google Scholar]
- Vigneron J P. et al Phys. Rev. 2007;76(E):031907. doi: 10.1103/PhysRevE.76.031907. [DOI] [Google Scholar]
- Graham-Rowe D. Nat. Photonics. 2009;3:551. doi: 10.1038/nphoton.2009.172. [DOI] [Google Scholar]
- Tabata H. Structural Colors in Biological Systems—Principles and Applications. In: Kinoshita S, , Yoshioka S, , editors. Structurally colored fibers and applications. Osaka Osaka University Press; 2005. p. p 297. [Google Scholar]
- Hammond P T. Adv. Mater. 2004;16:1271. doi: 10.1002/(ISSN)1521-4095. [DOI] [Google Scholar]
- Mönch W, Dehnert J, Jaufmann E. and Zappe H. Appl. Phys. Lett. 2006;89:164104. doi: 10.1063/1.2358811. [DOI] [Google Scholar]
- Convertino A, Capobianchi A, Valentini A. and Cirillo E N M. Adv. Mater. 2003;13:1103. doi: 10.1002/adma.200304777. [DOI] [Google Scholar]
- Calvo M E, Sánchez S O, Lozano G. and Míguez H. J. Mater. Chem. 2009;19:3144. doi: 10.1039/b902090j. [DOI] [Google Scholar]
- Wang Z. et al Adv. Funct. Mater. 2010;20:3784. doi: 10.1002/adfm.201001195. [DOI] [Google Scholar]
- Choi S Y, Mamak M, Von Freymann G, Chopra N. and Ozin G A. Nano Lett. 2006;6:2456. doi: 10.1021/nl061580m. [DOI] [PubMed] [Google Scholar]
- Fuertes M C, López-Alcaraz F J, Marchi M C, Troiani H E, Luca V, Míguez H. and Soler-Illia G J D A. Adv. Funct. Mater. 2007;17:1247. doi: 10.1002/(ISSN)1616-3028. [DOI] [Google Scholar]
- Wu Z, Lee D, Rubner M F. and Cohen R E. Small. 2007;3:1445. doi: 10.1002/(ISSN)1613-6829. [DOI] [PubMed] [Google Scholar]
- Edrington A C. et al Adv. Mater. 2001;13:421. doi: 10.1002/(ISSN)1521-4095. [DOI] [Google Scholar]
- Bockstaller M R, Mickiewicz R A. and Thomas E L. Adv. Mater. 2005;17:1331. doi: 10.1002/(ISSN)1521-4095. [DOI] [PubMed] [Google Scholar]
- Kang Y, Walish J J, Gorishnyy T. and Thomas E L. Nat. Mater. 2007;6:957. doi: 10.1038/nmat2032. [DOI] [PubMed] [Google Scholar]
- Valkama S, Kosonen H, Ruokolainen J, Haatainen T, Torkkeli M, Serimaa R, Brinke G T. and Ikkala O. Nat. Mater. 2004;3:872. doi: 10.1038/nmat1254. [DOI] [PubMed] [Google Scholar]
- Lu Y, Xia H, Zhang G. and Wu C. J. Mater. Chem. 2009;19:5952. doi: 10.1039/b905760a. [DOI] [Google Scholar]
- Haque M A, Kamita G, Kurokawa T, Tsujii K. and Gong J P. Adv. Mater. 2010;22:5110. doi: 10.1002/adma.201002509. [DOI] [PubMed] [Google Scholar]
- Ozin G A. and Arsenault A C. Cambridge RCS Publishing; 2008. Nanochemistry 2nd edn. [Google Scholar]
- Xia Y, Gates B, Yin Y. and Lu Y. Adv. Mater. 2000;12:693. doi: 10.1002/(ISSN)1521-4095. [DOI] [Google Scholar]
- Vlasov Y A, Bo X Z, Sturm J C. and Norris D J. Nature. 2001;414:289. doi: 10.1038/35104529. [DOI] [PubMed] [Google Scholar]
- Harun-ur-rashie M, Seki T. and Takeoka Y. Chem. Rec. 2009;9:87. doi: 10.1002/tcr.v9:2. [DOI] [PubMed] [Google Scholar]
- Marlow F, Muldarisnur, Sharifi P, Brinkmann R. and Mendive C. Angew. Chem., Int. Ed. Engl. 2009;48:6212. doi: 10.1002/anie.200900210. [DOI] [PubMed] [Google Scholar]
- Furumi S, Fudouzi H. and Sawada T. Laser Photon. Rev. 2010;4:205. doi: 10.1002/lpor.200910005. [DOI] [Google Scholar]
- Zhao Y, Zhao X. and Gu Z. Adv. Funct. Mater. 2010;20:2970. doi: 10.1002/adfm.201000098. [DOI] [Google Scholar]
- Aguirre C I, Reguera E. and Stein A. Adv. Funct. Mater. 2010;20:2565. doi: 10.1002/adfm.201000143. [DOI] [Google Scholar]
- Ge J. and Yin Y. Angew. Chem., Int. Ed. Engl. 2011;50:1492. doi: 10.1002/anie.200907091. [DOI] [PubMed] [Google Scholar]
- Kim S H, Lee S Y, Yang S M. and Yi G R. NPG Asia Mater. 2011;3:25. [Google Scholar]
- Galisteo-Lõpez J F, Ibisate M, Sapienza R, Froufe-Pérez L S, Blanco Ú. and Lõpez C. Adv. Mater. 2011;23:30. doi: 10.1002/adma.v23.1. [DOI] [PubMed] [Google Scholar]
- Sanders J V. Acta Crystallogr. 1968;24(A):427. doi: 10.1107/S0567739468000860. [DOI] [Google Scholar]
- Eckert A W. New York Wiley; 1997. The World of Opals. [Google Scholar]
- Nakano Y, Kamiyama K. and Kobayashi T. 1987 US Patent 4,703,020 [Google Scholar]
- Jiang P, Bertone J F, Hwang K S. and Colvin V L. Chem. Mater. 1999;11:2132. doi: 10.1021/cm990080+. [DOI] [Google Scholar]
- Gu Z Z, Fujishima A. and Sato O. Chem. Mater. 2002;14:760. doi: 10.1021/cm0108435. [DOI] [Google Scholar]
- Norris D J, Arlinghaus E G, Meng L L, Heiny R. and Scriven L E. Adv. Mater. 2004;16:1393. doi: 10.1002/(ISSN)1521-4095. [DOI] [Google Scholar]
- Fudouzi H. J. Colloid Interface Sci. 2004;275:277. doi: 10.1016/j.jcis.2004.01.054. [DOI] [PubMed] [Google Scholar]
- Arsenault A C, Miguez H, Kitaev V, Ozin G A. and Manners I. Adv. Mater. 2003;15:503. doi: 10.1002/adma.200390116. [DOI] [Google Scholar]
- Fudouzi H. and Xia Y N. Adv. Mater. 2003;15:892. doi: 10.1002/adma.200304795. [DOI] [Google Scholar]
- Rugge A, Ford W T. and Tolbert S H. Langmuir. 2003;19:7852. doi: 10.1021/la034617g. [DOI] [Google Scholar]
- Pursiainen O L J, Baumberg J J, Ryan K, Bauer J, Winkler H, Viel B. and Ruhl T. Appl. Phys. Lett. 2005;87:101902. doi: 10.1063/1.2032590. [DOI] [Google Scholar]
- Yoshino K, Kawagishi Y, Ozaki M. and Kose A. Japan. J. Appl. Phys. 2. 1999;38:L786. doi: 10.1143/JJAP.38.L786. [DOI] [Google Scholar]
- Takeoka Y. and Watanabe M. Adv. Mater. 2003;15:199. doi: 10.1002/adma.200390044. [DOI] [Google Scholar]
- Lee Y J. and Braun P V. Adv. Mater. 2003;15:563. doi: 10.1002/adma.200304588. [DOI] [Google Scholar]
- Arsenault A C. et al Nat. Mater. 2006;5:179. doi: 10.1038/nmat1588. [DOI] [Google Scholar]
- Hiltner P A. and Krieger I M. J. Phys. Chem. 1969;73:2386. doi: 10.1021/j100727a049. [DOI] [Google Scholar]
- Weissman J M, Sunkara H B, Tse A S. and Asher S A. Science. 1996;274:959. doi: 10.1126/science.274.5289.959. [DOI] [PubMed] [Google Scholar]
- Holtz J H. and Asher S A. Nature. 1997;389:829. doi: 10.1038/39834. [DOI] [PubMed] [Google Scholar]
- Hu Z, Lu X. and Gao J. Adv. Mater. 2001;13:1708. doi: 10.1002/(ISSN)1521-4095. [DOI] [Google Scholar]
- Iwayama Y, Yamanaka J, Takiguchi Y, Takasaka M, Ito K, Shinohara K T, Sawada T. and Yonese M. Langmuir. 2003;19:977. doi: 10.1021/la0207365. [DOI] [Google Scholar]
- Xia J, Ying Y. and Foulger S H. Adv. Mater. 2005;17:2463. doi: 10.1002/(ISSN)1521-4095. [DOI] [Google Scholar]
- Lawrence J R, Ying Y, Jiang P. and Foulger S H. Adv. Mater. 2006;18:300. doi: 10.1002/(ISSN)1521-4095. [DOI] [Google Scholar]
- Jiang P, Smith D W, Jr, Ballato J M. and Foulger S H. Adv. Mater. 2005;17:179. doi: 10.1002/(ISSN)1521-4095. [DOI] [Google Scholar]
- Kim H, Ge J, Kim J, Choi S, Lee H, Lee H, Park W, Yin Y. and Kwon S. Nat. Photon. 2009;3:534. doi: 10.1038/nphoton.2009.141. [DOI] [Google Scholar]
- Ge J, Hu Y. and Yin Y. Angew. Chem., Int. Ed. Engl. 2007;46:7428. doi: 10.1002/(ISSN)1521-3773. [DOI] [PubMed] [Google Scholar]
- Fudouzi H. and Xia Y. Langmuir. 2003;19:9653. doi: 10.1021/la034918q. [DOI] [Google Scholar]
- Fudouzi H. and Sawada T. Langmuir. 2006;22:1365. doi: 10.1021/la0521037. [DOI] [PubMed] [Google Scholar]
- Kim J H, Moon J H, Lee S Y. and Park J. Appl. Phys. Lett. 2010;97:103701. doi: 10.1063/1.3486115. [DOI] [Google Scholar]
- Potyrailo R A, Ghiradella H, Vertiatchikh A, Dovidenko K, Cournoyer J R. and Olson E. Nat. Photon. 2007;1:123. doi: 10.1038/nphoton.2007.2. [DOI] [Google Scholar]
- Kinoshita S. and Yoshioka S. Chem. Phys. Chem. 2005;6:1443. [Google Scholar]
- Michielsen K. and Stavenga D G. J. R. Soc. Interface. 2008;5:85. doi: 10.1098/rsif.2007.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranathana V, Osujib C O, Mochrieb S G J, Nohb H, Narayananf S, Sandyf A, Dufresneb E R. and Pruma R O. Proc. Natl. Acad. Sci. USA. 2010;107:11676. doi: 10.1073/pnas.0909616107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galusha J W, Jorgensen M R. and Bartl M H. Adv. Mater. 2010;22:107. doi: 10.1002/adma.v22:1. [DOI] [PubMed] [Google Scholar]
- Hajduk D A, Harper P E, Gruner S M, Honeker C C, Kim G, Thomas E L. and Fetters L J. Macromolecules. 1994;27:4063. doi: 10.1021/ma00093a006. [DOI] [Google Scholar]
- Chan V Z H, Hoffman J, Lee V Y, Iatrou H, Avgeropoulos A, Hadjichristidis N, Miller R D. and Thomas E L. Science. 1999;286:1716. doi: 10.1126/science.286.5445.1716. [DOI] [PubMed] [Google Scholar]


