Abstract
As most spinal cord injuries (SCIs) are incomplete, an important target for promoting neural repair and recovery of lost motor function is to promote the connections of spared descending spinal pathways with spinal motor circuits. Among the pathways, the corticospinal tract (CST) is most associated with skilled voluntary functions in humans and many animals. CST loss, whether at its origin in the motor cortex or in the white matter tracts subcortically and in the spinal cord, leads to movement impairments and paralysis. To restore motor function after injury will require repair of the damaged CST. In this review, I discuss how knowledge of activity-dependent development of the CST—which establishes connectional specificity through axon pruning, axon outgrowth, and synaptic competition among CST terminals—informed a novel activity-based therapy for promoting sprouting of spared CST axons after injur in mature animals. This therapy, which comprises motor cortex electrical stimulation with and without concurrent trans-spinal direct current stimulation, leads to an increase in the gray matter axon length of spared CST axons in the rat spinal cord and, after a pyramidal tract lesion, restoration of skilled locomotor movements. I discuss how this approach is now being applied to a C4 contusion rat model.
Keywords: corticospinal tract, motor cortex, electrical stimulation, spinal direct current stimulation, spinal cord injury, brain injury
Introduction
As most spinal cord injuries (SCIs) are incomplete, an important target for promoting neural repair and recovery of lost motor function is to augment and strengthen the connections of spared descending spinal pathways with spinal motor circuits. Among the pathways, the corticospinal tract (CST) is most associated with skilled voluntary functions in humans and many animals. CST loss, whether at its origin in the motor cortex or in the white matter tracts subcortically and in the spinal cord, leads to movement impairments and paralysis. To restore motor function after injury will, by necessity, require repair of the damaged CST.
Knowledge of the mechanisms of establishment of CST spinal connections during development and CST plasticity induced by partial injury in maturity has informed repair strategies that we have examined (Carmel and Martin, 2014). We have shown that development of CST spinal connections depends on the activity of the corticospinal motor system (Martin et al., 2009). Activation of the system during development leads to CST outgrowth, and inactivation promotes withdrawal of its axons and the spinal connections they make. Importantly, harnessing activity can repair the injured developing corticospinal system and restore motor functions.
CST plasticity after SCI in maturity may contribute to partial recovery of function (Weidner et al., 2001), but this recovery is limited. With serious injury, there are too few spared CST axons to enable effective transmission of control signals; they must be made stronger. In this review, focus is on CST repair after injury by enabling activity-dependent processes that lead both to axonal outgrowth and to establishment of new connections.
Activity-Dependent Development of the CST
The CST is a late-developing motor pathway in humans and animals. Human and cat CST grow into the spinal cord before and near term, respectively, whereas rodent CST develops postnatally. Irrespective of the time of the start of development, important refinement occurs at the last stages, when connectional specificity is established. In the different species, this is when skilled and highly adaptive motor behavior begins to be acquired. Normal CST development is characterized by concurrent axonal outgrowth and axon elimination (i.e., pruning).
A variety of manipulations reveal the importance of activity-dependent synaptic competition in establishment of CST connections during development. Unilateral motor cortex (MCX) inactivation during an early critical period in cats blocks development of a dense and strong contralateral CST (Friel and Martin, 2007). Concurrently, the active MCX/CST not only develops a dense contralateral CST but also an ipsilateral CST (Friel and Martin, 2007). The ipsilateral CST is not a misprojection, as seen in several genetic models of CST miswiring. Rather, it represents maintenance and further outgrowth of an early ipsilateral CST projection that normally is eliminated early in development. Bilateral MCX inactivation results in contralateral CST projections from each MCX. These data suggest that it is not activity per se that is steering CST development, but rather activity-dependent competition. Electrical stimulation of MCX maintains ipsilateral CST projections, at least into feline adolescent development (Salimi and Martin, 2004), and, interestingly, impedes somewhat development of the contralateral CST projection from the non-stimulated side. Again, this is consistent with activity-dependent competition, playing a key role.
Repair of CST Using a Model Injury to Study Sprouting
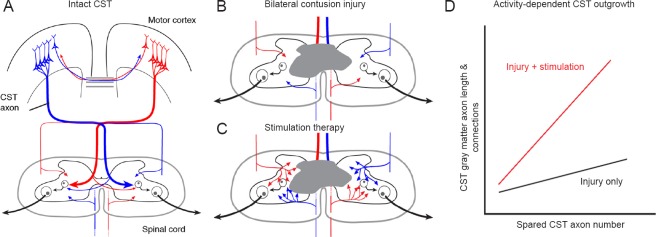
Our developmental findings provide a framework for using activity in maturity to promote CST repair and restoration of function after injury (Carmel and Martin, 2014). Spared axons are too sparse and too weak to contribute to restoration of serious impairments. A way to promote spared CST axon outgrowth is to stimulate the axons, to drive up their activity and their capacity to compete for spinal connections. In extending our developmental findings to maturity, we used the unilateral pyramidal tract lesion (PTX) model (Brus-Ramer et al., 2007). The predominantly contralateral mature CST is shown in Figure 1A. PTX eliminates all of CST from one hemisphere. The denervated side of the spinal cord receives sparse ipsilateral CST projections from the intact MCX. For example, if the pyramidal tract on the right side (red) is lesioned, then the spinal cord on the left would only receive sparse ipsilateral CST axons from the blue pathway. Whereas it is well known that spared ipsilateral axons can sprout in response to PTX, there is no recovery of the skilled locomotion task we study (horizontal ladder walking). We hypothesize that injury-dependent sprouting must be promoted for recovery to occur.
Figure 1.
Activity-based corticospinal tract (CST) repair.
(A) Schematic of CST organization; (B) sparse spared axons after contusion injury; (C) axonal outgrowth after stimulation; and (D) schematic relationship between spared CST axons in the white matter and CST axon length/connections in the gray matter.
Our studies showed that injury alone produces injury-dependent sprouting of ipsilateral CST axons into the denervated side of the spinal cord, which is neither significant over uninjured controls nor does it lead to significant improvement in the horizontal ladder walking task (Brus-Ramer et al., 2007; Carmel et al., 2010). Importantly, by stimulating the injured CST (6 hours of motor cortex epidural stimulation; each day for 10 days), either directly after injury or beginning 8 weeks later, resulted in significant ventral ipsilateral CST outgrowth and significant improvement in the ladder walking task (Carmel et al., 2010, 2014).
CST outgrowth and motor recovery shows a protracted time-course. We have observed that CST outgrowth appears to be several times greater 6 weeks compared with 2 weeks after injury, and motor recovery parallels this time-course of CST outgrowth. We conducted a critical test about our hypothesis. Reversible inactivation of the stimulated hemisphere abolished the recovery produced by stimulation through reinstating the impairment (Carmel et al., 2014).
Our findings show that MCX stimulation promotes CST outgrowth and recovery after PTX. It is important to consider that simulation-induced CST activation can be implemented in animals that have limited behavioral capacity and thus may be useful in humans with serious motor impairment.
Repairing the Injured CST after Cervical Contusion Injury
PTX is an important model for establishing the presence of CST axonal sprouting and has been essential for our demonstration of the importance of activity-dependent competition and simulation efficacy. But because it is a brain lesion, it is not known if stimulation can provoke outgrowth below a spinal lesion, where there is massive Wallerian degeneration and associated tissue reaction that could create an unfavorable environment for activity-dependent outgrowth. We extended our PTX studies to cervical contusion SCI, where it has been shown that a moderate injury spares a small number of CST axons bilaterally (Anderson et al., 2009). With the loss of most CST axons due to the bilateral contusion injury in a rat model (gray; loss of most of the dorsal CST bilaterally), there are only sparse projections from axons located in the lateral and ventral columns (Figure 1B).
In translating our stimulation approach, we were initially concerned that the CST sprouting induced by motor cortex stimulation might not be sufficiently robust caudal to a serious SCI to promote function. For this reason, we modified our stimulation paradigm in three important ways. First, we used intermittent theta burst stimulation (iTBS), which is a stimulation protocol that induces strong short-term physiological plasticity (Huang et al., 2005). The stimulation method we previously used did not induce this kind plasticity. Rather, it resulted in stable (non-augmenting) motor responses. Second, we stimulated bilaterally since we wished to repair the damaged CST on each side. Third, we augmented spinal activity using trans-spinal direct current stimulation (tsDCS) (Song et al., 2016). Cathodal tsDCS facilitates spinal responses (Song et al., 2016). In intact animals, we conducted a series of studies demonstrating that combined iTBS and tsDCS produced larger muscle evoked potentials and disproportionately strong correlations between motor cortex local field potential activity and contralateral forelimb EMG than either stimulation alone. Recently, we used this combined approach and showed motor recovery and CST outgrowth after PTX, equivalent to our earlier study, but with only 30 minutes of stimulation each day (Song et al., 2016).
Using a fourth segment cervical (C4) contusion model, we compared an injury-only group of rats (n = 13) after with animals that received stimulation beginning 1 week after injury (n = 14). As in our recent PTX study (Song et al., 2016) using combined MCX stimulation and tsDCS, we stimulated for 27 minutes each day for 10 days. We used several behavioral assays, but described only the forepaw manipulation task here, the Irvine, Beattie, and Bresnahan test (Irvine et al., 2014). Analysis of the other assays is in progress. The CST was traced 6 weeks post-injury, after the behavioral assays were completed. Our current findings show that the injured + stimulated animals had significantly increased manipulation scores at 6 weeks post-injury compared with the injury-only group. Interestingly, between 4 and 6 weeks post-contusion, the injury-only group did not show a significant improvement (paired t-test), whereas the stimulated group did. For the stimulated group, improvement of distal motor skills in this contusion injury was accompanied by several measures of greater CST outgrowth. Compared with the injured-only group, the injured + stimulated group showed an expanded spinal region at C6 where the CST gray matter axons are located and local CST axon density was greater (shown schematically in Figure 1C). To quantify CST outgrowth further, we examined the relationship between the number of spared CST axons in the white matter and axon length and presynaptic connections (assayed as axon varicosities) within the gray matter. We expected that there would be a longer CST gray matter axon length and more connections for larger numbers of CST axon spared in the white matter, whether or not an animal received treatment (Figure 1D shows this relationship schematically). In support of this idea, data analyzed thus far revealed that the slope of the linear regression was shallow in the injury-only group. By contrast, the slope of the linear regression line in the stimulated group was greater than that of the injured-only group.
Our initial studies are very encouraging, showing that our stimulation approach, based on the PTX lesion model, can be optimized for the more complex and physically damaging spinal injury. For example, the improvement after SCI was observed with only a brief daily therapy session (27 minutes) and for a brief treatment period (10 days); longer periods of daily stimulation or a longer treatment period may improve efficacy.
Overall Conclusion
We have developed a novel treatment approach for CST repair and motor recovery after cervical SCI in rats. This approach produces a durable form of plasticity. This is because stimulation produces CST structural plasticity, axon sprouting and formation of more connections with spinal motor circuits. The theoretical basis for this approach derives from our studies in development, where activity-dependent processes initially help establish strong contralateral CST connections. It is well-recognized that activity-dependent processes can be augmented through intensive behavioral approaches. However, motor functions are limited after serious injuries, reducing the efficacy of behavioral therapies. Instead, to augment the activity of the corticospinal system we leverage brain stimulation and neuromodulation approaches that do not depend on the behavioral capabilities of the injured person.
Acknowledgments
I want to recognize the participation of N. Zareen, M. Shinozaki, D. Ryan, H. Alexander, and S. Naeem in the spinal contusion injury experiments and M. Brus-Ramer and J. Carmel in earlier motor cortex electrical stimulation experiments.
Footnotes
Funding: Support provided by grants from the National Institutes of Health R01NS064004 and the New York State Department of Health Spinal Cord Injury Board C30606GG, C30835GG.
References
- Anderson KD, Sharp KG, Steward O. Bilateral cervical contusion spinal cord injury in rats. Exp Neurol. 2009;220:9–22. doi: 10.1016/j.expneurol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel JB, Martin JH. Motor cortex electrical stimulation augments sprouting of the corticospinal tract and promotes recovery of motor function. Front Integr Neurosci. 2014;8:51. doi: 10.3389/fnint.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel JB, Kimura H, Martin JH. Electrical stimulation of motor cortex in the uninjured hemisphere after chronic unilateral injury promotes recovery of skilled locomotion through ipsilateral control. J Neurosci. 2014;34:462–466. doi: 10.1523/JNEUROSCI.3315-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel KM, Martin JH. Bilateral activity-dependent interactions in the developing corticospinal system. J Neurosci. 2007;27:11083–11090. doi: 10.1523/JNEUROSCI.2814-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Irvine KA, Ferguson AR, Mitchell KD, Beattie SB, Lin A, Stuck ED, Huie JR, Nielson JL, Talbott JF, Inoue T, Beattie MS, Bresnahan JC. The Irvine, Beatties, and Bresnahan (IBB) Forelimb Recovery Scale: an assessment of reliability and validity. Front Neurol. 2014;5:116. doi: 10.3389/fneur.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Friel K, Salimi I, Chakrabarty S. Corticospinal Development. In: Squire L, editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. pp. 302–314. [Google Scholar]
- Salimi I, Martin JH. Rescuing transient corticospinal terminations and promoting growth with corticospinal stimulation in kittens. J Neurosci. 2004;24:4952–4961. doi: 10.1523/JNEUROSCI.0004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Amer A, Ryan D, Martin JH. Combined motor cortex and spinal cord neuromodulation promotes corticospinal system functional and structural plasticity and motor function after injury. Exp Neurol. 2016;277:46–57. doi: 10.1016/j.expneurol.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]