Keywords: nerve regeneration, ischemic stroke, brain structure abnormality, functional magnetic resonance imaging, gray matter density, voxel-based morphometry, Fugl-Meyer Motor Assessment, nervous functional deficiency scale, functional deficiency, neuroplasticity, neural reorganization, neural regeneration
Abstract
Our previous study used regional homogeneity analysis and found that activity in some brain areas of patients with ischemic stroke changed significantly. In the current study, we examined structural changes in these brain regions by taking structural magnetic resonance imaging scans of 11 ischemic stroke patients and 15 healthy participants, and analyzing the data using voxel-based morphometry. Compared with healthy participants, patients exhibited higher gray matter density in the left inferior occipital gyrus and right anterior white matter tract. In contrast, gray matter density in the right cerebellum, left precentral gyrus, right middle frontal gyrus, and left middle temporal gyrus was less in ischemic stroke patients. The changes of gray matter density in the middle frontal gyrus were negatively associated with the clinical rating scales of the Fugl-Meyer Motor Assessment (r = –0.609, P = 0.047) and the left middle temporal gyrus was negatively correlated with the clinical rating scales of the nervous functional deficiency scale (r = –0.737, P = 0.010). Our findings can objectively identify the functional abnormality in some brain regions of ischemic stroke patients.
Introduction
Ischemic stroke characterized by the occlusion of an arterial blood vessel in the brain accounts for more than 80% of all stroke cases (Jauch et al., 2013; Aprile et al., 2014). Ischemic stroke might be accompanied by aphasia, sensory disturbance, or cognitive disorders (Lloyd-Jones et al., 2009; Wolf et al., 2016; Zhong et al., 2016). According to the World Stroke Campaign of the World Stroke Organization, one in six individuals will have a stroke in their lifetime (Kaste, 2013; Mozaffarian et al., 2016), and 33% of all deaths in America are due to stroke (Willey et al., 2010).
As neuroimaging techniques becoming widely available, an increasing number of studies have attempted to explore the cerebral abnormalities in stroke patients. Park et al. (2011) used resting-state functional magnetic resonance imaging (fMRI) to study stroke, and found that functional connectivity of the ipsilesional primary motor cortex (M1) of stroke patients was different from that of healthy participants. Another study combined fMRI with diffusion tensor imaging (DTI), demonstrating that the functional organization of the residual distributed motor system is related to the degree of disruption in the corticospinal tract (Wei et al., 2013). Tuladhar et al. (2013) reported that, compared with healthy participants, stroke patients have impaired connectivity within the default mode network, which might lead to post-stroke cognitive dysfunction. All of thesestudies suggested that cerebral function and connectivity in stroke patients are certainly different from what is seen in healthy participants.
Recently, functional magnetic resonance imaging (fMRI) has played an integral role in defining the neural substrates and system-level neural mechanisms underlying recovery after stroke (Fazekas et al., 2015; Lefebvre et al., 2015; van de Ven et al., 2015). Cortical reorganization has been characterized by observing changes in cerebral activation during motor recovery following stroke (Ward et al., 2003; Tombari et al., 2004; Nair et al., 2005; Kim et al., 2006; Ward et al., 2006; Loubinoux et al., 2007; Chechlacz et al., 2013). For example, using voxel-based morphometry (VBM) analysis, Beal et al. (2013) found that the middle temporal gyrus and superior temporal sulcus were linked to visual and tactile extinction of stroke patients. Additionally, by comparing fluent children with those who stutter, they found that the pathogenesis of stuttering was closely related to lower gray matter density (GMD) in the bilateral inferior frontal gyrus and left putamen, and higher GMD in the right rolandic operculum and superior temporal gyrus.
VBM has been applied widely to various brain diseases. Our previous study showed that the activity in the cerebral regions of patients with ischemic stroke was significantly different from that in healthy individuals (Wu et al., 2015). However, how GMD changes after ischemic strokes is still unknown. The aim of this study was to use structural MRI to investigate whether cerebral GMD changes in patients with ischemic strokes. A secondary goal was to determine how changes in regional GMD are related to clinical variables.
Participants and Methods
Participants
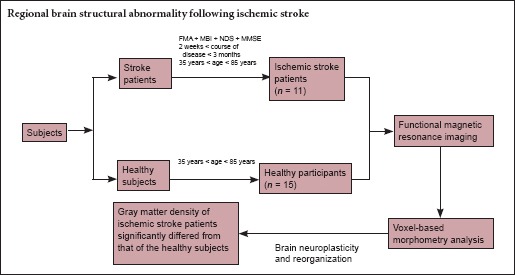
This study was approved by the Ethics Committee of Chengdu University of Traditional Chinese Medicine in China (No. 2011KL-002). The ischemic stroke patients were recruited at the outpatient and inpatient department of the Teaching Hospital, Chengdu University of Traditional Chinese Medicine in China from January 2011 to May 2013. All participants gave their written informed consent. The inclusion criteria for patients and healthy participants were described in detail previously (Wu et al., 2015). Briefly, the inclusion criteria for patients were as follows: (1) A first-time diagnosis of left lesions based on computed tomography or MRI. (2) Stroke had occurred within approximately 2 weeks to 3 months. (3) Dyskinesia in the right limb (upper or lower), as assessed by the Fugl-Meyer Motor Assessment (FMA)test (muscle strength > level II) (Duncan et al., 1983; Berglund and Fugl-Meyer, 1986; Sanford et al., 1993; Gladstone et al., 2002). (4) Being right handed, aged 35–85 years. (5) Absence of dementia, aphasia, neurological, or psychiatric disorders as determined by the Mini-Mental State Examination (MMSE) (Becker et al., 2016). No patients accepted any rehabilitation therapy. Patients who had contraindications for the MRI scan or who suffered from serious complications were excluded. The healthy participants were recruited by advertisement. Healthy people were gender- and age-matched with ischemic stroke patients, and were included only if they had no history of neurological or psychiatric diseases. People who did not satisfy the above conditions were excluded from the experiment.
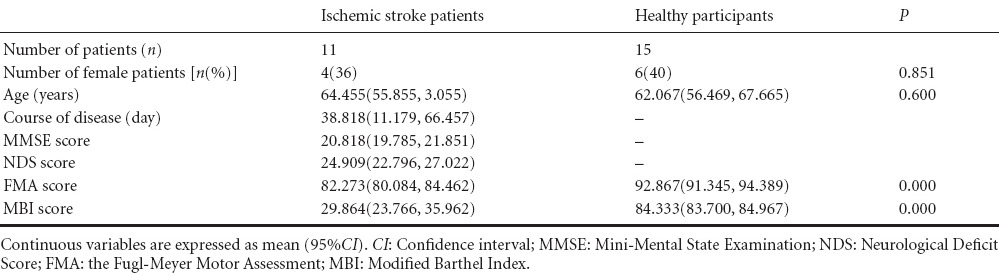
Eleven ischemic stroke patients (7 males and 4 females) and 15 age-matched healthy participants (9 males and 6 females) entered the final analysis. No significant differences in age or gender were found between the two groups (P > 0.05; Table 1).
Table 1.
Baseline characteristics of ischemic stroke patients and healthy participants
Clinical assessments
The FMA, neurological deficiency scale (NDS) and the modified Barthel index (MBI) were applied to assess patient motor skills, severity of neurological function deficits, and the ability of self-care independence, respectively.
The FMA has been widely employed in clinical trials that focus on motor outcomes after stroke. It has been found to be valid (Berglund and Fugl-Meyer, 1986; Gladstone et al., 2002) and reliable (Duncan et al., 1983; Sanford et al., 1993; Gladstone et al., 2002). FMA scores range from 0 to 100, with 0 indicating absence of motor deficits and higher scores indicating worsening of motor deficits. The NDS, revised based on the Scandinavian Stroke Scale, was used to observe and estimate the severity of neurological functional deficit after stroke (Chen et al., 2009a). Scores on this eight-item scale range from 0 to 45, with higher scores indicating worse neurological function. The ability of self-care independence was evaluated by the MBI, which consists of 10 domains, including help needed with feeding, transferring, grooming, using the toilet, bathing, walking, climbing stairs, or dressing, and the presence or absence of fecal or urinary incontinence. The maximum MBI score is 100, with higher scores indicating better independence in activities of daily living (Wei et al., 2016).
fMRI scan
All brain images were acquired on a 3T Siemens MRI scanner (MAGNETOM Trio Tim, Siemens, Amberg, Germany) at the Huaxi Magnetic Resonance Research Center, West China Hospital of Sichuan University, China. The VBM protocol used a spin-echo planar image sequence with the following parameters: repetition time/echo time = 1,900 ms/2.26 ms, flip angle = 9°; in-plane matrix resolution =256 × 256; slices = 176; field of view = 16 × 16 mm2; voxel size = 1 × 1 × 1 mm3). During the scan, each participant wore a foam cushion to prevent head translation and rotation. Additionally, their eyes were blindfolded and their ears were plugged. In order to avoid the probable influence on cerebral activity and size of menstrual cycles, all female participants were scanned during the week following their menstrual periods (Hagemann et al., 2011; Veldhuijzen et al., 2013).
Data analysis
Clinical variables
Data were analyzed using SPSS 19.0 (IBM Inc., Armonk, New York, USA) by two evaluators who were blinded to the experiment. A chi-square test and an independent samples t-test were applied to categorical variables and numerical variables, respectively. A value of P < 0.05 was considered a statistically significant difference.
VBM analysis
T1-weighted images were analyzed using the VBM toolbox (http://dbm.neuro.uni-jena.de/vbm.html) for Statistical Parametric Mapping 8 (SPM8) (Welcome Department of Cognitive Neurology, London, UK). A customized VBM approach was implemented by combining the VBM8 toolbox with Diffeomorphic Anatomical Registration using Exponentiated Lie Algebra (DARTEL) (Ashburner, 2007). DARTEL has been shown to produce a more accurate registration than the standard VBM procedure (Klein et al., 2009) and enables increased sensitivity to findings such as the correlation between gray matter volume and measures such as age and gender. First, the “New Segmentation” algorithm from SPM8 was applied to every T1-weighted MR image in order to extract tissue maps corresponding to gray matter, white matter, and cerebrospinal fluid. This algorithm, which is an improvement over the unified segmentation algorithm (Ashburner and Friston, 2005), uses a Bayesian framework to iteratively perform the probabilistic tissue classification and spatial non-linear deformation in terms of Montreal Neurological Institute (MNI) space. Next, these segmented tissue maps were input into DARTEL to create a customized, more population-specific template (Ashburner, 2007). DARTEL estimates the best set of smooth deformations working from each participant's tissue to their common average, applies the deformations to create a new average, and then reiterates the process until convergence is achieved. We used a set of standard MNI tissues maps and a multivariate tissue-affinity-registration algorithm provided by SPM and DARTEL for that process. At the end of the process, each participant's gray matter map was warped and each participant's gray matter segments were modulated for non-linear effects so that further analyses did not have to account for differences in head size. Finally, the warped and modulated gray matter images were smoothed by convolving an 8 × 8 × 8 mm3 full-width at half-maximum isotropic Gaussian kernel. After completing these image analyses, we obtained smoothed and modulated gray matter images to be used for statistical analysis. The significance of group differences was set at P < 0.05 using family-wise error correction.
Correlation analysis between FMA scores and GMD
We used a partial correlation analysis to assess the relationship between clinical variables (FMA, NDS, and MBI) scores and GMD. For each patient, we selected the peak voxel and the neighboring 100 voxels within each cluster showing different volumes as the region of interest for correlation analysis. The voxels not belonging to the same anatomic region within the cluster were discarded, and the volumes of the surviving voxels were extracted and averaged. Pearson correlation coefficients were calculated between the mean volumes of the clinical variables (FMA, NDS, and MBI scores) and the GMD.
Results
Participant characteristics
The lesion locations for the ischemic stroke patients were primarily in the left basal ganglia. FMA scale and MBI scale differed significantly between patients and healthy participants (P = 0.01; Table 1).
VBM results in ischemic stroke patients
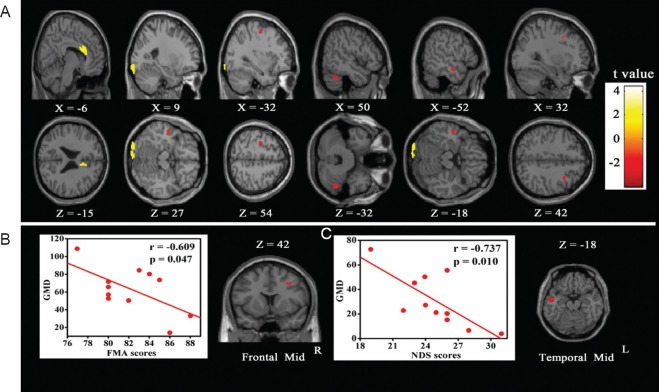
Compared with healthy participants, we observed significantly lower GMD in the left precentral gyrus, right cerebellum, right middle frontal gyrus, and left middle temporal gyrus in ischemic stroke patients. Additionally, greater GMD was observed in the left inferior occipital gyrus and right anterior cingulum of the patients compared with the healthy participants (Table 2, Figure 1A).
Table 2.
Significant differences of gray matter density between ischemic stroke patients and healthy participants
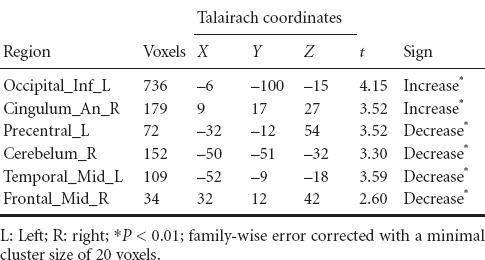
Figure 1.
Significant differences in GMD between ischemic stroke patients and healthy participants.
(A) Compared with healthy participants, the ischemic stroke patients showed lower GMD in the left precentral gyrus, right cerebellum, left middle temporal gyrus, and right middle frontal gyrus. Higher GMD was seen in the left inferior occipital gyrus and right anterior cingulum. (B) A significant negative correlation was found between the GMD index in the left middle temporal gyrus and NDS score (r = –0.737, P = 0.010). (C) A significant negative correlation was also found between the GMD index in the right middle frontal gyrus and FMA scores (r = –0.609, P = 0.047). GMD: Gray matter density; NDS: nervous functional deficiency scale; FMA: Fugl-Meyer Motor Assessment.
Correlations between GMD index and clinical scale scores
We detected a significant negative correlation between GMD index and NDS scores in the left middle temporal gyrus (r = –0.737, P = 0.010), and between GMD and FMA scores in the right middle frontal gyrus (r = –0.609, P = 0.047) (Figure 1B, C). GMD in other regions did not seem to correlate with the NDS, FMA or MBI scores.
Discussion
In this study, we found that GMD in the left precentral gyrus, right cerebellum, right middle frontal gyrus, and left middle temporal gyrus was lower in ischemic stroke patients. Simultaneously, the GMD in the left inferior occipital gyrus and right anterior cingulum were higher than in control participants. Our pervious diffusion tensor imaging study indicated that the ischemic stroke patients exhibited significantly decreased fractional anisotropy and increased axial and radial diffusivity compared with healthy participants (Li et al., 2015). Other studies (Carter et al., 2010; Zhu et al., 2014; Li et al., 2016) have reported that local lesions in ischemic stroke patients may lead to functional and structural recombination of perilesional and remote brain regions.
Moreover, the majority of regions, showing differences between groups in this study, such as the frontal gyrus, temporal gyrus, cerebellum, and cingulate, are the same cerebral regions in which we used regional homogeneity analysis to show that activity was significantly different between patients and stroke victims (Wu et al., 2015). We therefore predicted that the structural and functional changes in these regions might present motor, emotional, and cognitive abnormalities in ischemic stroke patients.
Motor regions
Motor function disorder is recognized as the most common symptom of ischemic stroke patients. Consistent with other VBM studies of ischemic stroke (Kim et al., 2006; Gauthier et al., 2012), the present study found lower GMD in the precentral gyrus, cerebellum, and middle frontal cortex, which are regions strongly associated with motor function. The precentral gyrus, located in the frontal lobe, is connected to primary motor cortex (M1) and non-motor cortex (Xu et al., 2014; Landsmann et al., 2016). Park et al. (2011) indicated that decreases in connectivity between M1 and other motor-related areas in the frontal lobe, as well as non-motor regions in the parietal and occipital cortices, might reflect plastic changes that compensate for impaired connectivity with the opposite foci of hemisphere. The cerebellum is widely thought to be involved in motor control, maintaining body balance, controlling posture and gait, modulatingmuscle tone, and coordinating the accuracy of voluntary movements (Stoodley et al., 2012). Additionally, cerebellar lobules IV–V and VIII are related to overt movement (Habas et al., 2009; Krienen and Buckner, 2009; O’Reilly et al., 2010; Stoodley and Schmahmann, 2010). Tang et al. (2016) found that the functional connection between the right cerebellum and the left precentral gyrus was significantly lower in left subcortical stroke patients, suggesting that abnormal functional connection in the right cerebellum may result in an impaired functional network and motor disorder. In our study, we observed the same cerebral changes; GMD in the right cerebellum and left precentral gyrus decreased. The motor deficiency of ischemic stroke patients in our study may therefore be closely connected to the changes in the right cerebellum and left precentral gyrus. Moreover, the frontal lobe is the site for motor planning and motor output and the middle frontal gyrus is connected to premotor cortex (Hortensius et al., 2016). Recent task-based fMRI studies have indicated that changes in the cerebellum and middle frontal cortex after stroke are related to motor recovery (Tombari et al., 2004; Puh et al., 2007).
Emotional and cognitive regions
Studies indicate that ischemic stroke patients are at increased risk of developing cognitive and emotional impairment (del Ser et al., 2005). A recent review reported that approximately one third of stroke survivors might suffer from depression during their follow-up (Hackett et al., 2005; Mittal et al., 2016). In the current study, we found significant differences in some brain regions associated with cognition and emotion, including the left middle frontal gyrus, middle temporal gyrus and inferior occipital gyrus, as well as right inferior cingulate gyrus and cerebellum.
The cerebellum and middle frontal gyrus are involved in cognitive processing other than motor control. Studies have shown that the cerebellum is involved in executive functions, such as abstract reasoning, working memory, and information updating (Collette et al., 2007; Monti et al., 2007; Habas et al., 2009). Additionally, the lower GMD in the right middle frontal cortex that we found was in line with another study of ischemic stroke patients (Chen et al., 2009b). The middle frontal gyrus located is connected to brain regions devoted to cognitive and emotional processing, and it is related to the management of cognitive load required for motor performance (Puh et al., 2007). In this study, lower GMD in the right middle frontal gyrus was significantly negatively correlated with the NDS scores, indicating that the deficiencies increase as the density of gray matter in this region is reduced. The NDS scales can assess patients’ neurological function in terms of consciousness, muscle strength of upper and lower limb, and ambulation. These results partly support findings related to middle frontal gyrus abnormalities. The middle temporal gyrus is linked to the default mode network, which involved in episodic memory processing (Tuladhar et al., 2013). Some studies have shown that compared with healthy participants, connectivity of middle temporal gyrus and default mode network was impaired in stroke patients (Tuladhar et al., 2013), and that GMD decrease in the middle temporal gyrus was related to visual and tactile extinction in stroke patients (Chechlacz et al., 2013).
To date, a large number of studies addressing human and animal models have shown that after stroke, some conditions such as injury, stimulation, and learning, can induce neurons and brain networks to reorganize themselves through neuroplastic changes (Clarkson et al., 2013; Karabanov et al., 2013). The activated and suppressed brain regions in the present study might be related to neuroplastic reorganization. Because the functional connections between the affected brain areas and other regions are damaged, the balance of interhemispheric inhibition is destroyed after stroke. Thus, some brain regions become hyperactivated, while others are suppressed (Manganotti et al., 2008; Clarkson et al., 2010). We hypothesize that the lower GMD that we observed in this study might result from a disorder of neuroplasticity and reorganization. Additionally, we found two cerebral regions in which GMD was higher in stroke victims. This was probably related to hyperactivation of brain neuroplasticity.
Conclusions
GMD of ischemic stroke patients significantly differed from that of healthy participants. The brain regions that differed included the left inferior occipital gyrus, right anterior cingulum, left precentral gyrus, right cerebellum, right middle frontal gyrus, and left middle temporal gyrus. These results were related to functional abnormalities (motor, sensory, and emotion). We propose that these regions might be potential targets for neuroplasticity and neural reorganization after stroke.
Limitations
The small sample size is the main limitation to this pilot study. Moreover, a more thorough understanding of the correlations between GMD in the middle temporal gyrus and FMA score, and between GMD in the middle frontal gyrus and NDS score could be achieved by increasing the sample size. Although it is a pilot study, the demonstration of GMD alterations in ischemic stroke patients could provide a new approach for future studies.
Acknowledgments
We thank Qie-zhu Wu from the Radiology Department, West China Hospital of Sichuan University, China for technical assistance in fMRI recordings, and Xi-mei Xie, Xiaoqing Zeng and Jie Li from Chengdu University of Traditional Chinese Medicine, China for help during clinical research.
Footnotes
Funding: This study was financially supported by the National Program on Key Basic Research Project of China (973 Program), No. 2012CB518501; and the National Natural Science Foundation of China, No. 81072864.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Phillips A, Raye W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Aprile I, Rabuffetti M, Padua L, Di Sipio E, Simbolotti C, Ferrarin M. Kinematic analysis of the upper limb motor strategies in stroke patients as a tool towards advanced neurorehabilitation strategies: a preliminary study. Biomed Res Int 2014. 2014 doi: 10.1155/2014/636123. 636123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Beal DS, Gracco VL, Brettschneider J, Kroll RM, De Nil LF. A voxel-based morphometry (VBM) analysis of regional grey and white matter volume abnormalities within the speech production network of children who stutter. Cortex. 2013;49:2151–2161. doi: 10.1016/j.cortex.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ, Tanzi P, Zierath D, Buckwalter MS. Antibodies to myelin basic protein are associated with cognitive decline after stroke. J Neuroimmunol. 2016;295-296:9–11. doi: 10.1016/j.jneuroim.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund K, Fugl-Meyer AR. Upper extremity function in hemiplegia. A cross-validation study of two assessment methods. Scand J Rehabil Med. 1986;18:155–157. [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DLW, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechlacz M, Terry A, Demeyere N, Douis H, Bickerton WL, Rotshtein P, Humphreys GW. Common and distinct neural mechanisms of visual and tactile extinction: A large scale VBM study in sub-acute stroke. Neuroimage Clin. 2013;2:291–302. doi: 10.1016/j.nicl.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Venketasubramanian N, Gan RN, Lambert C, Picard D, Chan BPL, Chan E, Bousser MG, Xuemin S. Danqi Piantang Jiaonang (DJ), a traditional Chinese medicine, in poststroke recovery. Stroke. 2009a;40:859–863. doi: 10.1161/STROKEAHA.108.531616. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen X, Xiao W, Mok VCT, Wong KS, Tang WK. Frontal lobe atrophy is associated with small vessel disease in ischemic stroke patients. Clin Neurol Neurosurg. 2009b;111:852–857. doi: 10.1016/j.clineuro.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, MacIsaac SE, Mody I, Carmichael ST. Reducing excessive GABAergic tonic inhibition promotes post-stroke functional recovery. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, López-Valdés HE, Overman JJ, Charles AC, Brennan KC, Thomas Carmichael S. Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. J Cereb Blood Flow Metab. 2013;33:716–723. doi: 10.1038/jcbfm.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Arigoni F, Delfiore G, Degueldre C, Luxen A, Salmon E. Mapping the updating process: common and specific brain activations across different versions of the running span task. Cortex. 2007;43:146–158. doi: 10.1016/s0010-9452(08)70452-0. [DOI] [PubMed] [Google Scholar]
- del Ser T, Barba R, Morin MM, Domingo J, Cemillan C, Pondal M, Vivancos J. Evolution of cognitive impairment after stroke and risk factors for delayed progression. Stroke. 2005;36:2670–2675. doi: 10.1161/01.STR.0000189626.71033.35. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Enzinger C, Schmidt R, Grittner U, Giese AK, Hennerici MG, Huber R, Jungehulsing GJ, Kaps M, Kessler C, Martus P, Putaala J, Ropele S, Tanislav C, Tatlisumak T, Thijs V, von Sarnowski B, Norrving B, Rolfs A. Brain magnetic resonance imaging findings fail to suspect Fabry disease in young patients with an acute cerebrovascular event. Stroke. 2015;46:1548–1553. doi: 10.1161/STROKEAHA.114.008548. [DOI] [PubMed] [Google Scholar]
- Gauthier LV, Taub E, Mark VW, Barghi A, Uswatte G. Atrophy of spared grey matter tissue predicts poorer motor recovery and rehabilitation response in chronic stroke. Stroke. 2012;43:453–457. doi: 10.1161/STROKEAHA.111.633255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Keller K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity network. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW, Gaser C. Changes in brain size during the menstrual cycle. PLoS One. 2011;6:e14655. doi: 10.1371/journal.pone.0014655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortensius R, Terburg D, Morgan B, Stein DJ, van Honk J, de Gelder B. The role of the basolateral amygdala in the perception of faces in natural contexts. Philos Trans R Soc Lond B Biol Sci. 2016:371. doi: 10.1098/rstb.2015.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H American Heart Association Stroke Council; Council on Cardiovascular Nursing, Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- Karabanov AN, Chao CC, Paine R, Hallett M. Mapping different intra-hemispheric parietal-motor networks using twin coil TMS. Brain Stimul. 2013;6:384–389. doi: 10.1016/j.brs.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaste M. Stroke: advances in thrombolysis. Lancet Neurol. 2013;12:2–4. doi: 10.1016/S1474-4422(12)70273-9. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Kwon YH, Hallett M, Kim JH, Jang SH. Longitudinal fMRI study for locomotor recovery in patients with stroke. Neurology. 2006;67:330–333. doi: 10.1212/01.wnl.0000225178.85833.0d. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsmann B, Pinter D, Pirker E, Pichler G, Schippinger W, Weiss EM, Mathie G, Gattringer T, Fazekas F, Enzinger C. An exploratory intervention study suggests clinical benefits of training in chronic stroke to be paralleled by changes in brain activity using repeated fMRI. Clin Interv Aging. 2016;11:97–103. doi: 10.2147/CIA.S95632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Dricot L, Laloux P, Gradkowski W, Desfontaines P, Evrard F, Peeters A, Jamart J, Vandermeeren Y. Neural substrates underlying stimulation-enhanced motor skill learning after stroke. Brain. 2015;138:149–163. doi: 10.1093/brain/awu336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu P, Liang F, Huang W. The microstructural status of the corpus callosum is associated with the degree of motor function and neurological deficit in stroke patients. PLoS One. 2015;10:e0122615. doi: 10.1371/journal.pone.0122615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang D, Zhang H, Wang Y, Wu P, Zhang H, Yang Y, Huang W. Changes of brain connectivity in the primary motor cortex after subcortical stroke: a multimodal magnetic resonance imaging study. Medicine. 2016;95:e2579. doi: 10.1097/MD.0000000000002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, De Boissezon X, Marque P, Pariente J, Albucher JF, Berry I, Chollet F. Prognostic value of fMRI in recovery of hand function in subcortical stroke patients. Cereb Cortex. 2007;17:2980–2987. doi: 10.1093/cercor/bhm023. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Acler M, Zanette GP, Smania N, Fiaschi A. Motor cortical disinhibition during early and late recovery after stroke. Neurorehabil Neural Repair. 2008;22:396–403. doi: 10.1177/1545968307313505. [DOI] [PubMed] [Google Scholar]
- Mittal N, Hurn PD, Schallert T. Exploring a need for improved preclinical models of post-stroke depression. Neural Regen Res. 2016;11:561–562. doi: 10.4103/1673-5374.180736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Osherson DN, Martinez MJ, Parsons LM. Functional neuroanatomy of deductive inference: a language-independent distributed network. Neuroimage. 2007;37:1005–1016. doi: 10.1016/j.neuroimage.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;119:480–486. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Nair DG, Fuchs A, Burkart S, Steinberg FL, Kelso JAS. Assessing recovery in middle cerebral artery stroke using functional MRI. Brain Inj. 2005;19:1165–1176. doi: 10.1080/02699050500149858. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, Kim YH. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42:1357–1362. doi: 10.1161/STROKEAHA.110.596155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puh U, Vovk A, Sevšek F, Šuput D. Increased cognitive load during simple and complex motor tasks in acute stage after stroke. Int J Psychophysiol. 2007;63:173–180. doi: 10.1016/j.ijpsycho.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73:447–454. doi: 10.1093/ptj/73.7.447. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Zhao Z, Chen C, Zheng X, Sun F, Zhang X, Tian J, Fan M, Wu Y, Jia J. decreased functional connectivity of homotopic brain regions in chronic stroke patients: a resting state f mri0 study. PLoS One. 2016;11:e0152875. doi: 10.1371/journal.pone.0152875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, Cassol E, Chollet F. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage. 2004;23:827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Tuladhar AM, Snaphaan L, Shumskaya E, Rijpkema M, Fernandez G, Norris DG, de Leeuw FE. Default mode network connectivity in stroke patients. PLoS One. 2013;8:e66556. doi: 10.1371/journal.pone.0066556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven RM, Schmand B, Groet E, Veltman DJ, Murre JMJ. The effect of computer-based cognitive flexibility training on recovery of executive function after stroke: rationale, design and methods of the TAPASS study. BMC Neurol. 2015;15:144. doi: 10.1186/s12883-015-0397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuijzen DS, Keaser ML, Traub DS, Zhuo J, Gullapalli RP, Greenspan JD. The role of circulating sex hormones in menstrual cycle dependent modulation of pain-related brain activation. Pain. 2013;154:548–559. doi: 10.1016/j.pain.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Longitudinal changes in cerebral response to proprioceptive input in individual patients after stroke: an FMRI study. Neurorehabil Neural Repair. 2006;20:398–405. doi: 10.1177/1545968306286322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Bai L, Wang J, Dai R, Tong RK, Zhang Y, Song Z, Jiang W, Shi C, Li M, Ai L, Tian J. A longitudinal study of hand motor recovery after sub-acute stroke: a study combined FMRI with diffusion tensor imaging. PLoS One. 2013;8:e64154. doi: 10.1371/journal.pone.0064154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YX, Zhao X, Zhang BC. Synergistic effect of moxibustion and rehabilitation training in functional recovery of post-stroke spastic hemiplegia. Complement Ther Med. 2016;26:55–60. doi: 10.1016/j.ctim.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Willey JZ, Disla N, Moon YP, Paik MC, Sacco RL, Boden-Albala B, Elkind MS, Wright CB. Early depressed mood after stroke predicts long-term disability: the Northern Manhattan Stroke Study (NOMASS) Stroke. 2010;41:1896–1900. doi: 10.1161/STROKEAHA.110.583997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf TJ, Polatajko H, Baum C, Rios J, Cirone D, Doherty M, McEwen S. Combined cognitive-strategy and task-specific training affects cognition and upper-extremity function in subacute stroke: an exploratory randomized controlled trial. Am J Occup Ther. 2016;70:7002290010p7002290011–7002290010p7002290010. doi: 10.5014/ajot.2016.017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Zeng F, Li YX, Yu BL, Qiu LH, Qin W, Li J, Zhou YM, Liang FR. Changes of resting cerebral activities in subacute ischemic stroke patients. Neural Regen Res. 2015;10:760–765. doi: 10.4103/1673-5374.156977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Qin W, Chen H, Jiang L, Li K, Yu C. Contribution of the resting-state functional connectivity of the contralesional primary sensorimotor cortex to motor recovery after subcortical stroke. PLoS One. 2014;9:e84729. doi: 10.1371/journal.pone.0084729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong CK, Zhong XY, Xu T, Zhang YH. Measures of abdominal adiposity and risk of stroke: a dose-response meta-analysis of prospective studies. Biomed Environ Sci. 2016;29:12–23. doi: 10.3967/bes2016.002. [DOI] [PubMed] [Google Scholar]
- Zhu D, Chang J, Freeman S, Tan Z, Xiao J, Gao Y, Kong J. Changes of functional connectivity in the left frontoparietal network following aphasic stroke. Front Behav Neurosci. 2014;8:167. doi: 10.3389/fnbeh.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]