Keywords: nerve regeneration, subcortical ischemic vascular dementia, carnosine, corpus callosum, neuron, internal capsule, oligodendrocyte, optic tract, white matter damage, neural regeneration
Abstract
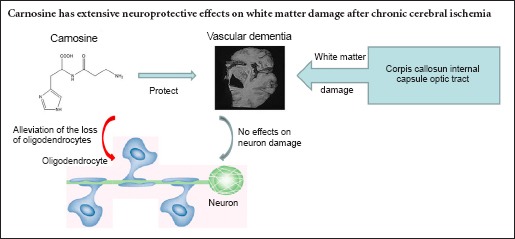
Carnosine is a dipeptide that scavenges free radicals, inhibits inflammation in the central nervous system, and protects against ischemic and hypoxic brain damage through its anti-oxidative and anti-apoptotic actions. Therefore, we hypothesized that carnosine would also protect against white matter damage caused by subcortical ischemic injury. White matter damage was induced by right unilateral common carotid artery occlusion in mice. The animals were treated with 200, 500 or 750 mg/kg carnosine by intraperitoneal injection 30 minutes before injury and every other day after injury. Then, 37 days later, Klüver-Barrera staining, toluidine blue staining and immunofluorescence staining were performed. Carnosine (200, 500 mg/kg) substantially reduced damage to the white matter in the corpus callosum, internal capsule and optic tract, and it rescued expression of myelin basic protein, and alleviated the loss of oligodendrocytes. However, carnosine at the higher dose of 750 mg/kg did not have the same effects as the 200 and 500 mg/kg doses. These findings show that carnosine, at a particular dose range, protects against white matter damage caused by chronic cerebral ischemia in mice, likely by reducing oligodendroglial cell loss.
Introduction
Vascular dementia is the second most prevalent form of dementia (Kalaria et al., 2008). Vascular dementia is a clinicopathological entity that is distinct from Lewy body dementia, Alzheimer's dementia and frontotemporal dementia. Subcortical ischemic vascular dementia (SIVD), induced by chronic cerebral hypoperfusion due to small-artery disease, is a common type of vascular dementia. It is often observed in patients with atherosclerosis or hypertension, as well as in the aging population (Areosa et al., 2005). Many factors, such as hypertension, hyperlipidemia, atrial fibrillation and diabetes, may increase the risk of the disease (Selnes and Vinters, 2006). However, the pathogenetic mechanisms of SIVD are unclear. The characteristic features of the disease include progressive demyelination, white matter damage and cognitive impairment. Computed tomography scans and magnetic resonance imaging frequently detect periventricular and subcortical white matter damage in SIVD patients (Hashimoto and Ikeda, 2015). This white matter damage has been found to be associated with the loss of myelin and the apoptotic death of oligodendrocytes (Johnson et al., 2013). The cerebral white matter contains oligodendrocytes and myelin sheaths formed by oligodendrocytes, which are sensitive to various adverse stimuli, including ischemia (Benarroch, 2009). Proteolipid protein (PLP) and myelin basic protein (MBP), which are found throughout the myelin sheath, have been demonstrated to be linked with vascular dementia (Barker et al., 2013).
Therapy for SIVD has received great attention (Olivares et al., 2012; Safouris et al., 2015). There are several compounds with different mechanisms of action that display mild efficacy in SIVD patients (Craig and Birks, 2006; Taguchi, 2011; Tomimoto, 2011). However, there are no drugs approved for halting the progression of SIVD (McGuinness et al., 2009). Vasodilators and acetylcholinesterase inhibitors are used for treating SIVD, but they exhibit limited efficacy (Craig and Birks, 2006). Therefore, it is imperative to develop more effective drugs for the prevention and treatment of SIVD.
Carnosine (β-alanyl-L-histidine) is a natural dipeptide found at high levels in the central nervous system (Usui et al., 2013). Carnosine has been assigned many putative functions, including free radical scavenger, anti-inflammatory agent and pH buffer (Kurata et al., 2006; Babizhayev and Yegorov, 2010). Carnosine protects mice and rats against ischemia-induced brain damage and hypoxia through its antioxidative actions and by attenuating apoptosis in transient cerebral ischemia (Jin et al., 2005; Shen et al., 2010). Therefore, we hypothesized that carnosine may protect against SIVD.
In the present study, experimental SIVD was induced by right unilateral common carotid artery occlusion, which better simulates the clinical cognitive impairment caused by white matter damage in SIVD (Yoshizaki et al., 2008; Ma et al., 2012). In addition, in our previous study (Ma et al., 2015), we showed that the pathological lesions after right unilateral common carotid artery occlusion share common features with SIVD. The aim of our study is to investigate the effect of carnosine on white matter damage in the SIVD model. Our novel model should help in the study of the mechanisms and therapy of SIVD.
Materials and Methods
Ethics statement and animals
The animal studies were approved by the committee for experimental animals of the Xinhua Hospital of Shanghai Jiao Tong University School of Medicine, and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment. Fifty male C57BL/6 mice (specific-pathogen-free/viral-antibody-free), 8-weeks-old, weighing 22–30 g, were purchased from Charles River (license No. SCXK 2011-0011). Mice were housed under a 12-hour light/dark cycle, and were allowed free access to food and water. The mice were equally and randomly divided into five groups: sham, SIVD, SIVD + carnosine 200 mg/kg, SIVD + carnosine 500 mg/kg, SIVD + carnosine 750 mg/kg.
SIVD model establishment
After anesthesia with sodium pentobarbital (60 mg/kg, intraperitoneally), the right common carotid artery was isolated from the adjacent vagus nerve, and then double-ligated with 6-0 silk sutures to achieve right unilateral common carotid artery occlusion. Mice in the sham group were subjected to the same procedure, except for carotid ligation. The mortality rate in this model was 0% (Ma et al., 2012).
Carnosine administration
Carnosine (Sigma, St. Louis, MO, USA), dissolved in sterile saline, was administered by intraperitoneal injection. Adult male mice were administered saline (0.1 mL) in the sham and SIVD group, and carnosine in the SIVD + carnosine 200 mg/kg, SIVD + carnosine 500 mg/kg and SIVD + carnosine 750 mg/kg group, 30 minutes before surgery and every other day until the mice were sacrificed. Sodium pentobarbital (60 mg/kg) was used for anesthesia. The mice were sacrificed on day 37 after model establishment, and the brain tissues were removed for Klüver-Barrera and histochemical staining.
Klüver-Barrera staining
Mice were deeply anesthetized and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4). The brains were removed quickly and stored in 4% paraformaldehyde at 4°C for 24 hours, and then in 30% sucrose for 3 days. Frozen brain sections (10-μm-thick) were made on a cryostat (SM2000R; LEICA, Wetzlar, Germany). The severity of white matter lesions was evaluated by examining fiber density by Klüver-Barrera staining, as in Wakita et al. (2008).
Toluidine blue staining
Toluidine blue staining was performed on day 37 after model establishment. Frozen brain sections (10-μm thick) were incubated three times with PBS, and stained in toluidine blue (Sigma, St. Louis, MO, USA) working solution for 2–3 minutes and washed in water. The sections were dipped in 0.5% hydrochloric acid/alcohol (75%) for 5 seconds. Finally, the sections were observed under a fluorescence microscope (BX51; Olympus, Tokyo, Japan).
Immunofluorescence staining
Individual brain sections were incubated with PBS containing 0.3% Triton X-100 and 3% normal donkey serum for 2 hours, and then with the appropriate primary antibodies overnight for 4°C as follows: rabbit anti-Oligo2 polyclonal antibody (1:500; Millipore, Billerica, MA, USA), rat anti-MBP polyclonal antibody (1:250; Millipore) and rat anti-PLP polyclonal antibody (1:250; Millipore). The sections were washed three times in PBS and incubated with Cy3-conjugated goat anti-rabbit IgG (1:400; Invitrogen, Carlsbad, CA, USA), Cy3-conjugated goat anti-rat IgG (1:400; Invitrogen) or fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG (1:400; Invitrogen) for 2 hours at room temperature. Finally, the sections were observed under a fluorescence microscope.
Statistical analysis
Data are presented as the mean ± SEM. Statistical analyses were done with SPSS 11.5 for Windows (SPSS, Chicago, IL, USA). One-way analysis of variance followed by the least significant difference or Dunnett's T3 post-hoc test (where equal variances were not assumed) was used for multiple comparisons.
Results
Effects of carnosine on myelin damage in the brain of SIVD model mice
Corpus callosum
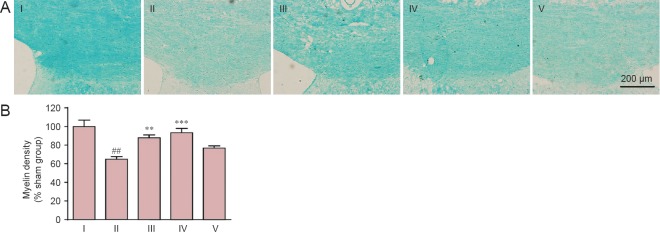
Myelin damage in the corpus callosum was examined by Klüver-Barrera staining. Myelin density in the corpus callosum was greatly reduced on day 37 after model establishment, compared with the sham group (P < 0.01). Carnosine significantly elevated myelin density to 87.84% (200 mg/kg: P < 0.01, vs. SIVD group) and 93.39% (500 mg/kg: P < 0.001, vs. SIVD group) of that in the sham group, as assessed by Klüver-Barrera staining. However, carnosine at 750 mg/kg had no effect on myelin damage in the corpus callosum (P > 0.05, vs. SIVD group; Figure 1).
Figure 1.
Effect of carnosine on white matter damage in the corpus callosum in the mouse model of SIVD.
(A) Effect of carnosine on white matter damage in the corpus callosum was evaluated by Klüver-Barrera staining after right unilateral common carotid artery occlusion. Klüver-Barrera staining revealed white matter rarefaction 37 days after carotid occlusion. The staining intensity of the myelinated fibers was reduced in the SIVD group, and was increased by administration of carnosine (200, 500 mg/kg). Scale bar: 200 μm. (B) Quantitative analysis was performed using the mean optical density value (mean ± SEM). n = 8–10 mice in each group. ##P < 0.01, vs. sham group; **P < 0.01, ***P < 0.001, vs. SIVD group. I–V: Sham, SIVD, SIVD + carnosine 200 mg/kg, SIVD + carnosine 500 mg/kg and SIVD + carnosine 750 mg/kg groups, respectively. SIVD: Subcortical ischemic vascular dementia.
Optic tract
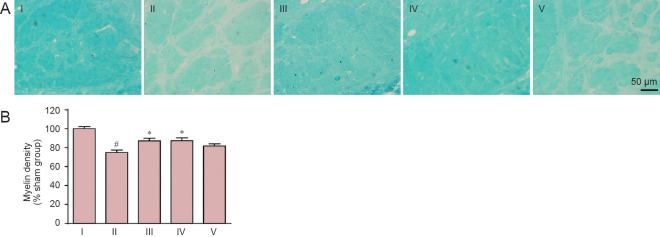
Myelin damage in the optic tract was examined by Klüver-Barrera staining. Myelin density in the optic tract was greatly lower at day 37 after model establishment compared with the sham group (P < 0.01). Carnosine at 500 mg/kg greatly increased the myelin density to the level in the sham group on day 37 after model establishment (P < 0.01, vs. SIVD group). However, carnosine at 200 and 750 mg/kg had no effect on myelin damage in the optic tract (P > 0.05, vs. SIVD group; Figure 2).
Figure 2.
Effect of carnosine on white matter damage in the optic tract in the mouse model of SIVD.
(A) Effect of carnosine on white matter damage in the optic tract was evaluated by Klüver-Barrera staining after right unilateral common carotid artery occlusion. Klüver-Barrera staining revealed white matter rarefaction 37 days after carotid occlusion. The staining intensity of the myelinated fibers was reduced in the SIVD group, and was increased by administration of carnosine (500 mg/kg). The areas between the two black lines represent the optic tract. Scale bar: 50 µm. (B) Quantitative analysis was performed using the mean optical density value (mean ± SEM). n = 8–10 mice in each group. #P < 0.05, vs. sham group; *P < 0.05, vs. SIVD group. I–V: Sham, SIVD, SIVD + carnosine 200 mg/kg, SIVD + carnosine 500 mg/kg and SIVD + carnosine 750 mg/kg groups, respectively. SIVD: Subcortical ischemic vascular dementia.
Internal capsule
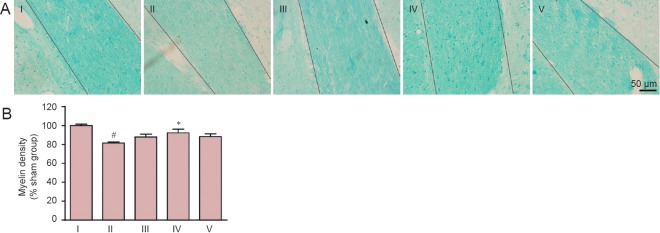
Myelin damage in the internal capsule was examined by Klüver-Barrera staining. Compared with the sham group, myelin density in the internal capsule was reduced to 74.89% of that in the sham group on day 37 after model establishment (P < 0.05). Carnosine at 200 and 500 mg/kg markedly elevated myelin density to 87.17% and 87.83% (P < 0.05, vs. SIVD group) of that in the sham group, respectively. However, carnosine at 750 mg/kg showed no such effect (P > 0.05, vs. SIVD group; Figure 3).
Figure 3.
Effect of carnosine on white matter damage in the internal capsule in the mouse model of SIVD.
(A) Effect of carnosine on white matter damage in the internal capsule was evaluated by Klüver-Barrera staining after right unilateral common carotid artery occlusion. Klüver-Barrera staining revealed white matter rarefaction on day 37 after surgery. The staining intensity of the myelinated fibers was reduced in the SIVD group, and was increased by administration of carnosine (200, 500 mg/kg). Scale bar: 50 µm. (B) Quantitative analysis was performed using the mean optical density value (mean ± SEM). n = 8–10 mice in each group. #P < 0.05, vs. sham group; *P < 0.05, vs. SIVD group. I–V: Sham, SIVD, SIVD + carnosine 200 mg/kg, SIVD + carnosine 500 mg/kg and SIVD + carnosine 750 mg/kg groups, respectively. SIVD: Subcortical ischemic vascular dementia.
Effects of carnosine on MBP expression in the corpus callosum of SIVD model mice
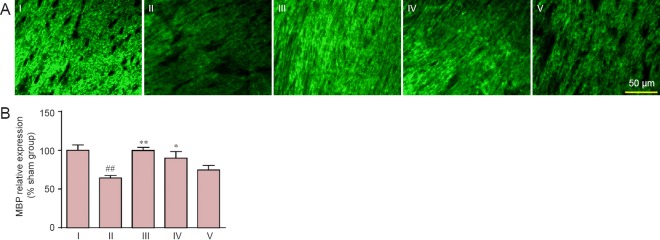
MBP is a major component of myelin, and reduced expression of the protein is associated with demyelination (Barker et al., 2013). We analyzed MBP expression in the corpus callosum after model establishment by immunofluorescence analysis. MBP expression was greatly reduced 37 days after model establishment compared with the sham group (P < 0.01). Carnosine (200 and 500 mg/kg) significantly improved MBP expression after model establishment (P < 0.01 or 0.05, vs. SIVD group; Figure 4).
Figure 4.
Effect of carnosine on MBP expression in the corpus callosum in the mouse model of SIVD.
(A) Effect of carnosine on MBP expression in the corpus callosum was evaluated using the mean fluorescence intensity after right unilateral common carotid artery occlusion. The fluorescence indicator is FITC (green). Scale bar: 50 µm. (B) Quantitative analysis of MBP expression. The staining intensity for MBP was reduced in the SIVD group, and was increased by administration of carnosine (200, 500 mg/kg). Data are expressed as the mean ± SEM. n = 8–10 mice in each group. ##P < 0.01, vs. sham group; *P < 0.05, **P < 0.01, vs. SIVD group. I–V: Sham, SIVD, SIVD + carnosine 200 mg/kg, SIVD + carnosine 500 mg/kg and SIVD + carnosine 750 mg/kg groups, respectively. SIVD: Subcortical ischemic vascular dementia; MBP: myelin basic protein; FITC: fluorescein isothiocyanate.
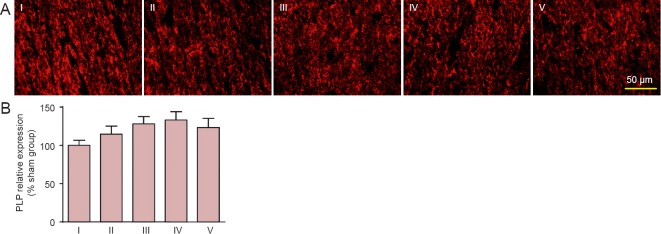
Effects of carnosine on PLP expression in the corpus callosum of SIVD model mice
PLP, a tetraspan membrane protein, is the most abundant component of central nervous system myelin (Barker et al., 2013). We analyzed PLP expression in the corpus callosum after model establishment using immunofluorescence staining. There was no obvious difference between the sham and SIVD groups in PLP fluorescence intensities (P > 0.05). Carnosine had no significant effect on PLP expression (P > 0.05, vs. SIVD group; Figure 5).
Figure 5.
Effect of carnosine on PLP expression in the corpus callosum in the mouse model of SIVD.
(A) Effect of carnosine on PLP expression in the corpus callosum was evaluated by mean fluorescence intensity after right unilateral common carotid artery occlusion. The fluorescent indicator was Cy3 (red). Scale bar: 50 µm. (B) Quantitative analysis of PLP expression. There were no obvious differences in PLP fluorescence intensity among the sham, SIVD and carnosine groups. Data are expressed as the mean ± SEM. n = 8–10 mice in each group. I–V: Sham, SIVD, SIVD + carnosine 200 mg/kg, SIVD + carnosine 500 mg/kg and SIVD + carnosine 750 mg/kg groups, respectively. PLP: Proteolipide protein; SIVD: subcortical ischemic vascular dementia.
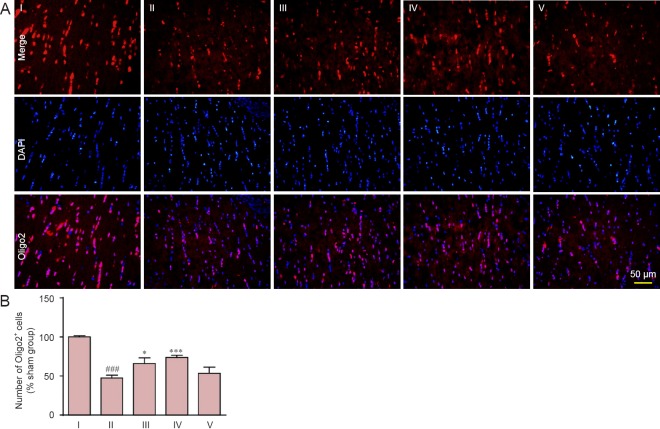
Effects of carnosine on the number of oligodendrocytes in the corpus callosum of SIVD model mice
We characterized the oligodendrocyte population on day 37 after model establishment, and we evaluated the effects of carnosine treatment. The number of oligodendrocytes (Oligo2+ cells) was strikingly decreased on day 37 after model establishment (P < 0.001, vs. sham group). We found that the administration of carnosine (200 and 500 mg/kg) increased the number of oligodendrocytes in SIVD mice (P < 0.05 or 0.001, vs. SIVD group; Figure 6).
Figure 6.
Effect of carnosine on oligodendrocyte damage in the corpus callosum.
(A) The total oligodendrocyte (Oligo2+) count after carnosine treatment was calculated as the percentage of total cells (labeled by DAPI) after right unilateral common carotid artery occlusion. Fluorescent indicator is Cy3 (red). Scale bar: 50 µm. (B) Quantitative analysis of Oligo2+ cells. The numbers of oligodendrocytes (Oligo2+ cells) was decreased on day 37 after artery occlusion, and was increased by administration of carnosine (200, 500 mg/kg). Data are expressed as the mean ± SEM. n = 8–10 mice in each group. ###P < 0.001, vs. sham group; *P < 0.05, ***P < 0.001, vs. SIVD group. I–V: Sham, SIVD, SIVD + carnosine 200 mg/kg, SIVD + carnosine 500 mg/kg and SIVD + carnosine 750 mg/kg groups, respectively. SIVD: Subcortical ischemic vascular dementia; DAPI: 4′,6-diamidino-2-phenylindole.
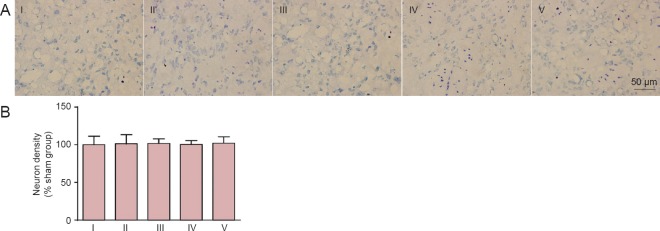
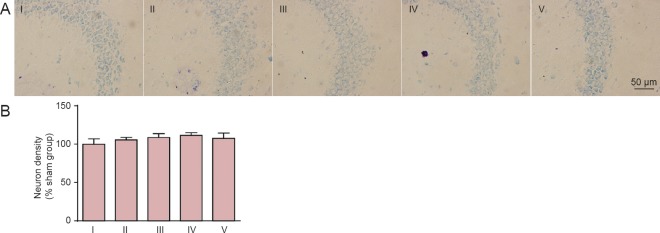
Lack of effect of carnosine on neuronal density in the brain of SIVD model mice
By toluidine blue staining, no obvious neuronal loss was observed in the corpus striatum (Figure 7) or hippocampus (Figure 8) after model establishment and carnosine treatment (P > 0.05, vs. sham group). We also examined neuronal density in the cortex, and obtained similar results (data not shown).
Figure 7.
Effect of carnosine on neuronal density in the corpus striatum in the mouse model of SIVD.
(A) Effect of carnosine on neuronal density in the corpus striatum was evaluated after right unilateral common carotid artery occlusion. Scale bar: 50 µm. (B) Quantitative analysis of cell density. There was no difference in neuronal number among these groups. Data are expressed as the mean ± SEM. n = 8–10 mice in each group. I–V: Sham, SIVD, SIVD + carnosine 200 mg/kg, SIVD + carnosine 500 mg/kg and SIVD + carnosine 750 mg/kg groups, respectively. SIVD: Subcortical ischemic vascular dementia.
Figure 8.
Effect of carnosine on neuronal density in the hippocampus in the mouse model of SIVD.
(A) Effect of carnosine on neuronal density in the hippocampus was evaluated after right unilateral common carotid artery occlusion. Scale bar: 50 µm. (B) Quantitative analysis of cell density. There was no difference in neuronal number among these groups. Data are expressed as the mean ± SEM. n = 8–10 mice in each group. I–V: Sham, SIVD, SIVD + carnosine 200 mg/kg, SIVD + carnosine 500 mg/kg and SIVD + carnosine 750 mg/kg groups, respectively. SIVD: Subcortical ischemic vascular dementia.
Discussion
In our previous studies, we have already found that after right unilateral common carotid arteries occlusion, carnosine significantly improved the learning and memory in the object recognition test, passive avoidance task and Morris water maze (Ma et al., 2012). In this study, we found that carnosine significantly ameliorated myelin damage in the corpus callosum, optic tract and internal capsule in the mouse model of SIVD. In addition, carnosine prevented the decrease in MBP expression, and it reduced oligodendrocyte loss, suggesting that it protects against white matter damage in SIVD.
Here, we observed extensive white matter damage in SIVD induced by right unilateral common carotid artery occlusion, particularly in the corpus callosum, which accords with a previous report (Yoshizaki et al., 2008). The corpus callosum is a white matter structure that is highly susceptible to ischemic damage. The accentuated vulnerability of the corpus callosum in ischemia-related white matter damage has also been observed by others (Cheng et al., 2015; Park et al., 2015). Farkas et al. (2004) reported that the optic tract seems to be particularly vulnerable to ischemia induced by bilateral common carotid artery occlusion. The corpus callosum is supplied by arterial blood from the anterior cerebral arteries, while the optic nerves and tract receive arterial blood directly from branches of the internal carotid arteries, and the internal capsule receives arterial blood from the middle cerebral artery (Farkas et al., 2004). Because the compensatory redistribution in the circle of Willis after carotid artery occlusion is not immediate or complete, our results suggest that specific brain regions may suffer more ischemic damage in different ischemic models. Case studies demonstrate that single, strategically placed lesions may result in SIVD, and the internal capsule is a key site (Tatemichi et al., 1992; Chui, 2007). Moreover, significant white matter damage in the internal capsule was observed in chronic cerebral ischemic injury induced by right unilateral common carotid artery occlusion in this study. We found that carnosine significantly ameliorated white matter damage in the corpus callosum, optic tract and internal capsule, indicative of a robust neuroprotective effect of carnosine against white matter damage. Thus, our results further demonstrate that carnosine may have therapeutic potential for the treatment of SIVD.
At present, the pathogenesis of SIVD is not clear. Recently, magnetic resonance imaging in patients with SIVD showed that white matter damage is progressive, often appearing before obvious cognitive dysfunction (Cavallari et al., 2015). The clinical and pathological changes in vascular dementia are different from Alzheimer's disease, Lewy body dementia and frontotemporal dementia. The most striking difference from Alzheimer's disease is that damage is primarily to the white matter, instead of the grey matter (Hashimoto and Ikeda, 2015). In this study, by toluidine blue staining, no obvious neuronal loss or apparent morphological change was observed in the cortex, striatum or hippocampus after model establishment and carnosine treatment, which further demonstrates that pathological changes in vascular dementia are mainly localized to the white matter. However, our data do not exclude the occurrence of neuronal dysfunction in SIVD, which will require additional studies to clarify.
Autopsy findings show that the main pathological changes in SIVD include damage to nerve fibers and the myelin sheath, which is produced by oligodendrocytes (Alvarez-Sabin and Roman, 2011). Although the pathogenesis of the white matter damage is still unclear, it frequently coincides with myelin degradation. Oligodendrocytes, myelin-forming glial cells of the central nervous system, are known to be quite vulnerable to ischemic stress, resulting in the loss of myelin after ischemia or hypoxia (Benarroch, 2009; Ihara et al., 2010). In the present study, we found that administration of carnosine (200 and 500 mg/kg) increased the number of oligodendrocytes after SIVD, which may contribute to its protective effect on the white matter, and was associated with more intense Klüver-Barrera staining and increased MBP expression. However, PLP expression was not changed on day 37 after model establishment, which suggests that PLP and MBP may play different roles in SIVD.
Although the neuroprotection by carnosine in SIVD has been investigated previously (Ma et al., 2012), the effects of carnosine at high doses had not been examined. Carnosine treatment has been reported to exhibit significant neuroprotection with wide therapeutic and clinically relevant time windows in both permanent and transient ischemic models (Bae et al., 2013; Bandyopadhyay et al., 2014; Dolu et al., 2014). Therefore, carnosine was considered well tolerated and without toxicity. However, we found that the neuroprotective effect of carnosine in SIVD is dose-dependent—the high dose of the dipeptide had no effect on white matter damage induced by right unilateral common carotid artery occlusion. Although the reason for this lack of effect is not clear, our study suggests that the administered dose is critical for neuroprotection. This finding should help in the development of treatment strategies involving carnosine.
In conclusion, carnosine displayed an extensive neuroprotective effect on white matter damage in the corpus callosum, optic tract and internal capsule induced by right unilateral common carotid artery occlusion, which may be mediated by its cytoprotection of oligodendrocytes. These findings suggest that carnosine may have therapeutic potential for the treatment of SIVD.
Footnotes
Funding: This study was funded by the National Natural Science Foundation of China, No. 81402904; the Foundation of Shanghai Jiao Tong University School of Medicine, No. 13XJ22001; the Foundation of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, No. 13YJ11; a grant from the Science and Technology Commission of Shanghai Municipality of China, No. 13ZR1426900, 15411963900.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Patel B, de Souza M, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Alvarez-Sabin J, Roman GC. Citicoline in vascular cognitive impairment and vascular dementia after stroke. Stroke. 2011;42:S40–43. doi: 10.1161/STROKEAHA.110.606509. [DOI] [PubMed] [Google Scholar]
- Areosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev. 2005:CD003154. doi: 10.1002/14651858.CD003154.pub3. [DOI] [PubMed] [Google Scholar]
- Babizhayev MA, Yegorov YE. Advanced drug delivery of N-acetylcarnosine (N-acetyl-beta-alanyl-L-histidine), carcinine (beta-alanylhistamine) and L-carnosine (beta-alanyl-L-histidine) in targeting peptide compounds as pharmacological chaperones for use in tissue engineering, human disease management and therapy: from in vitro to the clinic. Recent Pat Drug Deliv Formul. 2010;4:198–230. doi: 10.2174/187221110793237547. [DOI] [PubMed] [Google Scholar]
- Bae ON, Serfozo K, Baek SH, Lee KY, Dorrance A, Rumbeiha W, Fitzgerald SD, Farooq MU, Naravelta B, Bhatt A, Majid A. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke. 2013;44:205–212. doi: 10.1161/STROKEAHA.112.673954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay TK, Biswas A, Roy A, Guin DS, Gangopadhyay G, Sarkhel S, Ghoshal MK, Senapati AK. Neuropsychiatric profiles in patients with Alzheimer's disease and vascular dementia. Ann Indian Acad Neurol. 2014;17:325–330. doi: 10.4103/0972-2327.138520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R, Wellington D, Esiri MM, Love S. Assessing white matter ischemic damage in dementia patients by measurement of myelin proteins. J Cereb Blood Flow Metab. 2013;33:1050–1057. doi: 10.1038/jcbfm.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Oligodendrocytes: Susceptibility to injury and involvement in neurologic disease. Neurology. 2009;72:1779–1785. doi: 10.1212/WNL.0b013e3181a6b123. [DOI] [PubMed] [Google Scholar]
- Cavallari M, Hshieh TT, Guttmann CRG, Ngo LH, Meier DS, Schmitt EM, Marcantonio ER, Jones RN, Kosar CM, Fong TG, Press D, Inouye SK, Alsop DC on behalf of the SSG. Brain atrophy and white matter hyperintensities are not significantly associated with incidence and severity of postoperative delirium in older persons without dementia. Neurobiol Aging. 2015;36:2122–2129. doi: 10.1016/j.neurobiolaging.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Ren Y, Bai S, Wu Y, Xu Y, Pan J, Chen J, Zhu X, Qi Z, Shao W, Tang W, Liu M, Xie P, Huang W. Chronic cerebral ischemia induces downregulation of A1 adenosine receptors during white matter damage in adult mice. Cell Mol Neurobiol. 2015;35:1149–1156. doi: 10.1007/s10571-015-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui H. Subcortical ischemic vascular dementia. Neurol Clin. 2007;25:717–740. doi: 10.1016/j.ncl.2007.04.003. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig D, Birks J. Galantamine for vascular cognitive impairment. Cochrane Database Syst Rev. 2006:CD004746. doi: 10.1002/14651858.CD004746.pub2. [DOI] [PubMed] [Google Scholar]
- Dolu N, Acer H, Kara AY. Investigation of dose-related effects of carnosine on anxiety with sympathetic skin response and T-maze. Acta Medica (Hradec Kralove) 2014;57:112–118. doi: 10.14712/18059694.2014.49. [DOI] [PubMed] [Google Scholar]
- Farkas E, Donka G, de Vos RA, Mihály A, Bari F, Luiten PG. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004;108:57–64. doi: 10.1007/s00401-004-0864-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Ikeda M. White matter lesion and alzheimer's disease: the association between small vessel disease and neuropsychiatric symptoms in Alzheimer's disease. Brain Nerve. 2015;67:427–432. doi: 10.11477/mf.1416200158. [DOI] [PubMed] [Google Scholar]
- Ihara M, Polvikoski TM, Hall R, Slade JY, Perry RH, Oakley AE, Englund E, O’Brien JT, Ince PG, Kalaria RN. Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer's disease, and dementia with Lewy bodies. Acta Neuropathol. 2010;119:579–589. doi: 10.1007/s00401-009-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CL, Yang LX, Wu XH, Li Q, Ding MP, Fan YY, Zhang WP, Luo JH, Chen Z. Effects of carnosine on amygdaloid-kindled seizures in Sprague–Dawley rats. Neuroscience. 2005;135:939–947. doi: 10.1016/j.neuroscience.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry EK, Potocnik F, Prince M, Stewart R, Wimo A, Zhang ZX, Antuono P. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata H, Fujii T, Tsutsui H, Katayama T, Ohkita M, Takaoka M, Tsuruoka N, Kiso Y, Ohno Y, Fujisawa Y, Shokoji T, Nishiyama A, Abe Y, Matsumura Y. Renoprotective effects of l-carnosine on ischemia/reperfusion-induced renal injury in rats. J Pharmacol Exp Ther. 2006;319:640–647. doi: 10.1124/jpet.106.110122. [DOI] [PubMed] [Google Scholar]
- Ma J, Xiong JY, Hou WW, Yan HJ, Sun Y, Huang SW, Jin L, Wang Y, Hu WW, Chen Z. Protective effect of carnosine on subcortical ischemic vascular dementia in mice. CNS Neurosci Ther. 2012;18:745–753. doi: 10.1111/j.1755-5949.2012.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhang J, Hou WW, Wu XH, Liao RJ, Chen Y, Wang Z, Zhang XN, Zhang LS, Zhou YD, Chen Z, Hu WW. Early treatment of minocycline alleviates white matter and cognitive impairments after chronic cerebral hypoperfusion. Sci Rep. 2015;5:12079. doi: 10.1038/srep12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2009:CD003160. doi: 10.1002/14651858.CD003160.pub2. [DOI] [PubMed] [Google Scholar]
- Olivares D, Deshpande VK, Shi Y, Lahiri DK, Greig NH, Rogers JT, Huang X. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer's disease, vascular dementia and Parkinson's disease. Curr Alzheimer Res. 2012;9:746–758. doi: 10.2174/156720512801322564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Shin K, Choi EK, Choi Y, Jang JY, Kim J, Jeong HS, Lee W, Lee YB, Kim SU, Joo SS, Kim YB. Protective effects of N-acetyl-L-cysteine in human oligodendrocyte progenitor cells and restoration of motor function in neonatal rats with hypoxic-ischemic encephalopathy. Evid Based Complement Alternat Med 2015. 2015 doi: 10.1155/2015/764251. 764251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safouris A, Psaltopoulou T, Sergentanis TN, Boutati E, Kapaki E, Tsivgoulis G. Vascular risk factors and Alzheimer's disease pathogenesis: are conventional pharmacological approaches protective for cognitive decline progression? CNS Neurol Disord Drug Targets. 2015;14:257–269. doi: 10.2174/1871527314666150217123147. [DOI] [PubMed] [Google Scholar]
- Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neuro. 2006;2:538–547. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- Shen Y, He P, Fan YY, Zhang JX, Yan HJ, Hu WW, Ohtsu H, Chen Z. Carnosine protects against permanent cerebral ischemia in histidine decarboxylase knockout mice by reducing glutamate excitotoxicity. Free Radical Biol Med. 2010;48:727–735. doi: 10.1016/j.freeradbiomed.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Taguchi A. Cell-based therapy for patients with vascular dementia. Psychogeriatrics. 2011;11:113–115. doi: 10.1111/j.1479-8301.2010.00343.x. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Prohovnik I, Cross DT, Gropen TI, Mohr JP, Stern Y. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42:1966–1979. doi: 10.1212/wnl.42.10.1966. [DOI] [PubMed] [Google Scholar]
- Tomimoto H. Subcortical vascular dementia. Neurosci Res. 2011;71:193–199. doi: 10.1016/j.neures.2011.07.1820. [DOI] [PubMed] [Google Scholar]
- Usui T1, Kubo Y, Akanuma S, Hosoya K. B-alanine and l-histidine transport across the inner blood-retinal barrier: potential involvement in L-carnosine supply. Exp Eye Res. 2013;113:135–142. doi: 10.1016/j.exer.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Wakita H, Ruetzler C, Illoh KO, Chen Y, Takanohashi A, Spatz M, Hallenbeck JM. Mucosal tolerization to E-selectin protects against memory dysfunction and white matter damage in a vascular cognitive impairment model. J Cereb Blood Flow Metab. 2008;28:341–353. doi: 10.1038/sj.jcbfm.9600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, Wakita H. Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp Neurol. 2008;210:585–591. doi: 10.1016/j.expneurol.2007.12.005. [DOI] [PubMed] [Google Scholar]