Abstract
Aim: The efficacy of statin therapy in inducing coronary plaque regression may depend on baseline cholesterol levels. We aimed to determine the efficacy of statin therapy in inducing coronary plaque regression in statin-naïve patients with low cholesterol levels using serial intravascular ultrasound (IVUS) data from the treatment with statin on atheroma regression evaluated by virtual histology IVUS (TRUTH) study.
Methods: The TRUTH study is a prospective, multicenter trial, comparing the efficacies of pitavastatin and pravastatin in coronary plaque regression in 164 patients. All patients were statin-naïve and received statin therapy only after study enrollment. The primary endpoint was the observation of coronary plaque progression, despite statin therapy.
Results: Serial IVUS data, at baseline and after an 8-month follow-up, were available for 119 patients. The patients were divided into three groups based on non-high-density lipoprotein cholesterol (HDL-C) levels—low: ≤ 140 mg/dl, n = 38; moderate: 141–169 mg/dl, n = 42; and high: ≥ 170 mg/dl, n = 39. Coronary plaque progression was noted in the low cholesterol group, whereas plaque regression was noted in the moderate and high cholesterol groups [%Δplaque volume: 2.3 ± 7.4 vs. − 2.7 ± 10.7 vs. − 3.2 ± 7.5, p = 0.004 (analysis of variance)]. After adjusting for all variables, a low non-HDLC level (≤ 140 mg/dl) was identified as an independent predictor of coronary plaque progression [odds ratio, 3.7; 95% confidence interval, 1.5–9.1, p = 0.004].
Conclusion: Serial IVUS data analysis indicated that statin therapy was less effective in inducing coronary plaque regression in patients with low cholesterol levels but more effective in those with high cholesterol levels at baseline.
University Hospital Medical Information Network (UMIN) (UMIN ID: C000000311).
Keywords: Statin, Coronary plaque regression, Baseline cholesterol level
See editorial vol. 23: 1030–1032
Introduction
Statin therapy reduces the levels of atherogenic lipoproteins and risk of cardiovascular events in patients with coronary artery disease (CAD)1–4). Previous studies using intravascular ultrasound (IVUS) demonstrated that statin therapy prevents plaque progression or reduces plaque volume in patients with CAD5–8). Hence, statin therapy has been considered as the gold standard method for treating patients with CAD. Pivotal trials showed that statin therapy reduced the cardiovascular risk by 25%–50%1), but it did not prevent the majority of cardiovascular events. Therefore, the presence of a residual risk of cardiovascular events under statin therapy has been a matter of great concern for the last 10 years.
One concern regarding the use of statin therapy is its efficacy in patients with low cholesterol levels at baseline. It remains unknown whether statin therapy induces coronary plaque regression in patients with low baseline cholesterol levels. It is well known that a reduction in cholesterol level in patients receiving statin therapy depends on the baseline cholesterol level, and only a small reduction in cholesterol levels occurs after statin therapy in patients with low baseline cholesterol levels. Previous IVUS studies indicated that the effect of statin therapy in inducing coronary plaque regression depends on the reductions in cholesterol levels6–9). Based on these observations, along with the well-accepted fact that cholesterol level reduction by statin therapy depends on baseline cholesterol levels, we hypothesized that statin therapy would be less effective in inducing coronary plaque regression in patients with low baseline cholesterol levels but more effective in those with high baseline cholesterol levels.
Aim
We aimed to determine the efficacy of statin therapy in inducing coronary plaque regression in statin-naïve patients according to the baseline cholesterol level using serial IVUS data from the treatment with statin on atheroma regression evaluated by virtual histology (VH) - IVUS (TRUTH) study.
Methods
Protocol Design
The TRUTH trial is a prospective, open-labeled, randomized, multicenter trial conducted at 11 Japanese centers that compared the effects of pitavastatin versus pravastatin treatment for 8 months on coronary artery plaque composition using VH-IVUS10). In brief, 164 statin-naïve patients with stable or unstable angina pectoris were randomized to receive either pitavastatin (4 mg/d, intensive lipid lowering) or pravastatin (20 mg/d, moderate lipid lowering) therapy after successful percutaneous coronary intervention (PCI) under IVUS guidance. None of the patients were receiving statin or other lipid-lowering drugs before study enrollment, and they received the statin therapy over 8 months. All patients were scheduled to undergo repeat IVUS at the 8-month follow-up period. Among the 164 patients, three withdrew consent and seven were lost to follow-up. Moreover, seven patients experienced adverse events during the 8-month treatment period, whereas 28 did not undergo follow-up IVUS or had inappropriate IVUS data. Thus, serial IVUS data, at baseline and at the 8-month follow-up period, were available for 119 patients. All patients provided written informed consent. The trial was reviewed and approved by the institutional review committee of the respective institutions, and the study complied with the guidelines of the Declaration of Helsinki. Further details of the trial study designs and clinical results have been previously published10). The study was registered with the University Hospital Medical Information Network Clinical Trials Registry (ID: C000000311).
The present study is a subanalysis of the TRUTH study. In our study, we aimed to determine the efficacy of statin therapy in inducing coronary plaque regression in patients with low cholesterol levels at baseline. As non-high-density lipoprotein cholesterol (HDL-C) is a more reliable maker than low-density lipoprotein cholesterol (LDL-C) for assessing the data, the former was used in this study11–14). The patients were divided into three groups based on non-HDL-C levels (low: ≤ 140 mg/dl, n = 38; moderate: 140–169 mg/dl, n = 42; and high: ≥ 170 mg/dl, n = 39) to assess the gradual difference in coronary plaque volume change. The primary endpoint of our study was the observation of coronary plaque progression despite the use of statin therapy.
Patient Enrollment and Management
Patients who had a lesion that was either distal or proximal to the PCI site were enrolled in the trial if the lesion fulfilled the following inclusion criteria: the target segment of interest needed to have a plaque burden of ≥ 50% and a minimum length of 10 mm, based on IVUS data. The exclusion criteria were as follows: acute myocardial infarction or unstable angina of class IIIB Braunwald Unstable Angina Classification; emergent PCI; angiographically apparent thrombi; established treatment with statin or other lipid-lowering drugs such as fibrate, ezetimibe, nicotinic acid, cholestyramine, and probucol; bypass graft at the PCI site; previous PCI lesion at the planned IVUS evaluation site; angular span of the acoustic shadow of calcification or attenuation by non-calcified tissues of > 90°; failed PCI or cardiogenic shock; or ineligibility for the study as per the investigator.
The initial doses of pitavastatin and pravastatin were 2 mg/d and 10 mg/d, respectively, for the first 1–2 weeks. After safety evaluations were performed based on laboratory tests and patient history, the doses were increased to 4 mg/d for pitavastatin and 20 mg/d for pravastatin. Blood examinations were performed before treatment and after 8 months of statin therapy. An independent event assessment committee evaluated the occurrence of major adverse cardiovascular events such as death, myocardial infarction, and target lesion revascularization.
IVUS Imaging and Analysis
After PCI of the culprit lesion, IVUS examination was performed for the non-culprit lesion, including angiographic lesions with lumen narrowing of < 50% on both the distal and proximal sides of the culprit lesion. IVUS imaging was performed after the intracoronary administration of nitroglycerin using a motorized pullback system (0.5 mm/s), as well as with contemporary, commercial scanners with a 20-MHz, 2.9-F phase-array IVUS catheter (Eagle Eye Gold; Volcano Co.). IVUS data were acquired from the distal segment to the ostium for a safe duration.
After 8 months of statin therapy, IVUS examinations were repeated in the same coronary artery under conditions identical to the pretreatment conditions. The IVUS gray-scale images were copied to a digital media CD-R and DVD and subsequently sent for analysis to the Toyohashi Heart Center Core Laboratory (Toyohashi, Japan), which was blinded to the treatment arm. All baseline and follow-up analyses were performed by an independent and experienced investigator (M. T.) at the core laboratory. Details of the IVUS analysis protocol have been previously published10). Both the distal and proximal ends of the target segment were identified based on the presence of reproducible anatomic landmarks such as the side branch, vein, and stent edge15, 16). The plaques adjacent to the PCI site—i.e., within 5 mm—were excluded. Using IVUS Lab software (version 2.2; Volcano Co.), the external elastic membrane (EEM), plaque and media, and lumen cross-sectional areas were measured at 1-mm intervals from the distal to proximal side of the lesion. A volumetric index (mm3/mm) was calculated using Simpson's rule for subsegments after deleting frames containing a significant side branch (> 2 mm) or significant calcification that precluded EEM measurement. This volumetric index was used to assess EEM, plaque and media, and lumen volumes. Percent atheroma volume was calculated using general methods" ref-type="bibr">and lumen volumes. Percent atheroma volume was calculated using general methods5). VH-IVUS data analysis was based on grayscale border contour calculation, and relative and absolute amounts of different coronary artery plaque components were measured using IVUS Lab version 2.2 (Volcano Co.).
The investigators performing all IVUS measurements were blinded to information on randomization, risk factors, and procedure characteristics. Quantitative IVUS analysis was performed according to the guidelines of the American College of Cardiology Clinical Expert Consensus Document on Standard for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies17). The accuracy of IVUS measurement was verified by intraobserver analysis, as previously reported10).
Statistical Analysis
Categorical variables are presented as frequencies and percentages and were compared between groups using the chi-square or Fisher exact test, as appropriate. Continuous variables are expressed as mean ± standard deviation and were compared between the groups using 2-tailed, unpaired t tests; in case of non-normal distribution of parameters, the variables are expressed as median [interquartile range (IQR)] and were compared using the Mann – Whitney test. Analysis of variance or Kruskal – Wallis test was used for comparisons among the three groups, as appropriate. Continuous variables were compared between the post-procedure and follow-up stages using 2-tailed, paired t tests; in case of non-normal distribution of parameters, the Wilcoxon test was used. Correlations were analyzed using Pearson or Spearman correlation coefficient, as appropriate. Univariate and multivariate logistic regression analyses were performed to identify independent predictors of coronary plaque progression. Variables with p < 0.1 on univariate analysis were entered into the multiple logistic regression analysis model to adjust for baseline differences. Multiple linear regression analysis was also performed to identify factors correlated with coronary plaque variation. All variables were entered into the model in their original form, without transformation. Statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL). Differences were considered to be statistically significant when p < 0.05.
Results
Baseline Characteristics
The patients in the low cholesterol group (≤ 140 mg/dl) were older and had a lower body mass index (BMI) as compared to those in the high cholesterol group (≥ 170 mg/dl; p = 0.00 2 and 0.006, respectively; Table 1). Other coronary risk factors, including unstable or stable angina, angiographic characteristics, and medications at admission, were similar among the three groups.
Table 1. Baseline Clinical Characteristics.
| Variable | Non-HDL-Cholesterol (mg/dl) |
|||
|---|---|---|---|---|
| ≦ 140 n = 38 | 141–169 n = 42 | ≧ 170 n = 39 | p value | |
| Age years | 70.3 ± 8.8 | 67.0 ± 8.9 | 62.9 ± 11.6 | 0.006 |
| Men | 33 (86.8%) | 33 (78.6%) | 33 (84.6%) | 0.62 |
| Body mass index (kg/mm2) | 23.5 ± 2.8 | 23.9 ± 2.8 | 25.9 ± 4.3 | 0.004 |
| Diabetes Mellitus | 14 (36.8%) | 15 (35.7%) | 21 (53.8%) | 0.19 |
| Hypertension | 26 (68.4%) | 25 (59.5%) | 24 (61.5%) | 0.69 |
| Treatment allocation | ||||
| Pitavastatin | 21 (55.3%) | 19 (45.2%) | 18 (46.2%) | 0.62 |
| Pravastatin | 17 (44.7%) | 23 (54.8%) | 21 (53.8%) | |
| Stable angina pectoris | 30 (78.9%) | 31 (73.8%) | 22 (56.4%) | 0.077 |
| Unstable angina pectoris | 8 (21.1%) | 11 (26.2%) | 17 (43.6%) | |
| Target vessel | ||||
| Left anterior descending | 23 (60.5%) | 22 (52.4%) | 22 (56.4%) | 0.72 |
| Left circumflex | 1 (2.6%) | 1 (2.4%) | 3 (7.7%) | |
| Right | 14 (36.8%) | 19 (45.2%) | 14 (35.9%) | |
| Type of stent | ||||
| Bare metal stent | 5 (13.2%) | 10 (23.8%) | 5 (12.8%) | 0.34 |
| Drug-eluting stent | 33 (86.8%) | 32 (76.2%) | 34 (87.2%) | |
| Medications | ||||
| Aspirin | 37 (97.4%) | 41 (97.6%) | 39 (100%) | 0.77 |
| Thienopyridine | 37 (97.4%) | 41 (97.6%) | 38 (97.4%) | 0.99 |
| ACE-I or ARB | 23 (60.5%) | 18 (42.9%) | 20 (51.3%) | 0.29 |
| β-blocker | 5 (13.2%) | 2 (4.8%) | 6 (15.4%) | 0.25 |
| Calcium channel blocker | 23 (60.5%) | 20 (47.6%) | 17 (43.6%) | 0.30 |
| Follow-up duration (days) | 236 ± 37 | 221 ± 35 | 225 ± 37 | 0.15 |
Values are means ± SDs or numbers (percentages); HDL, high-density lipoprotein; ACE-I indicates, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker
Biomarker Levels at Baseline and Follow-Up
Non-HDL-C levels significantly decreased in the three groups over the 8-month period (Table 2). The variation in non-HDL-C levels was significantly lower in the low cholesterol group than that in the high cholesterol group (Δ: − 35 ± 19 vs. − 69 ± 26, p < 0.001). Moreover, the %Δnon-HDL-C values were significantly lower in the low cholesterol group than in the high cholesterol group (− 28% ± 15% vs. − 35% ± 11%, p = 0.028). The non-HDL-C level at baseline was significantly correlated with the variation in non-HDL-C levels (r = −0.58, p < 0.001). Similar results were observed for LDL-C levels (Δ: − 33 ± 19 vs. − 62 ± 22, p < 0.001). The triglyceride levels at baseline were significantly higher in the high cholesterol group than those in the low cholesterol group (p < 0.001); however, the variation in triglyceride levels was similar between the two groups (p = 0.40). HDL-C levels at baseline were similar between the low and high cholesterol groups (p = 0.13). HDL-C levels increased during the 8-month treatment period in the two groups (p = 0.001 and 0.04, respectively). The variation in HDL-C levels was similar between the two groups (p = 0.46).
Table 2. Risk factor control at baseline and follow-up.
| Variable | Non-HDL cholesterol (mg/dl) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≦140 (n = 38) |
141–169 (n = 42) |
≧170 (n = 39) |
|||||||
| Baseline | 8-month | p value | Baseline | 8-month | p value | Baseline | 8-month | p value | |
| Non-HDL cholesterol | 122 ± 14 | 87 ± 19 | < 0.001 | 155 ± 8 | 108 ± 23 | < 0.001 | 197 ± 29 | 127 ± 24 | < 0.001 |
| (mg/dl) | Δ | − 35 ± 19 | − 48 ± 23 | − 69 ± 26 | |||||
| LDL cholesterol | 100 ± 15 | 67 ± 18 | < 0.001 | 132 ± 13 | 87 ± 21 | < 0.001 | 162 ± 28 | 100 ± 24 | < 0.001 |
| (mg/dl) | Δ | − 33 ± 19 | − 45 ± 21 | − 62 ± 22 | |||||
| Triglycerides (mg/dl) | 98 ± 30 | 89 ± 32 | 0.12 | 122 ± 51 | 109 ± 51 | 0.18 | 174 ± 79 | 151 ± 81 | 0.15 |
| Δ | − 8 ± 34 | − 12 ± 58 | − 23 ± 99 | ||||||
| HDL cholesterol | 48 ± 12 | 54 ± 14 | 0.001 | 47 ± 12 | 51 ± 13 | 0.06 | 45 ± 10 | 48 ± 10 | 0.04 |
| (mg/dl) | Δ | 5 ± 9 | 4 ± 13 | 4 ± 11 | |||||
| Apo A-I (mg/dl) | 119 ± 22 | 133 ± 27 | < 0.001 | 118 ± 19 | 134 ± 26 | < 0.001 | 117 ± 19 | 129 ± 22 | 0.002 |
| Δ | 15 ± 18 | 16 ± 23 | 12 ± 21 | ||||||
| Apo B (mg/dl) | 80 ± 10 | 60 ± 13 | < 0.001 | 103 ± 8 | 75 ± 15 | < 0.001 | 128 ± 16 | 85 ± 15 | < 0.001 |
| Δ | − 19 ± 14 | − 28 ± 14 | − 43 ± 15 | ||||||
| hs-CRP (µg/ml) | 2.4 [0.8, 7.0] | 0.6 [0.3, 2.4] | 0.007 | 3.9 [0.8, 7.6] | 0.4 [0.2, 1.3] | < 0.001 | 5.7 [2.4, 12.3] | 0.8 [0.4, 2.1] | < 0.001 |
| Δ | − 0.8 [− 4.5, − 0.1] | − 2.7 [− 6.8, − 0.5] | − 3.1 [− 8.3, − 1.9] | ||||||
Values are mean ± SDs or median [interquartile range]; HDL, high-density lipoprotein; LDL, low-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein
Moreover, high-sensitivity C-reactive protein (hs-CRP) levels significantly decreased in the three groups over the 8-month treatment period. The variation in hs-CRP levels was significantly lower in the low cholesterol group than that in the high cholesterol group (p = 0.014).
IVUS Measurements at Baseline and the 8-Month Follow-Up Period
The IVUS measurements before statin therapy and after the 8-month follow-up period are shown in Table 3. The volume of the vessel, lumen, and plaque at baseline was similar among the three groups. Negative remodeling of the vessel was observed in the moderate and high cholesterol groups, whereas the vessel volume remained unchanged in the low cholesterol group. The lumen volume was similar between the baseline and 8-month follow-up period in the moderate and high cholesterol groups (p = 0.73 and 0.74, respectively); however, a significant loss in the lumen volume was observed in the low cholesterol group (p = 0.006). The variation in the absolute lumen volume did not significantly differ (p = 0.20), although the %Δlumen volume tended to be different among the three groups (p = 0.14; Fig. 1). The absolute coronary plaque volume at baseline was similar among the three groups (p = 0.81). The coronary plaque volume increased in the low cholesterol group (p = 0.083), whereas it decreased in the moderate and high cholesterol groups (p = 0.079 and 0.008, respectively). The variation in absolute coronary plaque volume was significantly different among the three groups (p = 0.007). The %Δcoronary plaque volume was also significantly different among the groups; in particular, it was higher in the low cholesterol group and lower in the moderate and high cholesterol groups (p = 0.004; Fig. 1).
Table 3. IVUS measurements at baseline and at 8-month follow-up.
| Lesion | Non-HDL cholesterol (mg/dl) |
|||
|---|---|---|---|---|
| ≦ 140 (n = 38) | 141-169 (n = 42) | ≧ 170 (n = 39) | p value* | |
| Vessel VI, mm3/mm | ||||
| Baseline | 16.3 ± 5.5 | 16.8 ± 5.6 | 15.9 ± 4.6 | 0.88 |
| 8-month follow-up | 16.1 ± 5.3 | 16.5 ± 5.7 | 15.6 ± 4.7 | 0.87 |
| Δ | − 0.19 ± 0.79 | − 0.24 ± 1.15 | − 0.33 ± 0.89 | 0.71 |
| p value# | 0.21 | 0.042 | 0.076 | |
| Lumen VI, mm3/mm | ||||
| Baseline | 7.5 ± 2.9 | 7.5 ± 2.5 | 7.2 ± 2.3 | 0.80 |
| 8-month follow-up | 7.2 ± 2.8 | 7.6 ± 2.7 | 7.1 ± 2.4 | 0.76 |
| Δ | − 0.32 ± 0.75 | 0.06 ± 0.89 | − 0.045 ± 0.85 | 0.20 |
| p value# | 0.006 | 0.73 | 0.74 | |
| Plaque VI, mm3/mm | ||||
| Baseline | 8.8 ± 3.2 | 9.3 ± 3.6 | 8.7 ± 3.0 | 0.81 |
| 8-month follow-up | 8.9 ± 3.1 | 9.0 ± 3.4 | 8.4 ± 3.0 | 0.74 |
| Δ | 0.13 ± 0.67 | − 0.30 ± 1.03 | − 0.28 ± 0.62 | 0.007 |
| p value# | 0.083 | 0.079 | 0.008 | |
| PAV | ||||
| Baseline | 54.2 ± 7.8 | 54.9 ± 6.6 | 54.6 ± 6.7 | 0.9 |
| 8-month follow-up | 55.7 ± 7.4 | 54.0 ± 6.0 | 53.9 ± 7.6 | 0.56 |
| Δ | 1.5 ± 3.5 | − 0.85 ± 4.6 | − 0.65 ± 3.8 | 0.011 |
| p value# | 0.010 | 0.24 | 0.29 | |
| Plaque progression | 25 (65.8%) | 14 (33.3%) | 12 (30.8%) | 0.002** |
Values are means ± SDs;
Analysis of variance or Kruskal-Wallis test as appropriate
Paired t-test or Wilcoxon test as appropriate
Chi-square test; HDL, high-density lipoprotein; VI, volume index; PAV, Percent atheroma volume
Fig 1.
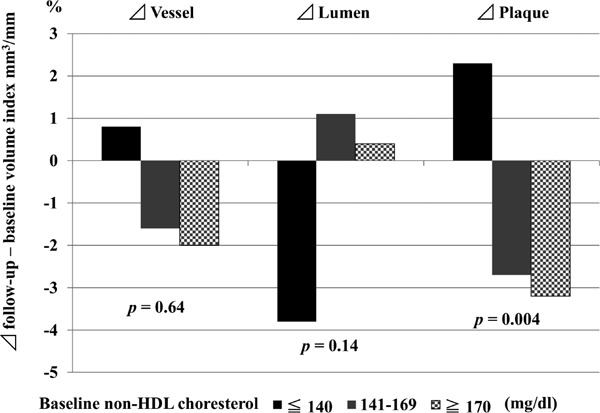
Comparison of %Δ of vessel, lumen, and plaque volume among three groups
The volume of the vessel, lumen, and plaque at baseline were similar among the three groups. A small change in the vessel wall was observed after 8 months of statin therapy. Negative remodeling of the vessel was observed in the moderate and high cholesterol groups. In contrast, positive remodeling of the vessel was noted in the low cholesterol group. Lumen enlargement was detected in the moderate and high cholesterol groups, whereas lumen loss was observed in the low cholesterol group; however, these differences were not significant among the groups (p = 0.14). The coronary plaque volume increased in the low cholesterol group and decreased in the moderate and high cholesterol groups (p = 0.004).
Further, %Δcoronary plaque was not different between patients treated with pitavastatin and those treated with pravastatin in each baseline non-HDL-C level (≦ 140, 0.010 ± 0.05 vs. 0.040 ± 0.009, p = 0.25; 141–169, − 0.031 ± 0.10 vs. − 0.023 ± 0.12, p = 0.80; ≧ 170, − 0.041 ± 0.05 vs. − 0.025 ± 0.09, p = 0.51, respectively).
Percent atheroma volume decreased in the moderate and high cholesterol groups; however, it increased in the low cholesterol group (Table 3); %Δatheroma volume was significantly different among the three groups (p = 0.011).
In total, 51 patients (42.9%) had coronary plaque progression (primary endpoint), whereas 68 (57.1%) had coronary plaque regression after 8 months of statin therapy. Coronary plaque progression was most frequently observed in the low cholesterol group (Table 3).
VH-IVUS Assessment
VH-IVUS measurements at baseline and 8 months are shown in Table 4. The absolute amounts of each plaque component—dense calcium, necrotic-core, fibrous tissue, fibro-fatty tissue—were similar at baseline. Further, %Δfibrous component was significantly higher in low cholesterol group than in the moderate and high cholesterol groups (p = 0.021 and 0.001, respectively). %Δ of other components were similar among the three groups (dense calcium, p = 0.65; necrotic-core, p = 0.46; fibro-fatty, p = 0.55).
Table 4. VH-IVUS measurements at baseline and at 8-month follow-up.
| Lesion | Non-HDL cholesterol (mg/dl) |
|||
|---|---|---|---|---|
| ≦ 140 (n = 38) | 141–169 (n = 42) | ≧ 170 (n = 39) | p value* | |
| DC (VI, mm3/mm) | ||||
| Baseline | 0.51 ± 0.56 | 0.45 ± 0.34 | 0.33 ± 0.29 | 0.18 |
| 8-month follow-up | 0.67 ± 0.62 | 0.56 ± 0.52 | 0.41 ± 0.27 | 0.18 |
| Δ | 0.16 ± 0.31 | 0.11 ± 0.36 | 0.08 ± 0.22 | 0.55 |
| %Δ | 81.1 ± 144 | 62.7 ± 131 | 81.9 ± 145 | 0.65 |
| p value# | 0.004 | 0.061 | 0.024 | |
| NC (VI, mm3/mm) | ||||
| Baseline | 0.83 ± 0.60 | 0.83 ± 0.60 | 0.57 ± 0.42 | 0.062 |
| 8-month follow-up | 0.93 ± 0.65 | 0.90 ± 0.60 | 0.79 ± 0.50 | 0.67 |
| Δ | 0.10 ± 0.52 | 0.07 ± 0060 | 0.22 ± 0.54 | 0.47 |
| %Δ | 42.1 ± 98 | 45.5 ± 104 | 279 ± 173 | 0.46 |
| p value# | 0.25 | 0.47 | 0.014 | |
| FI (VI, mm3/mm) | ||||
| Baseline | 3.1 ± 1.6 | 3.5 ± 2.0 | 3.3 ± 1.9 | 0.66 |
| 8-month follow-up | 3.2 ± 1.5 | 3.2 ± 1.8 | 2.9 ± 1.7 | 0.45 |
| Δ | 0.13 ± 0.85 | − 0.24 ± 0.79 | − 0.3 ± 0.73 | 0.004 |
| %Δ | 9.4 ± 26.7 | − 2.7 ± 33.5 | − 7.6 ± 26.6 | 0.003 |
| p value# | 0.37 | 0.054 | 0.005 | |
| FF (VI, mm3/mm) | ||||
| Baseline | 0.98 ± 0.66 | 1.1 ± 1.2 | 1.1 ± 0.84 | 0.54 |
| 8-month follow-up | 0.72 ± 0.53 | 0.87 ± 0.84 | 0.86 ± 0.80 | 0.99 |
| Δ | − 0.26 ± 0.53 | − 0.22 ± 0.79 | − 0.27 ± 0.77 | 0.61 |
| %Δ | − 8.7 ± 62.3 | 5.6 ± 87.2 | 11.5 ± 189 | 0.55 |
| p value# | 0.004 | 0.074 | 0.037 | |
Values are means ± SDs;
Analysis of variance or Kruskal-Wallis test as appropriate
Paired t-test or Wilcoxon test as appropriate; VH-IVUS, virtual histology intravascular ultrasound; DC, dense-calcium; NC, necrotic-core; FI, fibrous; FF, fibro-fatty; HDL, high-density lipoprotein; VI, volume index; PAV, Percent atheroma volume
Correlation between Non-HDL-C Levels at Baseline and %Δcoronary Plaque Volume
A negative relationship was observed between non-HDL-C levels at baseline and %Δcoronary plaque volume (r = − 0.25, p = 0.006) and also between LDL-C levels at baseline and %Δcoronary plaque volume (r = − 0.23, p = 0.013). However, Δnon-HDL-C was not correlated with %Δcoronary plaque volume (r = 0.093, p = 0.32).
Baseline HDL-C levels, 8-month HDL-C levels, and ΔHDL-C were not correlated with %Δcoronary plaque (r = 0.029, p = 0.76; r = − 0.005, p = 0.96; r = 0.14, p = 0.13, respectively).
Predictors of Coronary Plaque Progression on Multivariate Analysis
Table 5 shows the predictors of coronary plaque progression, identified via univariate and multivariate logistic regression analyses. After adjusting for all variables, low baseline non-HDL-C levels (≤ 140 mg/dl) were found to be an independent predictor of coronary plaque progression under statin therapy [odds ratio, 3.689; 95% confidence interval (CI), 1.502 − 9.059; p = 0.004]; old age was another predictor of coronary plaque progression under statin therapy (odds ratio, 1.048; 95% CI, 1.003–1.095; p = 0.036).
Table 5. Multivariable predictors of coronary plaque progression.
| Variable | Univariable Analysis |
Multivariable Analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p value | OR | 95%CI | p value | |
| Low cholesterol at baseline* | 4.068 | 1.798–9.205 | 0.001 | 3.689 | 1.502–9.059 | 0.004 |
| Age | 1.064 | 1.022–1.108 | 0.002 | 1.048 | 1.003–1.095 | 0.036 |
| Men | 0.707 | 0.270–1.853 | 0.48 | |||
| Body mass index (kg/mm2) | 0.956 | 0.853–1.072 | 0.45 | |||
| Diabetes Mellitus | 1.907 | 0.909–3.999 | 0.088 | 1.973 | 0.851–4.574 | 0.11 |
| Hypertension | 2.889 | 1.294–6.451 | 0.010 | 2.369 | 0.940–5.971 | 0.067 |
| Allocated Pitavastatin | 0.674 | 0.325–1.400 | 0.29 | |||
| Unstable angina pectoris | 0.791 | 0.356–1.757 | 0.57 | |||
| Left anterior descending | 1.105 | 0.532–2.296 | 0.79 | |||
| Left circumflex | 1.131 | 0.182–7.029 | 0.90 | |||
| Right | 0.884 | 0.421–1.857 | 0.75 | |||
| Drug-eluting stent use | 0.707 | 0.270–1.853 | 0.48 | |||
| ACE-I or ARB | 2.265 | 1.077–4.765 | 0.031 | 1.248 | 0.516–3.017 | 0.62 |
| β-blocker | 1.162 | 0.366–3.693 | 0.80 | |||
| Calcium channel blocker | 1.810 | 0.868–3.773 | 0.11 | |||
| Glucose (mg/dl) at baseline | 1.003 | 0.992–1.015 | 0.55 | |||
| hs-CRP (µg/ml) at baseline | 1.003 | 9.90–1.016 | 0.62 | |||
| Baseline plaque volume (mm3) | 0.999 | 0.997–1.002 | 0.646 | |||
non-high-density lipoprotein cholesterol ≦ 140mg/dl; ACE-I indicates, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; hs-CRP, high-sensitivity C-reactive protein
Multiple linear regression analysis indicated that low baseline non-HDL-C levels (≤ 140 mg/dl) were independently correlated with the continuous variable of %Δcoronary plaque volume (β = 0.223, p = 0.015) after adjusting for all the variables shown in Table 5.
Discussion
In the present study, we evaluated the efficacy of statin therapy in inducing coronary plaque regression in statin-naïve patients with low cholesterol levels. The major findings of the present study are as follows: (1) non-HDL-C levels at baseline are inversely related to coronary plaque volume changes under statin therapy; (2) low non-HDL-C levels at baseline are an independent predictor of coronary plaque progression under statin therapy; (3) the coronary lumen volume decreases and plaques progress in patients with low cholesterol levels, whereas the coronary lumen volume remains unchanged and plaques regress in patients with moderate or high cholesterol levels after statin therapy; and (4) percent atheroma volume decreases in patients with moderate and high cholesterol levels; however, it increases in those with low cholesterol levels.
The results of the present study demonstrate that statin therapy is less effective in inducing coronary plaque regression in patients with low cholesterol levels but more effective in those with high cholesterol levels at baseline. Hence, statin therapy is insufficient in patients with CAD and low cholesterol levels at baseline. Novel targets and therapeutic strategies need to be determined in these patients to effectively reduce this residual risk.
Reliability of Non-HDL-C
Non-HDL-C includes not only LDL but also all other atherogenic lipoproteins. There is considerable evidence that non-HDL-C is more reliable than LDL-C for assessing cardiovascular events11–14). The reliability of non-HDL-C is stated in the Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 201218). Accordingly, non-HDL-C was used for assessing all data in the present study.
Statin Therapy for Patients with Low Cholesterol Levels
To the best of our knowledge, no randomized trial has assessed the efficacy of statin therapy in patients with low cholesterol levels at baseline. However, several subanalyses from large registries or trials have focused on the impact of baseline cholesterol levels on clinical outcomes19–23). These studies demonstrated that statin use in patients with low cholesterol levels at baseline was associated with improved clinical outcomes. Thus, previous studies support the use of statins in patients with CAD, including those with low cholesterol levels at baseline.
A reduction in non-HDL-C12) and LDL-C24) levels is associated with a reduction in the rate of cardiovascular events under statin therapy. A subanalysis from the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) study indicated that statin-naïve patients with baseline LDL-C levels of ≤ 66 mg/dl did not seem to show reduced cardiovascular events with intensive lipid-lowering therapy as compared to those with moderate lipid-lowering therapy25). One potential reason for this discrepancy is that LDL-C levels are reduced to a generally lesser extent in patients with low baseline LDL-C levels. Hence, the effect of statin therapy depends not only on the reduction in cholesterol required but also on the cholesterol level at baseline.
Effect of Statin Therapy on Coronary Plaque Volume Change
Considerable evidence has been obtained from IVUS studies that statin therapy prevents plaque progression or reduces plaque volume in patients with CAD5–8). It is still difficult to predict which patient would receive greater benefit from statin therapy in terms of coronary plaque regression. A subanalysis from the Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin versus Atorvastatin study indicated that the average non-HDL-C level during treatment was positively associated with the change in coronary plaque volume26); however, the study did not assess baseline non-HDL-C levels. In rosuvastatin-treated groups, in comparison with atorvastatin-treated groups, coronary plaque regression was more evident in patients with higher baseline LDL-C levels. Thus, a more intensive statin therapy may be less effective in patients with lower baseline cholesterol levels. These results are consistent with those reported by the PROVE IT-TIMI 22 study, wherein statin-naïve patients with low baseline LDL-C levels did not show any major benefit with intensive lipid-lowering therapy as compared with moderate lipid-lowering therapy25).
Pivotal trials showed that a majority of CAD patients exhibited coronary plaque regression following intensive statin therapy; however, one-third of patients still exhibited coronary plaque progression even after receiving intensive statin therapy5, 7). This may be related to the residual risk of cardiovascular events in CAD patients receiving statin therapy. In the present study, 43% patients had coronary plaque progression after 8 months of statin therapy; these patients were randomized to receive either intensive or moderate lipid-lowering therapy. In the previousstudy, Okazaki et al. reported that the percent reduction in LDL-C levels was significantly correlated with the percent change in plaque volume8). A recent meta-analysis of randomized controlled trials indicated that the effect of statin therapy in inducing coronary plaque regression depended on the reduction in LDL-C levels9). It is well recognized that the reduction of cholesterol levels is lower in patients with low baseline cholesterol levels than in those with high baseline cholesterol levels, both in terms of absolute and %change. Based on the findings from previous studies, it appears that the effect of statin therapy in reducing cholesterol levels and inducing coronary plaque regression is lower in patients with lower baseline cholesterol levels. Hence, the results of previous studies support our finding that baseline non-HDL-C levels are inversely correlated with coronary plaque volume change and are an important predictor of coronary plaque progression under statin therapy.
A previous study suggested that HDL-C level was associated with a change in carotid atherosclerosis27). However, baseline HDL-C levels, 8-month HDL-C levels, and ΔHDL-C were not correlated with %Δcoronary plaque. The change in HDL-C level was relatively small in the present study protocol. A more intensive statin therapy and longer duration may be needed to determine the relationship between ΔHDL-C and %Δcoronary plaque. Previous studies suggested that low serum docosahexaenoic acid level or docosahexaenoic acid/arachidonic acid ratio was associated with changes in coronary plaque volume28, 29). These factors may be residual risks in coronary artery disease patients under statin therapy.
Assessment from VH-IVUS Analyses
The data from VH ∓ IVUS provided some insights into the mechanism underlying the less effective response to statins in patients with low cholesterol levels. %Δfibrous component was different, whereas %Δ of other components was similar among the three groups (Table 4). Fibrous component decreased in the moderate and high cholesterol groups; however, it significantly increased in the low cholesterol group.
It is believed that fibrous tissue is the main component in plaque progression30). On the contrary, the present study suggests that fibrous tissue is the main component in plaque regression under statin therapy. Only 8 months of follow-up and relatively low dose of statin therapy did not decrease the necrotic-core and fibro-fatty components in the present study. As reported by a previous study31), the amount of necrotic-core or fibro-fatty components may be associated with remnant lipoprotein or with lipoproteins other than LDL-C and HDL-C. It may be another explanation that cholesterol-lowering therapy with statin did not decrease necrotic-core and fibro-fatty components.
Other components may not possibly have statistical power in the present study. Further mechanisms should be addressed in the future.
Impact of hs-CRP on Coronary Plaque Regression
In the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin trial, statin therapy was found to reduce cardiovascular events in the healthy population with normal cholesterol levels and high hs-CRP levels32). Statin therapy reduces hs-CRP levels, and such an improvement in systemic inflammation may reflect the efficacy of statin therapy. In the present study, the variation in hs-CRP levels was significantly lower in the low cholesterol group than that in the high cholesterol group, suggesting that the efficacy of statin treatment was greater in the high cholesterol group. This may represent another mechanism through which statin therapy was less effective in inducing coronary plaque regression in patients with lower baseline cholesterol levels.
Study Limitations
The present study had certain limitations. First, this study was a post-hoc analysis of the TRUTH trial. Second, IVUS examinations were performed only for the non-culprit lesion in the culprit vessel. In this case, mechanical interventions may have affected atheroma measurements. Third, patients with acute coronary syndrome who required emergent coronary angiography and PCI were excluded from the TRUTH study. Finally, the statistical power and follow-up duration (8 months) may not be sufficient to demonstrate the correlation between lipid parameters and plaque volume change.
Conclusions
In serial IVUS analysis, statin therapy was less effective in inducing coronary plaque regression in statin-naïve patients with low cholesterol levels but more effective in those with high cholesterol levels at baseline. Hence, novel targets and alternative therapeutic strategies need to be identified to effectively reduce this residual risk.
Acknowledgments
We are indebted to all participants in the clinical trial for their contributions to the study and acknowledge the contributions of Mr Yasuyoshi Suzuki and Mr Yoshiaki Nakayama in the IVUS core-laboratory management.
Disclosures
Conflict of Interest: none.
References
- 1). Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists' (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005; 366: 1267-1278 [DOI] [PubMed] [Google Scholar]
- 2). Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998; 339: 1349-1357 [DOI] [PubMed] [Google Scholar]
- 3). Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994; 344: 1383-1389 [PubMed] [Google Scholar]
- 4). Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996; 335: 1001-1009 [DOI] [PubMed] [Google Scholar]
- 5). Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM, ASTEROID Investigators Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006; 295: 1556-1565 [DOI] [PubMed] [Google Scholar]
- 6). Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria AN, REVERSAL Investigators Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004; 291: 1071-1080 [DOI] [PubMed] [Google Scholar]
- 7). Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M, Wolski K, Nissen SE. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011; 365: 2078-2087 [DOI] [PubMed] [Google Scholar]
- 8). Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, Daida H. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation. 2004; 110: 1061-1068 [DOI] [PubMed] [Google Scholar]
- 9). Takagi H, Umemoto T. Atorvastatin reduces coronary plaque volume in dependence on reductions in low-density lipoprotein: a meta-analysis and meta-regression of randomized controlled trials. Int J Cardiol. 2012; 157: 114-116 [DOI] [PubMed] [Google Scholar]
- 10). Nozue T, Yamamoto S, Tohyama S, Umezawa S, Kunishima T, Sato A, Miyake S, Takeyama Y, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Sozu T, Terashima M, Michishita I. Statin treatment for coronary artery plaque composition based on intravascular ultrasound radiofrequency data analysis. Am Heart J. 2012; 163: 191-199 [DOI] [PubMed] [Google Scholar]
- 11). Boekholdt SM1, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, Simes RJ, Durrington P, Hitman GA, Welch KM, DeMicco DA, Zwinderman AH, Clearfield MB, Downs JR, Tonkin AM, Colhoun HM, Gotto AM, Jr, Ridker PM, Kastelein JJ. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012; 307: 1302-1309 [DOI] [PubMed] [Google Scholar]
- 12). Robinson JG, Wang S, Smith BJ, Jacobson TA. Meta-analysis of the relationship between non-high-density lipoprotein cholesterol reduction and coronary heart disease risk. J Am Coll Cardiol. 2009; 53: 316-322 [DOI] [PubMed] [Google Scholar]
- 13). Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008; 51: 1512-1524 [DOI] [PubMed] [Google Scholar]
- 14). Blaha M, Blumenthal R, Brinton E, Jacobson T, on behalf of the National Lipid Association Taskforce on Non-HDL Cholesterol The importance of non-HDL cholesterol reporting in lipid management. J Clin Lipidol. 2008; 2: 267-273 [DOI] [PubMed] [Google Scholar]
- 15). García-García HM, Mintz GS, Lerman A, Vince DG, Margolis MP, van Es GA, Morel MA, Nair A, Virmani R, Burke AP, Stone GW, Serruys PW. Tissue characterization using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention. 2009; 5: 177-189 [DOI] [PubMed] [Google Scholar]
- 16). Mintz GS, Garcia-Garcia HM, Nicholls SJ, Weissman NJ, Bruining N, Crowe T, Tardif JC, Serruys PW. Clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound regression/progression studies. EuroIntervention. 2011; 6: 1123-1130 [DOI] [PubMed] [Google Scholar]
- 17). Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, Yock PG. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001; 37: 1478-1492 [DOI] [PubMed] [Google Scholar]
- 18).Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovacular Diseases. 2012. [Google Scholar]
- 19). Lee KH, Jeong MH, Kim HM, Ahn Y, Kim JH, Chae SC, Kim YJ, Hur SH, Seong IW, Hong TJ, Choi DH, Cho MC, Kim CJ, Seung KB, Chung WS, Jang YS, Rha SW, Bae JH, Cho JG, Park SJ, KAMIR (Korea Acute Myocardial Infarction Registry) Investigators Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-densitylipoprotein cholesterol. J Am Coll Cardiol. 2011; 58: 1664-1671 [DOI] [PubMed] [Google Scholar]
- 20). Spencer FA, Goldberg RJ, Gore JM, Fox KA, Avezum A, Agnelli G, Kritharides L, Anderson FA, Goodman SG, FitzGerald G, Allegrone J, Brieger D, GRACE Investigators Comparison of utilization of statin therapy at hospital discharge and six-month outcomes in patients with an acute coronary syndrome and serum low-density lipoprotein ≥ 100 mg/dl versus < 100 mg/dl. Am J Cardiol. 2007; 100: 913-918 [DOI] [PubMed] [Google Scholar]
- 21). MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebocontrolled trial. Lancet 2002; 360: 7-22 [DOI] [PubMed] [Google Scholar]
- 22). Leeper NJ, Ardehali R, deGoma EM, Heidenreich PA. Statin use in patients with extremely low low-density lipoprotein levels is associated with improved survival. Circulation. 2007; 116: 613-618 [DOI] [PubMed] [Google Scholar]
- 23). Tsai TT, Nallamothu BK, Mukherjee D, Rubenfire M, Fang J, Chan P, Kline-Rogers E, Patel A, Armstrong DF, Eagle KA, Goldberg AD. Effect of statin use in patients with acute coronary syndromes and a serum low-density lipoprotein < or = 80 mg/dl. Am J Cardiol. 2005; 96: 1491-1493 [DOI] [PubMed] [Google Scholar]
- 24). Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM, IMPROVE-IT Investigators Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015; 372: 2387-2397 [DOI] [PubMed] [Google Scholar]
- 25). Giraldez RR, Giugliano RP, Mohanavelu S, Murphy SA, McCabe CH, Cannon CP, Braunwald E. Baseline low-density lipoprotein cholesterol is an important predictor of the benefit of intensive lipid-lowering therapy: a PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) analysis. J Am Coll Cardiol. 2008; 52: 914-920 [DOI] [PubMed] [Google Scholar]
- 26). Puri R, Nissen SE, Ballantyne CM, Barter PJ, Chapman MJ, Erbel R, Libby P, Raichlen JS, St John J, Wolski K, Uno K, Kataoka Y, Nicholls SJ. Factors underlying regression of coronary atheroma with potent statin therapy. Eur Heart J. 2013; 34: 1818-1825 [DOI] [PubMed] [Google Scholar]
- 27). Ishigaki Y, Kono S, Katagiri H, Oka Y, Oikawa S, NTTP investigators Elevation of HDL-C in response to statin treatment is involved in the regression of carotid atherosclerosis. J Atheroscler Thromb. 2014; 21: 1055-1065 [DOI] [PubMed] [Google Scholar]
- 28). Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Nozato T, Miyake S, Takeyama Y, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Terashima M, Michishita I. Low serum docosahexaenoic acid is associated with progression of coronary atherosclerosis in statin-treated patients with diabetes mellitus: results of the treatment with statin on atheroma regression evaluated by intravascular ultrasound with virtual histology (TRUTH) study. Cardiovasc Diabetol. 2014; 13: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Nozato T, Miyake S, Takeyama Y, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Terashima M, Michishita I. Comparison of effects of serum n-3 to n-6 polyunsaturated fatty acid ratios on coronary atherosclerosis in patients treated with pitavastatin or pravastatin undergoing percutaneous coronary intervention. Am J Cardiol. 2013; 111: 1570-1575 [DOI] [PubMed] [Google Scholar]
- 30). Qian J, Maehara A, Mintz GS, Margolis MP, Biro S, Stone GW, Leon MB. Relation between individual plaque components and overall plaque burden in the prospective, multicenter virtual histology intravascular ultrasound registry. Am J Cardiol 2009; 104: 501-506 [DOI] [PubMed] [Google Scholar]
- 31). Matsuo N, Matsuoka T, Onishi S, Yamamoto H, Kato A, Makino Y, Kihara S. Impact of Remnant Lipoprotein on Coronary Plaque Components. J Atheroscler Thromb. 2015; 22: 783-795 [DOI] [PubMed] [Google Scholar]
- 32). Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008; 359: 2195-2207 [DOI] [PubMed] [Google Scholar]


