Abstract
Nanometer-scale architectures assembled on cell surface receptors from smaller macromolecular constituents generated a large amplification of fluorescence. A targeted dendrimer was synthesized from a cystamine-core G4 PAMAM dendrimer, and contained an anti-BrE3 monoclonal antibody as the targeting group, several fluorophores and an average of 12 aldehyde moieties as complementary bio-orthogonal reactive sites for the covalent assembly. A cargo dendrimer, derived from a PAMAM G4 dendrimer, contained several fluorophores as the cargo for delivery and five hydrazine moieties as complimentary bio-orthogonal reactive sites. The system is designed to be flexible and allow for facile incorporation of a variety of targeting ligands.
The usefulness of currently available bioreceptor-directed agents is often limited by the low abundance of targets present in and around the tumor site, making selective delivery of imaging and therapeutic modalities a difficult task.1 Nanometer or micron size particles offer an attractive strategy for signal amplification,2 but such particles may suffer from unfavorable biodistribution in vivo.3 Inspired in part by reports of self-assembly of drugs4 and pre-targeted amplification experiments with radiolabels,5 we prototype here the on-site construction of particles by administration of building blocks that do not exceed a specific size criterion, in this case chosen as 40 Å, which is the approximate cutoff for renal clearance of xenobiotics.6 In a two-step approach, administration of a targeted molecule containing multivalent expression of a bio-orthogonal reactive chemical functional group is followed by a second molecule with a complementary reactive group and a cargo. We prototype the method by delivery of fluorescent dyes to cells in tissue culture, but the strategy could be utilized to amplify the localization of various imaging and/or therapeutic modalities at targeted sites. Our method resembles pre-targeting7 or secondary antibody labeling8 but offers amplification with relatively small building blocks that can be easily functionalized with different modalities.
Polyamidoamine (PAMAM) and cystamine core G4 dendrimers were selected for this study as platforms for multivalent self-assembly since they have been functionalized with imaging and therapeutic modalities.9 Low-generation (e.g., G4) cationic PAMAM dendrimers avoid lytic behavior of higher generation dendrimers,10 meet our size criterion.11 Elegant dendrimer self-assembly strategies have been examined.12 We chose covalent self-assembly using small organic functional groups (as opposed to avidin-biotin and similar approaches) to minimize the contribution of linker atoms to the size of the final particle. Among several interesting options, simple aldehyde/hydrazine chemistry is readily available and reversible,13 potentiating subsequent clearance. Although aldehydes may form Schiff bases with intracellular proteins, such adducts are reversible, weakly stable and favor dissociation (K~40 M−1).14
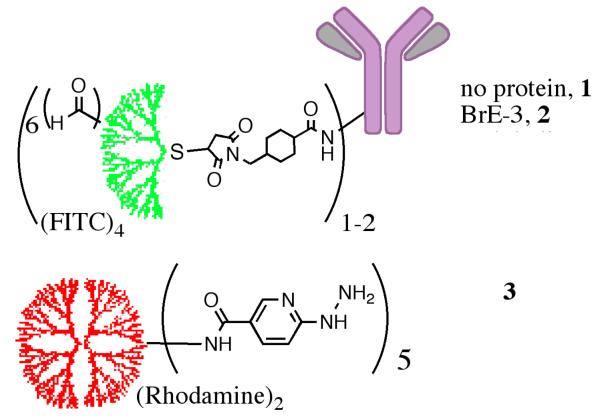
A targeted dendron (2) was prepared from a G4 cys-core dendrimer with attached fluorescein dyes (fluorescein isothiocyanate, FITC, average of eight), formylbenzoates (sixteen) and linked to an antibody via sulfosuccinimidyl 4-N-maleimidomethyl cyclohexane-1-carboxylate (sulfo-SMCC, Pierce). This dendrimer was targeted to an over-expressed receptor on a tumor cell surface by attachment to an antibody. After localization of the targeted dendrimer, a cargo-carrying dendrimer (3), containing two rhodamine dyes and five pyridylhydrazine moieties was introduced. Several cargo-carrying dendrimers can potentially react covalently with the targeted dendrimer, as each contains complimentary reactive groups (aryl aldehyde and pyridylhydrazine respectively), essentially creating a “dendrimer of dendrimers” and resulting in a dramatic amplification of signal. Preliminary 1HNMR experiments showed that dendrons could be assembled in solution (Figure S4, Supporting Information), and fluorescence microscopy indicated that assembly could be accomplished on solid supports (Figure 3).
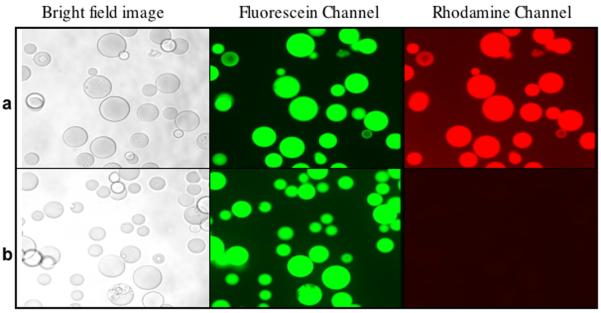
Figure 3.
(a) Compound 1 was linked covalently via the thiol to Sulfolink Coupling Resin (porous 6% crosslinked beaded agarose that has been activated with iodoacetyl groups). After washing, the beads are incubated with 3. (b) Cystamine core dendrimer, containing FITC and devoid of any aldehyde moieties (Cyscore(FITC)8), covalently linked to the resin. After washing, the beads are incubated with 3. The binding of 4 only occurred when both complementary bio-orthogonal functional groups were present.
In order to test the self-assembly strategy on clinically relevant carcinomas, the targeting dendrimer contained a humanized anti-BrE3 monoclonal antibody (HuBrE3) (2) as the targeting group, and HuBrE3 directly labeled with FITC (HuBrE3(FITC)4) was used as negative control. The BrE3 antibody targets the MUC1 transmembrane receptor, found in over 90% of pancreatic cancers.15 Human PANC-1 cells were cultured in a 96-well plate and grown to 50% confluence. Fixed cells were washed twice with PBS buffer, and incubated with 2, HuBrE3(FITC)4 or the cargo dendrimer, 3, at 1.0×10−7 M for 5 minutes at 37 °C. After washing, the cells were visualized on a Leica fluorescence microscope via the appropriate fluorescein and rhodamine channels. Binding of HuBrE3 was confirmed in the fluorescein channel, while cells incubated with 3 showed no green or red fluorescence. The washed cells that had been exposed to 2 or HuBrE3(FITC)4 were subsequently incubated with 3 at 1.0×10−7 M for 1 minute at 37 °C. After washing to remove any unbound dendrimer, the cells exposed to both 2 and 3 showed both red and green fluorescence, as seen in Figure 4. Qualitative inspection of the corresponding images suggested a dramatic increase in the amount of red vs. green color. While the optical properties (extinction coefficients, quantum yields) of the chosen dyes are not identical, the much greater intensity of red color suggests the presence of a greater amount of 3 over 2. This observation suggests the presence of multiple copies of aldehyde moieties on 2, leading to amplified assembly of 3.
Figure 4.
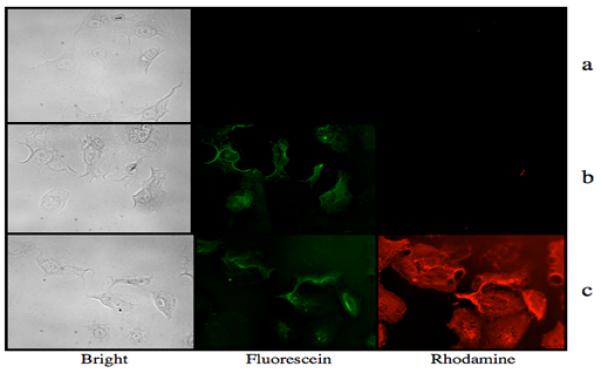
Bright field, fluorescein filtered, and rhodamine filtered images of human PANC1 cells incubated with (a) 3 only, 1.0 × 10−7 M, (b) HuBrE3(FITC)4, 1.0 × 10−7 M, washed to remove excess compound, and then incubated with 3 and (c) 2, 1.0 × 10−7 M, washed to remove excess, unbound dendrimer, and then incubated with 3 at 1.0 × 10 7 M and washed.
To confirm the amplification observed by microscopy, similarly treated live cells were analyzed by flow cytometry. Live PANC-1 cells incubated with 2 or 3 at 1.0×10−7 M for 1h at 37 °C with gentle shaking were washed with PBS. Cells exposed to 2 were re-suspended in 1x PBS or 1 mL of 3 at 1.0×10−7 M and then incubated for 1h at 37 °C with gentle shaking and washed to remove unbound dendrimer.
The large increase in mean fluorescence intensity (rhodamine channel, Figure 5) between cells treated with both 2 and 3, when compared to 3 alone, correlates with the increase in red color observed by microscopy. Despite the relatively small level of labeling by 3 alone, the directed self-assembly with 2 and 3 occurred 13±6 times more efficiently. Live cells are known to spontaneously internalize cationic dendrimers16 and we attribute the relatively low level of fluorescence reported upon incubation of 3 only to this non-specific uptake. Comparison of experimental results obtained on different days, show slight variations in the degree of labeling by 2.
Figure 5.
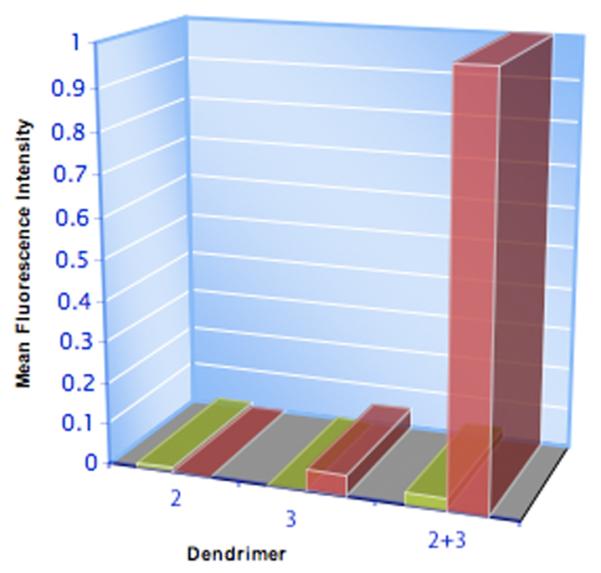
Normalized fluorescence intensities of PANC1 cells exposed to either 2, 3 or 2 and 3.
This amplification strategy has the following advantages: (i) The system is modular and flexible; (ii) the self-assembly strategy allows for use of relatively small dendrimers; (iii) amplification of targets is achieved upon successful delivery of the primary targeted dendrimer. In principle, delivery of just one targeted dendrimer, 2 (each containing an average of 6 aldehydes per dendron as indicated in Figure 2), can result in an 6 fold increase in potential targets for 3. We observed an order of magnitude increase in delivery of 3. This is only a fraction of the maximum possible amplification: A PAMAM G4 cys core targeting dendron could display up to 32 aldehydes if solubility would permit. The PAMAM G4 dendrimer with five pyridylhydrazine units could carry up to 59 cargo units. Practical issues like solubility and reactivity of more heavily labeled dendrimer may limit loading, but it is clear that significant amplification is available by this strategy (up to 1888 per Ab conjugation, and multiple conjugations are also possible). The complimentary reactive sites on each dendrimer are bio-orthogonal and advantageous in several ways. First, these units are far smaller in size and molecular weight when compared to previously reported pre-targeting strategies including biotin/streptavidin or complementary DNA analog noncovalent assembly.17 Second, the functional groups are known to be reactive with each other but unreactive with functional groups present in a physiological environment (i.e., “bio-orthogonal”).18 Finally, although the resultant pyridylhydrazine benzaldehyde imine linkage is covalent and stable, it is nevertheless still reversible with an equilibrium constant of 104,13a and will allow for eventual disassembly and clearance.
Figure 2.
Compounds used in this study. Functionalized targeted G4 Dendrimer containing (1) no targeting group (negative control), (2) humanized anti-BrE3 antibody as the targeting moiety.
We chose to use rhodamine as the cargo molecule as it is a readily visualizable model for organic drugs. Given the flexibility of the amplification system, any number of cargo can be substituted for rhodamine, or simply conjugated to the remaining unmodified amines, present on 3. We have generated data that indicate that the self-assembly scheme works with a marked amplification in detection of the clinically relevant carcinoma human PANC-1 cells. Given the expression of this epitope in breast and ovarian cancer,15 we expect these findings can be applied to the potential early detection of these three carcinomas after the generation of in vivo studies.
Supplementary Material
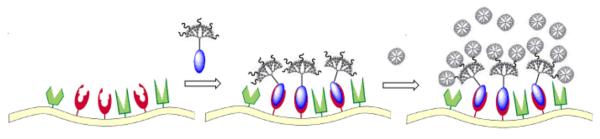
Figure 1.
Schematic showing amplified, two-step delivery to targeted cell surface receptors.
Acknowledgments
We are grateful to Peter Brooks (Maine Medical Research Institute) for helpful discussions. We thank the NSF (ODISSEI-1332411), the NYU Cancer Institute, the NYU Department of Radiology, and the American Society for Neuroradiology for financial support.
Footnotes
Supplementary data
Experimental procedures, structural proofs, and spectral data.
References
- 1.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Proc. Natl. Acad. Sci. USA. 2004;101:17867. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson EA, Isaacman S, Peabody DS, Wang EY, Canary JW, Kirshenbaum K. Nano Lett. 2006;6:1160. doi: 10.1021/nl060378g. [DOI] [PubMed] [Google Scholar]
- 3.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan RJ. Controlled Release. 2000;65:133. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 4 (a).Rideout D. Cancer Invest. 1994;12:189. doi: 10.3109/07357909409024874. [DOI] [PubMed] [Google Scholar]; (b) Rideout D, Calogeropoulou T, Jaworski J, McCarthy M. Biopolymers. 1990;29:247. doi: 10.1002/bip.360290129. [DOI] [PubMed] [Google Scholar]
- 5 (a).He J, Liu G, Gupta S, Zhang Y, Rusckowski M, Hnatowich DJ. J. Nucl. Med. 2004;45:1087. [PubMed] [Google Scholar]; (b) Sharkey RM, Cardillo TM, Rossi EA, Chang C-H, Karacay H, McBride WJ, Hansen HJ, Horak ID, Goldenberg DM. Nat. Med. 2005;11:1250. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 6.Burne MJ, Osicka TM, Comper WD. Kidney Int. 1999;55:261. doi: 10.1046/j.1523-1755.1999.00234.x. [DOI] [PubMed] [Google Scholar]
- 7 (a).Devaraj NK, Weissleder R, Hilderbrand SA. Bioconjugate Chem. 2008;19:2297. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Angew. Chem. Int. Ed. 2009;48:7013. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu T, Nedrow-Byers JR, Hopkins MR, Wu LY, Lee J, Reilly PTA, Berkman CE. Bioorg. Med. Chem. Lett. 2012;22:3931. doi: 10.1016/j.bmcl.2012.04.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aime S, Botta M, Terreno E. Adv. Inorg. Chem. 2005;57:173. [Google Scholar]
- 9 (a).Kobayashi H, Kawamoto S, Jo S-K, Bryant HL, Brechbiel MW, Star RA. Bioconjugate Chem. 2003;14:388. doi: 10.1021/bc025633c. [DOI] [PubMed] [Google Scholar]; (b) Wiener E, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA, Lauterbur PC. Magn. Reson. Med. 1994;31:1. doi: 10.1002/mrm.1910310102. [DOI] [PubMed] [Google Scholar]; (c) Patri AK, Kukowska-Latallo JF, Baker JR., Jr Adv. Drug Del. Rev. 2005;57:2203. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]; (d) Rudovský J, Botta M, Hermann P, Hardcastle KI, Lukeš I, Aime S. Bioconjugate Chem. 2006;17:975. doi: 10.1021/bc060149l. [DOI] [PubMed] [Google Scholar]; (e) Tomalia DA, Huang B, Swanson DR, Brothers HM, Ii, Klimash JW. Tetrahedron. 2003;59:3799. [Google Scholar]
- 10.Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D’Emanuele A. Int. J. Pharm. 2003;252:263. doi: 10.1016/s0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 11.Svenson S, Tomalia DA. Adv. Drug Del. Rev. 2005;57:2106. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 12 (a).Percec V, Imam MR, Peterca M, Cho W-D, Heiney PA. Isr. J. Chem. 2009;49:55. [Google Scholar]; (b) Rudick JG, Percec V. Acc. Chem. Res. 2008;41:1641. doi: 10.1021/ar800086w. [DOI] [PubMed] [Google Scholar]
- 13 (a).Dirksen A, Dawson PE. Bioconjugate Chem. 2008;19:2543. doi: 10.1021/bc800310p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Egli S, Nussbaumer MG, Balasubramanian V, Chami M, Bruns N, Palivan C, Meier W. J. Am. Chem. Soc. 2011;133:4476. doi: 10.1021/ja110275f. [DOI] [PubMed] [Google Scholar]; (c) Wang X, Canary JW. Bioconjugate Chem. 2012;23:2329. doi: 10.1021/bc300430k. [DOI] [PubMed] [Google Scholar]; (d) Achilles K, Kiedrowski G. v. Bioorg. Med. Chem. Lett. 2005;15:1229. doi: 10.1016/j.bmcl.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 14.Sander EG, Jencks WP. J. Am. Chem. Soc. 1968;90:6154. doi: 10.1021/ja01016a040. [DOI] [PubMed] [Google Scholar]
- 15.Ng B, Kramer E, Liebes L, Wasserheit C, Hochster H, Blank E, Ceriani R, Furmanski P. Cancer Res. 2001;61:2996. [PubMed] [Google Scholar]
- 16.Albertazzi L, Serresi M, Albanese A, Beltram F. Mol. Pharm. 2010;7:680. doi: 10.1021/mp9002464. [DOI] [PubMed] [Google Scholar]
- 17.He J, Rusckowski M, Wang Y, Dou S, Liu X, Zhang S, Liu G, Hnatowich D. Mol. Imag. Biol. 2007;9:17. doi: 10.1007/s11307-006-0071-2. [DOI] [PubMed] [Google Scholar]
- 18 (a).Saxon E, Bertozzi CR. Science. 2000;287:2007. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]; (b) Zeng Y, Ramya TNC, Dirksen A, Dawson PE, Paulson JC. Nat. Methods. 2009;6:207. doi: 10.1038/nmeth.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.