Abstract
Aim: Resveratrol is a popular ingredient in dietary supplements. Some patients concomitantly use dietary supplements and medicines in Japan. In the present study, we determined whether trans-resveratrol and melinjo (Gnetum gnemon L.) seed extract (MSE), which contains resveratrol dimers, interacted with drugs using a mouse model.
Methods: Male C57BL/6J mice were fed experimental diets containing 0.005%, 0.05%, or 0.5% (w/w) trans-resveratrol or MSE for 1 or 12 weeks. The expression of liver cytochrome P-450 (CYP) mRNA and activity of liver microsomal CYP were measured. To determine the influence of resveratrol or MSE on drug efficacy, the anticoagulant activity of warfarin was examined in mice that were fed diets containing trans-resveratrol or MSE for 12 weeks.
Results: When the mice were fed experimental diets for 1 week, none of the doses of trans-resveratrol and MSE affected body weight, liver weight, or plasma AST and ALT levels. Trans-resveratrol also did not affect CYP1A1, CYP1A2, CYP2C, or CYP3A activities. In contrast, 0.5% MSE slightly increased CYP1A1 activity. When the mice were fed experimental diets for 12 weeks, 0.05% trans-resveratrol increased CYP1A1, CYP2C, and CYP3A activities, whereas 0.5% MSE suppressed CYP3A activity. Under these conditions, 0.5% trans-resveratrol enhanced the anticoagulant activity of warfarin, although CYP2C activity increased. However, MSE did not affect the anticoagulant activity of warfarin.
Conclusion: The 0.05% trans-resveratrol did not interact with warfarin in a mouse model, whereas 0.5% trans-resveratrol may have enhanced the anticoagulant activity of warfarin.
Keywords: Cytochrome P-450 (CYP), Dietary supplement, Drug, Trans-resveratrol, Warfarin
Introduction
Trans-resveratrol (trans-3,4′,5-trihydroxystilbene), which is found in various foods including grapes, cranberries, and peanuts, has become a popular ingredient in dietary supplements in Japan because it has been shown to have antiaging effects in various animal models1). Melinjo (Gnetum gnemon L.), a species belonging to the family Gnetaceae, is extensively cultivated in Southeast Asia. Its seeds are nutritious and are the main staple food in some areas2). In Japan, melinjo seed extract (MSE) is also used as an ingredient in dietary supplements. Melinjo seeds are very rich in resveratrol dimers (such as gnetin C and its glucosides, gnemonoside A, and gnemonoside D)3) (Table 1). However, it is reported that dimers of resveratrol show different properties4) and effects5) to those of trans-resveratrol.
Table 1. Composition of the melinjo seed extract (MSE).
| Components | /100 g MSE |
|---|---|
| Nutrients | |
| Moisture | 4.8 g |
| Protein | 9.3 g |
| Fat | 0.9 g |
| Ash | 5.2 g |
| Available carbohydrate | 79.8 g |
| Dietary fiber | <0.5 g |
| Sodium | 5.0 mg |
| Phosphorus | 205 mg |
| Iron | 0.33 mg |
| Calcium | 10.9 mg |
| Potassium | 2.44 g |
| Magnesium | 167 mg |
| Copper | 2.59 mg |
| Zinc | 0.60 mg |
| Manganese | 1.07 mg |
| Selenium | 9 mg |
| Chlorine | 179 mg |
| Trans-resveratrol and its derivatives | |
| trans-resveratrol | 0.11 g |
| Gnetin C | 2.84 g |
| Gnemonoside A | 13.68 g |
| Gnemonoside D | 4.95 g |
| Gnemonoside C | 1.98 g |
| Gnetin L | 0.25 g |
| trans-piceid | 0.55 g |
| Isorhapontigenin | 0.20 g |
The population of Japan is rapidly aging, and as a result, chronic diseases associated with aging, such as diabetes mellitus, cardiovascular disease, hypertension, osteoporosis, and cancer, have become a widely recognized social issue. Against this background, an increase in health consciousness has prompted people to use dietary supplements to maintain health and prevent diseases. In addition to its antiaging effects, resveratrol has been suggested to be effective against obesity, diabetes mellitus6), atherosclerosis7, 8), hypertension9), Alzheimer's disease10), and cancers11).
The concurrent use of dietary supplements and drugs has recently become prevalent12). Physicians and pharmacists need to be aware of which of their patients are concomitantly using dietary supplements and medicines because many herbs (e.g., black cohosh, coleus forskohlii, echinacea, garlic, ginkgo, ginseng, green tea, kava, milk thistle, and St. John's wort)13–18) and ingredients (e.g., catechins19), curcuminoids20), isoflavones21), quercetin22), and polyphenols23)) affect drug-metabolizing enzymes. Resveratrol24) also affects the drug-metabolizing enzyme cytochrome P-450 (CYP). Resveratrol was initially found to inhibit CYP1A1 in human HepG2 hepatoma cells25), with inhibition of human recombinant CYP1B126), CYP3A4, CYP3A527), and others subsequently being reported. Resveratrol was also shown to inhibit the enzyme activities of CYP2D6, CYP2C9, and CYP3A4 in a healthy volunteer28). In contrast, the influence of MSE on drug-metabolizing enzymes has not yet been examined.
The ingredients in dietary supplements have to be absorbed and exist in their active forms to interact with drugs. Some ingredients are not absorbed or are metabolized immediately after their absorption. The concentrations of these ingredients at the sites of interactions are also an important factor. Ingredients have to exist at an effective dose in the blood or target organs to interact with drugs. Furthermore, even if dietary supplements affect drug metabolism and blood levels, drug efficacy is not affected if the drug exists at a therapeutic dose in the blood. Therefore, not only the ingredients but also their concentrations and duration of intake are important.
In the present study, we examined the effects of the intake of several amounts of trans-resveratrol and MSE for short and long periods on major CYP subtypes such as Cyp1a1, 1a2, 2c29, and 3a11 in a mouse model (human homologues Cyp1a1, 1a2, 2c9, and 3a4, respectively) as well as the interaction between trans-resveratrol or MSE and warfarin in vivo.
Materials and Methods
Animals
Male C57BL/6J mice were purchased from Japan SLC Inc. (Shizuoka, Japan) and were maintained under specific pathogen-free conditions in a temperaturecontrolled room (22°C ± 2°C) with a 12-h light/dark cycle. Animals had free access to a normal laboratory diet (CRF-1) and water until 10 weeks of age, when experiments were initiated. The mice were fed an AIN-93 semi-purified diet or diet containing several doses of trans-resveratrol or MSE for 1 or 12 weeks. The composition of MSE was shown in Table 129). To evaluate the interaction between trans-resveratrol or MSE and drugs, the mice were fed an AIN-93 semipurified diet or diet containing several doses of trans-resveratrol or MSE for 12 weeks and then administered warfarin racemate (A2250; Sigma-Aldrich Inc., St Louis, MO) (0.33 mg/kg) dissolved in 0.5% carboxymethylcellulose or vehicle via an intragastric injection for the last 2 days of the treatment regimen. Each group comprised five mice. All animal experiments were conducted with the approval of the National Institute of Health and Nutrition Laboratory Animal Ethics Committee.
Determination of Trans-Resveratrol or MSE doses in Diets
The diets used in this experiment were AIN-93 semi-purified diets containing 0.005%, 0.05%, or 0.5% trans-resveratrol or MSE. According to the Japan Health and Nutrition Food Association, the recommended daily doses of total resveratrol in dietary supplements are 2–100 mg/day (approximately 0.033–1.7 mg/kg BW) for humans. MSE, which we used in this study, contains approximately 25% total resveratrol (trans-resveratrol and its derivatives), and then the recommended daily doses of MSE in dietary supplements are 8–400 mg/day (approximately 0.13–6.7 mg/kg BW). This dose translated to 0.4–20 mg/kg BW of resveratrol or 1.6–80 mg/kg BW of MSE for mice using the body surface area normalization method30). Because mice (25 g BW) consume approximately 4 g diet/day, 0.05% (w/w) in the diet is similar to 80 mg/kg BW intake. Therefore, we examined the effects of 0.005%, 0.05%, or 0.5% trans-resveratrol or MSE in the diet.
Plasma Chemistry
After 1 or 12 weeks of feeding, the mice were killed under isoflurane anesthesia after overnight fasting. Blood samples were obtained from the abdominal aorta, and plasma samples were prepared immediately. Plasma levels of AST (transaminase CII-test Wako, Wako Pure Chemical Industries, Ltd., Osaka, Japan), ALT (transaminase CII-test Wako, Wako Pure Chemical Industries, Ltd.), and ALP (LabAssay ALP, Wako Pure Chemical Industries, Ltd.) were determined with enzymatic methods, respectively.
Quantitative RT-PCR
After 1 or 12 weeks of feeding, total RNA was extracted from the liver using the TRIzol Plus RNA Purification System (Life Technologies, Carlsbad, CA) and reverse transcribed with PrimeScript RT Master Mix (Takara Bio Inc., Shiga, Japan). Quantitative RTPCR was performed on 96-well plates with the SYBR Green PCR Master Mix and Thermal Cycler Dice Real Time System Single (Takara Bio Inc.). Results were expressed as the copy number ratio of the target mRNA to Gapdh mRNA. The following mouse-specific primer pairs were used:
Gapdh forward 5′-TGATGCTGGTGCTGAGTATGTCGT-3′;
reverse 5′-TCTCGTGGTTCACACCCATCACAA-3′;
Cyp1a1 forward 5′-AGCTTGGCCTGGATTACTGT-3′;
reverse 5′-AACCCCATCAACCCCAGTAG-3′;
Cyp1a2 forward 5′-ACATCACAAGTGCCCTGTTCAAGC-3′;
reverse 5′-ATCTTCCTCTGCACGTTAGGCCAT-3′;
Cyp2c29 forward 5′-AGCCTACTGTCATATTGCACGGGT-3′;
reverse 5′-CATGCCCAAATTTCGCAGGGTCAT-3′;
Cyp3a11 forward 5′-AGGCAGAAGGCAAAGAAAGGCAAG-3′;
reverse 5′-TGAGGGAATCCACGTTCACTCCAA-3′;
Preparation of the Liver Microsomal Fraction
Livers were homogenized in 50 mM Tris-HCl buffer containing 0.25 M sucrose (pH7.4) with a polytron homogenizer. The homogenate was centrifuged at 10,000 ×g for 30 min at 4°C, and the supernatant was collected. The supernatant was re-centrifuged at 105,000 ×g for 60 min at 4°C, and the supernatant was discarded. The pellet was re-suspended in 50 mM Tris-HCl buffer (pH7.4) and used as the liver microsomal fraction. Protein concentrations were determined using the BCA protein assay kit (Pierce, Rockford, IL).
Measurement of CYP Activity
The activity of each CYP subtype in the liver microsomal fraction was measured using a luminescent method using the P450-Glo™ CYP1A1 System (Luciferin-CEE) Assay, CYP1A2 System (Luciferin-1A2) Assay, CYP2C9 System (Luciferin-H) Assay, CYP3A4 System (Luciferin-PPXE) Assay, and NADPH Regeneration System with GloMax-Multi + Detection System (Promega Co., Madison, WI). CYP activity was adjusted by protein concentrations, and results were represented as a percentage of the control.
Measurement of Anticoagulant Activity
Blood samples were immediately centrifuged at 800 ×g at 4°C for 15 min to prepare plasma. Coagulation parameters [prothrombin time (PT), activated partial thromboplastin time (APTT), and thrombotest Owren (TTO)] were measured using an automated blood coagulation analyzer (CA-50; Sysmex Co., Hyogo, Japan) according to the manufacturer's protocol. PT and TTO are indicators of the extrinsic and common pathways of the coagulation cascade, respectively, and are used to monitor warfarin therapy. APTT is an indicator of both the intrinsic and common pathways of the coagulation cascade.
Statistical Analysis
Data are presented as the mean ± SEM. Comparisons of data from multiple groups were performed by a one-way ANOVA with a Bonferroni post hoc test (SPSS 18.0J for Windows, IBM Co. Armonk, NY). P < 0.05 was considered to be significant.
Results
Effects of Trans-Resveratrol and MSE for a Short Period (1 Week of Feeding)
When the mice were fed experimental diets containing 0.005%, 0.05%, or 0.5% trans-resveratrol or MSE for 1 week, neither trans-resveratrol nor MSE affected the body or liver weights (Table 2). Moreover, neither trans-resveratrol nor MSE affected the plasma levels of AST, ALT, or ALP (Table 2). Under these conditions, trans-resveratrol did not affect Cyp1a1, 1a2, 2c29, or 3a11 mRNA expression levels in the liver (Fig. 1A) or their activities in liver microsomal fractions (Fig. 2A). In contrast, 0.5% MSE significantly suppressed Cyp3a11 mRNA expression levels and increased CYP1A1 activity, whereas 0.005% and 0.05% MSE did not (Fig. 1B and 2B).
Table 2. Body weight, liver weight, and liver functional markers in plasma with 1 week of feeding.
| Control | 0.005% | 0.05% | 0.5% | ||
|---|---|---|---|---|---|
| Body weight (g) | Res | 20.8 ± 0.4 | 20.8 ± 0.4 | 20.8 ± 0.4 | 20.5 ± 0.1 |
| MSE | 21.5 ± 0.4 | 21.2 ± 0.4 | 21.4 ± 0.2 | 21.6 ± 0.4 | |
| Liver weight (g) | Res | 0.83 ± 0.01 | 0.81 ± 0.02 | 0.84 ± 0.02 | 0.81 ± 0.03 |
| MSE | 0.86 ± 0.02 | 0.85 ± 0.02 | 0.83 ± 0.01 | 0.81 ± 0.01 | |
| AST (IU/L) | Res | 12.4 ± 0.6 | 14.6 ± 1.1 | 14.2 ± 1.9 | 15.3 ± 0.6 |
| MSE | 12.1 ± 0.4 | 13.5 ± 0.5 | 15.6 ± 2.4 | 13.2 ± 0.4 | |
| ALT (IU/L) | Res | 3.7 ± 0.2 | 5.0 ± 0.8 | 3.8 ± 0.2 | 4.7 ± 0.1 |
| MSE | 3.6 ± 0.3 | 4.0 ± 0.4 | 4.4 ± 0.3 | 4.5 ± 0.4 | |
| ALP (IU/L) | Res | 64.1 ± 1.5 | 65.7 ± 4.5 | 72.9 ± 2.1 | 68.5 ± 3.3 |
| MSE | 63.9 ± 1.3 | 72.2 ± 4.3 | 70.0 ± 1.4 | 66.8 ± 0.9 | |
Data presented as the mean ± SEM. Body weight, liver weight, and plasma levels of AST, ALT, and ALP were measured 1 week after the initiation of the experimental diet. Res; resveratrol, MSE; melinjo seed extract. n = 5 in each group.
Fig. 1.
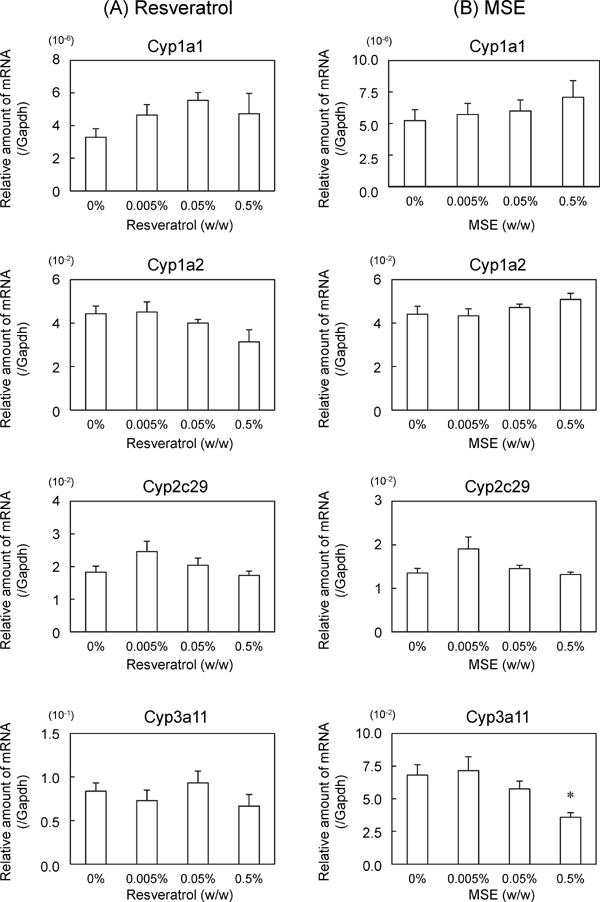
Effects of trans-resveratrol and MSE on CYP mRNA expression (1 week of feeding). C57BL/6J mice (male, 10 weeks of age) were fed experimental diets containing 0%, 0.005%, 0.05%, or 0.5% (w/w) trans-resveratrol (A) or MSE (B) for 1 week. After overnight fasting, the mice were killed, and the mRNA expression levels of the major CYP subtypes in the liver were measured using real-time qPCR methods. Data are presented as a percentage of the control. Bars show SEM. *P < 0.05 vs. 0% MSE by a one-way ANOVA with a Bonferroni post hoc test. n = 5 in each group.
Fig. 2.
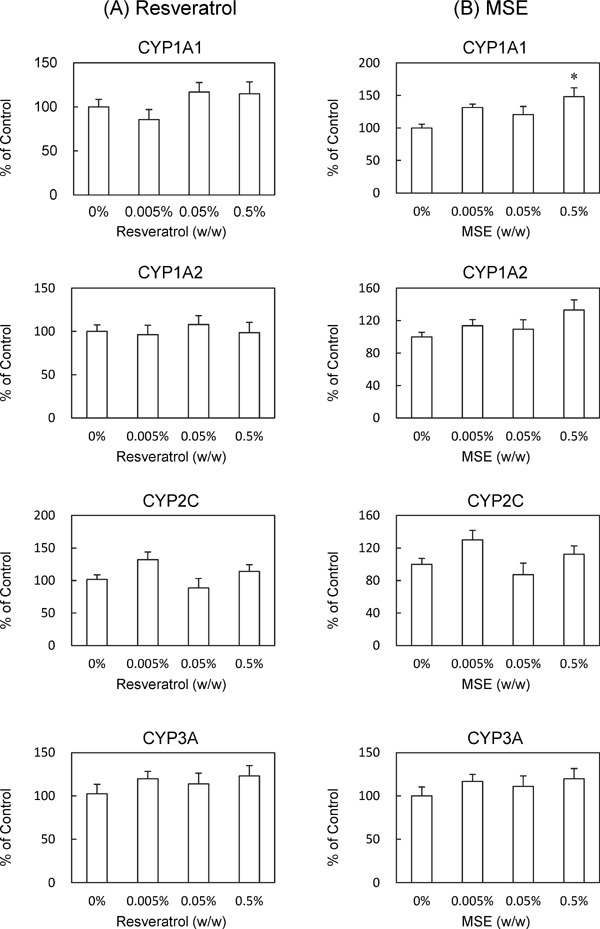
Effects of trans-resveratrol and MSE on CYP subtype activities (1 week of feeding). C57BL/6J mice were fed experimental diets containing 0%, 0.005%, 0.05%, or 0.5% (w/w) trans-resveratrol (A) or MSE (B) for 1 week. Liver microsomal CYP activities were measured. Data are presented as a percentage of the control. Bars show SEM. *P < 0.05 vs. 0% MSE by a one-way ANOVA with a Bonferroni post hoc test. n = 5 in each group.
Effects of Trans-Resveratrol and MSE for a Long Period (12 Weeks of Feeding)
We examined the effects of 12 weeks of feeding diets with 0.05% or 0.5% trans-resveratrol and MSE. When the mice were fed experimental diets for 12 weeks, trans-resveratrol and MSE slightly suppressed body weight gain (Table 3). In addition, liver weights were significantly lower in mice that were fed the 0.5% MSE diet than in control mice (Table 3). Trans-resveratrol and MSE suppressed the plasma levels of AST but did not affect those of ALT. In contrast, 0.05% trans-resveratrol significantly increased the plasma levels of ALP but within normal ranges. Under these conditions, 0.05% trans-resveratrol slightly increased Cyp3a11 mRNA expression levels in the liver (Fig. 3) and significantly increased CYP1A1, 2C, and 3A activities in liver microsomal fractions (Fig. 4), whereas 0.5% trans-resveratrol did not. In contrast, MSE did not affect CYP mRNA expression levels (Fig. 3), whereas 0.5% MSE significantly suppressed CYP3A activity (Fig. 4).
Table 3. Body weight, liver weight, liver functional markers in plasma with 12 weeks of feeding.
| Control | Resveratrol |
Melinjo seed extract |
|||
|---|---|---|---|---|---|
| 0.05% | 0.5% | 0.05% | 0.5% | ||
| Body weight (g) | 36.5 ± 0.9 | 34.0 ± 1.3 | 34.7 ± 1.7 | 35.6 ± 1.5 | 34.2 ± 0.6 |
| Liver weight (g) | 1.16 ± 0.03 | 1.10 ± 0.04 | 1.11 ± 0.05 | 1.08 ± 0.06 | 0.97 ± 0.01 * |
| Liver functional markers | |||||
| AST (IU/L) | 23.4 ± 4.6 | 13.7 ± 0.8 * | 19.9 ± 1.3 | 16.2 ± 2.1 | 13.5 ± 0.7 |
| ALT (IU/L) | 1.8 ± 0.2 | 1.4 ± 0.6 | 1.9 ± 0.3 | 1.8 ± 0.5 | 1.4 ± 0.3 |
| ALP (IU/L) | 42.1 ± 2.1 | 51.6 ± 2.0* | 44.7 ± 2.6 | 39.1 ± 0.9 | 39.5 ± 1.9 |
Data presented as the mean ± SEM. Body weight, liver weight, and plasma levels of AST, ALT, and ALP were measured 12 weeks after the initiation of the experimental diet.
P < 0.05 vs. the control by a one-way ANOVA with a Bonferroni post hoc test.
n = 5 in each group.
Fig. 3.
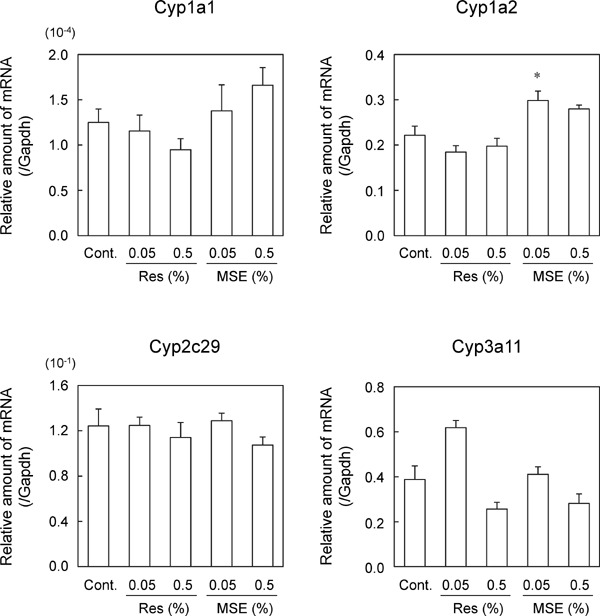
Effects of trans-resveratrol and MSE on CYP mRNA expression levels (12 weeks of feeding). C57BL/6J mice were fed experimental diets containing 0%, 0.05%, or 0.5% (w/w) trans-resveratrol or MSE for 12 weeks. The major CYP subtype mRNA expression levels in the liver were measured using real-time qPCR methods. Data are presented as a percentage of the control. Bars show SEM. *P < 0.05 vs. 0% MSE by a one-way ANOVA with a Bonferroni post hoc test. n = 5 in each group.
Fig. 4.
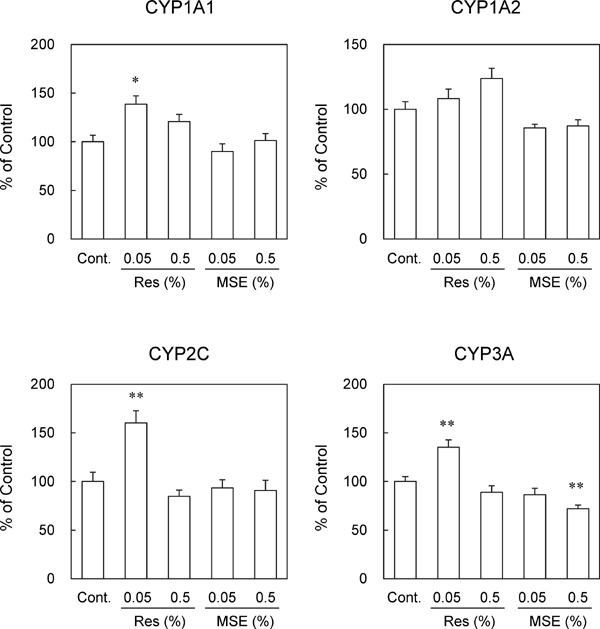
Effects of trans-resveratrol and MSE on CYP subtype activities (12 weeks of feeding). C57BL/6J mice were fed experimental diets containing 0%, 0.05%, or 0.5% (w/w) trans-resveratrol or MSE for 12 weeks. Liver microsomal CYP activities were measured. Data are presented as a percentage of the control. Bars show SEM. *P < 0.05, **P < 0.01 vs. control by a one-way ANOVA with a Bonferroni post hoc test. n = 5 in each group.
Interaction of Trans-Resveratrol or MSE with Warfarin
Trans-resveratrol (0.05%, 12 weeks) significantly increased the activities of the major CYPs, including CYP2C, and may have influenced the efficacy of the drugs metabolized by CYP2C. To address this issue, the mice were fed an experimental diet containing 0.05% or 0.5% trans-resveratrol or MSE for 12 weeks, and warfarin racemate or vehicle was then administrated on the last 2 days. The co-administration of trans-resveratrol and warfarin did not affect the body and liver weights (Table 4). Under these conditions, trans-resveratrol itself did not affect PT, APTT, or TTO (Fig. 5A). However, in the presence of warfarin, PT and APTT were higher with 0.5% trans-resveratrol than with 0% trans-resveratrol (Fig. 5A). In contrast, MSE slightly decreased the body and liver weights with or without warfarin (Table 4). Under these conditions, 0.5% MSE itself slightly shortened APTT (Fig. 5B). In the presence of warfarin, PT, APTT, and TTO were slightly higher with 0.5% MSE than with 0% MSE, but it was not statistically significance (Fig. 5B).
Table 4. Body weight and liver weight with 12 weeks of feeding.
| Control |
Warfarin |
|||||
|---|---|---|---|---|---|---|
| 0% | 0.05% | 0.5% | 0% | 0.05% | 0.5% | |
| Resveratrol | ||||||
| Body weight (g) | 28.4 ± 0.5 | 31.6 ± 0.7 | 30.4 ± 0.9 | 30.8 ± 0.6 | 31.5 ± 0.9 | 29.8 ± 0.5 |
| Liver weight (g) | 1.09 ± 0.03 | 1.08 ± 0.04 | 1.04 ± 0.06 | 1.10 ± 0.03 | 1.13 ± 0.04 | 0.99 ± 0.06 |
| MSE | ||||||
| Body weight (g) | 35.9 ± 1.2 | 33.2 ± 1.5 | 33.4 ± 2.5 | 34.7 ± 1.1 | 30.8 ± 0.8* | 31.2 ± 0.4 |
| Liver weight (g) | 1.07 ± 0.05 | 0.99 ± 0.05 | 1.01 ± 0.07 | 1.07 ± 0.04 | 0.97 ± 0.02 | 0.98 ± 0.02 |
Data presented as the mean ± SEM. Body and liver weights were measured 12 weeks after the initiation of the experimental diet.
P < 0.05 vs. 0% resveratrol or MSE in each treatment by a one-way ANOVA with a Bonferroni post hoc test.
n = 4 or 5 in each group.
Fig. 5.
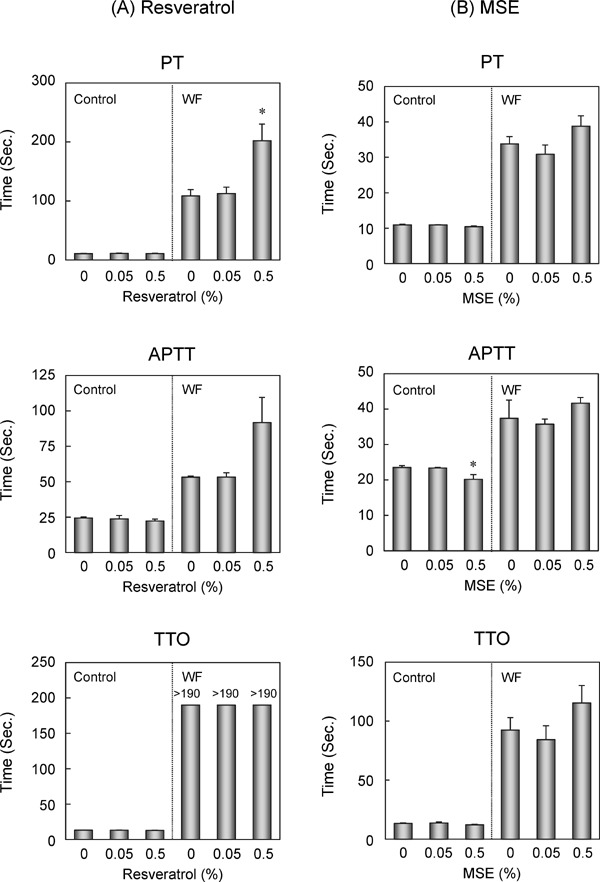
Effects of trans-resveratrol and MSE on anticoagulant activities of warfarin (12 weeks of feeding). C57BL/6J mice were fed experimental diets containing 0%, 0.05%, or 0.5% (w/w) trans-resveratrol (A) or MSE (B) for 12 weeks. Warfarin racemate (0.33 mg/kg) dissolved in 0.5% carboxymethylcellulose or vehicle was administrated via an intragastric injection for the last 2 days of the treatment regimen. After overnight fasting, the mice were killed, and PT, APTT, and TTO were measured. Bars show SEM. *P < 0.05 vs. 0% trans-resveratrol or MSE in each treatment by a one-way ANOVA with a Bonferroni post hoc test. n = 4 or 5 in each group.
Discussion
In the present study, 0.05% trans-resveratrol in the diet enhanced the activities of CYP1A1, 2C, and 3A but did not affect the anticoagulant activity of warfarin. In contrast, 0.5% trans-resveratrol in the diet did not affect the activities of CYP, but enhanced the anticoagulant activity of warfarin, even though trans-resveratrol itself did not exhibit anticoagulant activity. MSE did not affect the anticoagulant activity of warfarin.
Two possibilities have been suggested for why trans-resveratrol enhanced the anticoagulant activity of warfarin, whereas MSE did not in this experiment. MSE is mainly composed of resveratrol derivatives, the characteristics of which differ from those of trans-resveratrol4, 5). Therefore, the abilities of trans-resveratrol and MSE (the mixture of active compounds, which including trans-resveratrol and resveratrol derivatives) to interact with drugs differ. The second possibility, MSE contains approximately 25% of trans-resveratrol and its derivatives, indicating that 0.5% MSE in diet is similar to 0.125% active compounds in diet. In the present study, 0.5% MSE slightly, but not significantly, increased PT, APTT, and TTO. Therefore, there is a possibility that more than 0.5% of MSE in diet may enhance the anticoagulant activity of warfarin. However, when people use MSE dietary supplements within the recommended daily dose, MSE could not interact with warfarin.
We previously surveyed dietary supplement usage in patients in Japan and found that some had been concomitantly taking dietary supplements and medicines12). However, approximately 70% of these patients did not declare the use of dietary supplements to their physicians12). Therefore, health issues caused by interactions between dietary supplements and drugs may occur. Interactions between dietary supplements and drugs have mainly been observed at the absorption and metabolism steps31). ATP-binding cassette (ABC) transporters are associated with the absorption of drugs in the intestines. ABCB1, also known as P-glycoprotein, has been shown to play an important role in drug efflux32). Previous studies showed that resveratrol inhibited P-glycoprotein activity both in vitro33) and in vivo34) models. However, it is reported that the absorption of warfarin was independent of P-glycoprotein activity35). In contrast, CYPs are known to play an important role in drug metabolism. The effects of resveratrol on CYP1A1, 1A2, 1B1, and 3A4 have been extensively investigated24), whereas few studies have examined CYP2C. In the present study, trans-resveratrol did not affect or increase CYP2C activity, which depended on the time and dose administered. In this study, we used warfarin racemate, and the anticoagulant property of R-warfarin is less than that of S-warfarin36). S-warfarin is metabolized by CYP2C9, whereas R-warfarin is metabolized by CYP1A1, 1A2, and 3A4 in humans36, 37). In the present study, the activities of CYP1A1, 2C, and 3A were increased by 0.05% resveratrol. Therefore, the metabolism of S-warfarin and R-warfarin may have been enhanced. However, 0.05% resveratrol did not affect the anticoagulant activity of warfarin, whereas 0.5% resveratrol increased it (prolonged PT and APTT), even though the activities of CYPs did not change by 0.5% resveratrol. A previous study reported that trans-resveratrol was metabolized by CYP1A1, 1A2, and 1B138). In contrast, it was also reported that trans-resveratrol inhibited CYP1A1, 1B1, and 1A239). Previous studies showed that resveratrol suppressed platelet aggregation40, 41) and prevented atherosclerosis by inhibiting platelet aggregation in a mouse model42). This antiplatelet activity of resveratrol may have had an impact on the results obtained in the present study.
Resveratrol is regarded as a candidate for disease therapies against obesity, diabetes mellitus6), atherosclerosis7, 8), Alzheimer's disease10), and cancers11). In addition to animal studies, clinical studies have also been conducted. Resveratrol was found to improve insulin sensitivity and postprandial plasma glucose in subjects with impaired glucose tolerance43). Its administration was also reported to significantly improve mean hemoglobin A1c, systolic blood pressure, and total cholesterol levels in patients with type 2 diabetes mellitus44, 45). Furthermore, a resveratrol-enriched grape extract treatment significantly improved the lipid profile46) and inflammatory cytokine levels47) in the blood. Resveratrol has also been shown to induce dose-dependent increases in cerebral blood flow without influencing cognitive function48). A previous study reported that it reduced tumor cell proliferation by 5% in patients with colorectal cancer49). In contrast, few studies have examined MSE, with only two demonstrating its safety29, 50).
Conclusion
In the present study, 0.05% trans-resveratrol or MSE did not interact with warfarin, whereas 0.5% trans-resveratrol enhanced the anticoagulant effects of warfarin in a mouse model. Although we used a mouse model, our results indicate that care is needed regarding the concomitant use of resveratrol and drugs by patients.
Acknowledgments
This research was supported by Yamada Bee Company Inc. (Okayama, Japan).
Conflicts of Interest
This research was supported by Yamada Bee Company Inc. (Okayama, Japan). Y. Kimura and T. Tatefuji are employees of Yamada Bee Company, Inc. No other authors declare any potential conflicts of interest.
References
- 1). Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011; 1215: 138-143 [DOI] [PubMed] [Google Scholar]
- 2). Verheij EWM, Sukendar EV. Gnetum genmon L. In: Plant resources of South East Asia no. 2, ed by Verheij EWM, Coronel RE, pp182-184, Eddible Frouits and nuts. Prosea Foundation, Bogor, Indonesia: 1991 [Google Scholar]
- 3). Kato E, Tokunaga Y, Sakan F. Stilbenoids isolated from the seeds of melinjo (gnetum gnemon l.) and their biological activity. J Agric Food Chem. 2009; 57: 2544-2549 [DOI] [PubMed] [Google Scholar]
- 4). Piver B, Berthou F, Dreano Y, Lucas D. Differential inhibition of human cytochrome p450 enzymes by epsilonviniferin, the dimer of resveratrol: Comparison with resveratrol and polyphenols from alcoholized beverages. Life Sci. 2003; 73: 1199-1213 [DOI] [PubMed] [Google Scholar]
- 5). Zghonda N, Yoshida S, Ezaki S, Otake Y, Murakami C, Mliki A, Ghorbel A, Miyazaki H. Epsilon-viniferin is more effective than its monomer resveratrol in improving the functions of vascular endothelial cells and the heart. Biosci Biotechnol Biochem. 2012; 76: 954-960 [DOI] [PubMed] [Google Scholar]
- 6). Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010; 635: 1-8 [DOI] [PubMed] [Google Scholar]
- 7). Das S, Das DK. Resveratrol: A therapeutic promise for cardiovascular diseases. Recent Pat Cardiovasc Drug Discov. 2007; 2: 133-138 [DOI] [PubMed] [Google Scholar]
- 8). Voloshyna I, Hussaini SM, Reiss AB. Resveratrol in cholesterol metabolism and atherosclerosis. J Med Food. 2012; 15: 763-773 [DOI] [PubMed] [Google Scholar]
- 9). Dolinsky VW, Chakrabarti S, Pereira TJ, Oka T, Levasseur J, Beker D, Zordoky BN, Morton JS, Nagendran J, Lopaschuk GD, Davidge ST, Dyck JR. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim Biophys Acta. 2013; 1832: 1723-1733 [DOI] [PubMed] [Google Scholar]
- 10). Davinelli S, Sapere N, Zella D, Bracale R, Intrieri M, Scapagnini G. Pleiotropic protective effects of phytochemicals in alzheimer's disease. Oxid Med Cell Longev. 2012; 2012: 386527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007; 224: 274-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Chiba T, Sato Y, Nakanishi T, Yokotani K, Suzuki S, Umegaki K. Inappropriate usage of dietary supplements in patients by miscommunication with physicians in japan. Nutrients. 2014; 6: 5392-5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Madabushi R, Frank B, Drewelow B, Derendorf H, Butterweck V. Hyperforin in st. John's wort drug interactions. Eur J Clin Pharmacol. 2006; 62: 225-233 [DOI] [PubMed] [Google Scholar]
- 14). Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: An updated systematic review. Drugs. 2009; 69: 1777-1798 [DOI] [PubMed] [Google Scholar]
- 15). Hermann R, von Richter O. Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions. Planta Med. 2012; 78: 1458-1477 [DOI] [PubMed] [Google Scholar]
- 16). Shord SS, Shah K, Lukose A. Drug-botanical interactions: A review of the laboratory, animal, and human data for 8 common botanicals. Integr Cancer Ther. 2009; 8: 208-227 [DOI] [PubMed] [Google Scholar]
- 17). Yokotani K, Chiba T, Sato Y, Taki Y, Yamada S, Shinozuka K, Murata M, Umegaki K. Hepatic cytochrome p450 mediates interaction between warfarin and coleus forskohlii extract in vivo and in vitro. J Pharm Pharmacol. 2012; 64: 1793-1801 [DOI] [PubMed] [Google Scholar]
- 18). Virgona N, Yokotani K, Yamazaki Y, Shimura F, Chiba T, Taki Y, Yamada S, Shinozuka K, Murata M, Umegaki K. Coleus forskohlii extract induces hepatic cytochrome p450 enzymes in mice. Food Chem Toxicol. 2012; 50: 750-755 [DOI] [PubMed] [Google Scholar]
- 19). Muto S, Fujita K, Yamazaki Y, Kamataki T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome p450. Mutat Res. 2001; 479: 197-206 [DOI] [PubMed] [Google Scholar]
- 20). Bamba Y, Yun YS, Kunugi A, Inoue H. Compounds isolated from curcuma aromatica salisb. Inhibit human p450 enzymes. J Nat Med. 2011; 65: 583-587 [DOI] [PubMed] [Google Scholar]
- 21). Nakajima M, Itoh M, Yamanaka H, Fukami T, Tokudome S, Yamamoto Y, Yamamoto H, Yokoi T. Isoflavones inhibit nicotine c-oxidation catalyzed by human cyp2a6. J Clin Pharmacol. 2006; 46: 337-344 [DOI] [PubMed] [Google Scholar]
- 22). Chen Y, Xiao P, Ou-Yang DS, Fan L, Guo D, Wang YN, Han Y, Tu JH, Zhou G, Huang YF, Zhou HH. Simultaneous action of the flavonoid quercetin on cytochrome p450 (cyp) 1a2, cyp2a6, n-acetyltransferase and xanthine oxidase activity in healthy volunteers. Clin Exp Pharmacol Physiol. 2009; 36: 828-833 [DOI] [PubMed] [Google Scholar]
- 23). Kimura Y, Ito H, Ohnishi R, Hatano T. Inhibitory effects of polyphenols on human cytochrome p450 3a4 and 2c9 activity. Food Chem Toxicol. 2010; 48: 429-435 [DOI] [PubMed] [Google Scholar]
- 24). Detampel P, Beck M, Krahenbuhl S, Huwyler J. Drug interaction potential of resveratrol. Drug Metab Rev. 2012; 44: 253-265 [DOI] [PubMed] [Google Scholar]
- 25). Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of cyp1a1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998; 58: 5707-5712 [PubMed] [Google Scholar]
- 26). Chang TK, Lee WB, Ko HH. Trans-resveratrol modulates the catalytic activity and mrna expression of the procarcinogen-activating human cytochrome p450 1b1. Can J Physiol Pharmacol. 2000; 78: 874-881 [PubMed] [Google Scholar]
- 27). Chang TK, Yeung RK. Effect of trans-resveratrol on 7-benzyloxy-4-trifluoromethylcoumarin o-dealkylation catalyzed by human recombinant cyp3a4 and cyp3a5. Can J Physiol Pharmacol. 2001; 79: 220-226 [PubMed] [Google Scholar]
- 28). Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, Perloff M, Crowell JA, Alberts DS. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila). 2010; 3: 1168-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Tatefuji T, Yanagihara M, Fukushima S, Hashimoto K. Safety assessment of melinjo (gnetum gnemon l.) seed extract: Acute and subchronic toxicity studies. Food Chem Toxicol. 2014; 67: 230-235 [DOI] [PubMed] [Google Scholar]
- 30). Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22: 659-661 [DOI] [PubMed] [Google Scholar]
- 31). Cho HJ, Yoon IS. Pharmacokinetic interactions of herbs with cytochrome p450 and p-glycoprotein. Evid Based Complement Alternat Med. 2015; 2015: 736431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Zakeri-Milani P, Valizadeh H. Intestinal transporters: Enhanced absorption through p-glycoprotein-related drug interactions. Expert Opin Drug Metab Toxicol. 2014; 10: 859-871 [DOI] [PubMed] [Google Scholar]
- 33). Nabekura T, Kamiyama S, Kitagawa S. Effects of dietary chemopreventive phytochemicals on p-glycoprotein function. Biochem Biophys Res Commun. 2005; 327: 866-870 [DOI] [PubMed] [Google Scholar]
- 34). Choi JS, Choi BC, Kang KW. Effect of resveratrol on the pharmacokinetics of oral and intravenous nicardipine in rats: Possible role of p-glycoprotein inhibition by resveratrol. Pharmazie. 2009; 64: 49-52 [PubMed] [Google Scholar]
- 35). Wadelius M, Sorlin K, Wallerman O, Karlsson J, Yue QY, Magnusson PK, Wadelius C, Melhus H. Warfarin sensitivity related to cyp2c9, cyp3a5, abcb1 (mdr1) and other factors. Pharmacogenomics J. 2004; 4: 40-48 [DOI] [PubMed] [Google Scholar]
- 36). Schwarz UI, Stein CM. Genetic determinants of dose and clinical outcomes in patients receiving oral anticoagulants. Clin Pharmacol Ther. 2006; 80: 7-12 [DOI] [PubMed] [Google Scholar]
- 37). Wadelius M, Pirmohamed M. Pharmacogenetics of warfarin: Current status and future challenges. Pharmacogenomics J. 2007; 7: 99-111 [DOI] [PubMed] [Google Scholar]
- 38). Piver B, Fer M, Vitrac X, Merillon JM, Dreano Y, Berthou F, Lucas D. Involvement of cytochrome p450 1a2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem Pharmacol. 2004; 68: 773-782 [DOI] [PubMed] [Google Scholar]
- 39). Chang TK, Chen J, Lee WB. Differential inhibition and inactivation of human cyp1 enzymes by trans-resveratrol: Evidence for mechanism-based inactivation of cyp1a2. J Pharmacol Exp Ther. 2001; 299: 874-882 [PubMed] [Google Scholar]
- 40). Bertelli AA, Giovannini L, Giannessi D, Migliori M, Bernini W, Fregoni M, Bertelli A. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995; 17: 1-3 [PubMed] [Google Scholar]
- 41). Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin Chim Acta. 1995; 235: 207-219 [DOI] [PubMed] [Google Scholar]
- 42). Fukao H, Ijiri Y, Miura M, Hashimoto M, Yamashita T, Fukunaga C, Oiwa K, Kawai Y, Suwa M, Yamamoto J. Effect of trans-resveratrol on the thrombogenicity and atherogenicity in apolipoprotein e-deficient and low-density lipoprotein receptor-deficient mice. Blood Coagul Fibrinolysis. 2004; 15: 441-446 [DOI] [PubMed] [Google Scholar]
- 43). Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012; 67: 1307-1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, Meszaros LG, Sumegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the akt pathway in type 2 diabetic patients. Br J Nutr. 2011; 106: 383-389 [DOI] [PubMed] [Google Scholar]
- 45). Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012; 32: 537-541 [DOI] [PubMed] [Google Scholar]
- 46). Tome-Carneiro J, Gonzalvez M, Larrosa M, Garcia-Almagro FJ, Aviles-Plaza F, Parra S, Yanez-Gascon MJ, Ruiz-Ros JA, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Consumption of a grape extract supplement containing resveratrol decreases oxidized ldl and apob in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol Nutr Food Res. 2012; 56: 810-821 [DOI] [PubMed] [Google Scholar]
- 47). Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol. 2012; 110: 356-363 [DOI] [PubMed] [Google Scholar]
- 48). Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A, Haskell CF. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr. 2010; 91: 1590-1597 [DOI] [PubMed] [Google Scholar]
- 49). Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, Brown K. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010; 70: 7392-7399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Tani H, Hikami S, Iizuna S, Yoshimatsu M, Asama T, Ota H, Kimura Y, Tatefuji T, Hashimoto K, Higaki K. Pharmacokinetics and safety of resveratrol derivatives in humans after oral administration of melinjo (gnetum gnemon l.) seed extract powder. J Agric Food Chem. 2014; 62: 1999-2007 [DOI] [PubMed] [Google Scholar]


