Keywords: nerve regeneration, vascular endothelial growth factor, transfection, neural stem/progenitor cells, transplantation, hypoxic-ischemic brain damage, cerebral cortex, animal model, neuroprotection, neural regeneration
Abstract
Neural stem/progenitor cell (NSC) transplantation has been shown to effectively improve neurological function in rats with hypoxic-ischemic brain damage. Vascular endothelial growth factor (VEGF) is a signaling protein that stimulates angiogenesis and improves neural regeneration. We hypothesized that transplantation of VEGF-transfected NSCs would alleviate hypoxic-ischemic brain damage in neonatal rats. We produced and transfected a recombinant lentiviral vector containing the VEGF165 gene into cultured NSCs. The transfected NSCs were transplanted into the left sensorimotor cortex of rats 3 days after hypoxic-ischemic brain damage. Compared with the NSCs group, VEGF mRNA and protein expression levels were increased in the transgene NSCs group, and learning and memory abilities were significantly improved at 30 days. Furthermore, histopathological changes were alleviated in these animals. Our findings indicate that transplantation of VEGF-transfected NSCs may facilitate the recovery of neurological function, and that its therapeutic effectiveness is better than that of unmodified NSCs.
Introduction
Hypoxic-ischemic encephalopathy is a common neurological disease among neonates, with a high mortality rate ranging from 10% to 60%. Furthermore, 25% of affected patients suffer permanent neurological sequelae, such as cerebral palsy, mental retardation, learning difficulties and epilepsy (Meberg et al., 2004). Despite considerable advances in perinatal medicine, such as hypothermia, the management of hypoxic-ischemic encephalopathy is still mainly focused on symptomatic and supportive treatments. Therefore, there is a pressing need for more effective interventions for the disease. Because one of the major consequences of hypoxic-ischemic brain damage (HIBD) is the loss of neural cells, stimulation of the production of endogenous cells and supplementation with exogenous cells are potentially effective treatment strategies.
Neural stem/progenitor cells (NSCs), which are characterized by self-renewal, multilineage differentiation and non-immunogenicity, are ideal for hypoxic-ischemic encephalopathy treatment (Li et al., 2015). However, because of the poor survival rate and limited regenerative capacity of normal NSCs following transplantation, numerous studies have focused on genetically-modified NSCs expressing exogenous genes (Kim et al., 2004; Daadi et al., 2010; Zhu et al., 2011).
Vascular endothelial growth factor (VEGF) is a signaling protein that stimulates angiogenesis and improves neural regeneration. Administration of angiogenic factors, such as VEGF, combined with NSC transplantation, has been shown to be a very promising treatment strategy for enhancing vascularization and neuroprotection in ischemic diseases (Tan et al., 2014). Zhu et al. (2005), for example, transplanted VEGF-transfected NSCs to treat transient cerebral ischemia in adult rats, and found that these NSCs migrated in the host brain tissue and could express the gene product. Because this strategy combines both an abundant cell source and a niche factor, which is critical for cell survival, it results in better local vascularization and neuronal morphology in vitro. However, no study to date has evaluated the effects of a similar intervention for the treatment of brain injury in neonatal rats. The neonatal brain is in a developmental state with immature immune function, which might facilitate the survival of genetically modified NSCs and allow for better proliferation and migration of these cells. Because of these advantages, we hypothesized that genetically modified NSCs might have therapeutic potential for treating neonatal brain damage.
Previously, we created a recombinant lentiviral vector containing the VEGF165 gene (pGC-FU-VEGF165), which was transfected into cultured NSCs for transplantation into the brain in an animal model (Zhang et al., 2011). In the present study, we evaluated the therapeutic efficacy of genetically modified NSCs for functional recovery in neonatal animals post HIBD. We examined VEGF165 mRNA and protein levels in the transplanted brain region, and we also assessed neurological functions, including motor behavior, working memory, emotional behavior and object recognition, in neonatal rats. Our findings provide insight into the therapeutic efficacy of VEGF165-transfected NSC transplantation for neonatal HIBD, and they should help researchers develop new treatment strategies for perinatal hypoxic-ischemic injury.
Materials and Methods
NSC isolation and culture
Two pregnant (gestation days 14–16) Sprague-Dawley rats (weighing 256 g and 280 g) for rat embryo collection were provided by the Department of Animal Experiments of Xiangya Hospital of Central South University, China (license No. SCXK (Xiang) 2010-0002). The animal experimental procedures followed NIH guidelines and were approved by the Animal Ethics Committee of Central South University, China.
NSCs were isolated from Sprague-Dawley rat embryos as previously described (Liu et al., 2009). In brief, the dams, at 14–16 days of pregnancy, were intraperitoneally anesthetized with 3% chloral hydrate, and the embryos were sterilely removed. Single-cell suspensions were prepared by trypsinization of dissected brain tissue and filtration of digested cells. After washing with PBS, NSCs were cultured in conditioned medium (Dulbecco's modified Eagle's medium/F12) containing basic fibroblast growth factor (20 ng/mL; Changsha Lixin Biological Technology Co., Ltd., Changsha, China), epidermal growth factor (20 ng/mL; PeproTech, London, UK), B27 (20 μL/mL; Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) and insulin (100 ng/mL). NSCs were collected 14 days after primary culture, and identified using indirect immunofluorescence, based on their expression of the specific NSC antigen, nestin (Figure 1).
Figure 1.
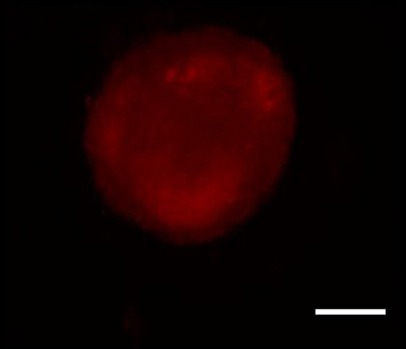
Neural stem/progenitor cells identified by immunofluorescence using the marker nestin (red fluorescence).
Scale bar: 20 µm.
Construction of the recombinant lentiviral vector pGC-FU-VEGF165 and transfection of NSCs
VEGF165 gene fragments were synthesized by polymerase chain reaction (PCR), with primers designed using GenBank sequence information (No. AF486837), as previously described by our group (Zhang et al., 2011). The target gene was introduced into a lentiviral vector (pGC-FU) to produce a recombinant lentiviral vector carrying the VEGF165 gene (pGC-FU-VEGF165). Reverse transcription (RT)-PCR and sequencing analysis confirmed that the gene was correctly cloned into the lentiviral vector. The fourth passage of NSCs was incubated with pGC-FU-VEGF165 (multiplicity of infection: 50) for 8 hours. The culture medium was replaced with fresh NSC culture medium, and the infection was continued for an additional 48 hours. The transfected cells were selected with G418 (400 μg/mL) for 2 weeks, and the positive clones were expanded for 2 more weeks in the presence of G418.
Animal experiment
Healthy 7-day-old Sprague-Dawley rats, of either sex, weighing 12–15 g, were randomly divided into four groups of 20 in each group, as follows: (1) sham-operated (control group, CON); (2) PBS transplantation (injection); (3) VEGF-transfected NSC transplantation (transgene NSCs); and (4) normal NSC transplantation (NSCs). All transplanted animals were subjected to hypoxia-ischemia surgery 3 days prior to NSC transplantation (see below).
Establishment of the HIBD model
The HIBD model was established in accordance with the method of Rice et al. (1981). Sprague-Dawley rats were anesthetized by ether inhalation and fixed in the supine position. The left common carotid artery was ligated 2 hours before the rats were exposed to a low oxygen environment. Animals were placed in a plexiglass chamber at 37°C for 2 hours to induce hypoxia. The nitrogen-oxygen mixture contained 8.0 ± 0.1% oxygen and was supplied at a rate of 1–2 L/min. The chamber bottom was covered with soda lime to absorb CO2 and moisture. Rats were returned to their cages for breastfeeding at the end of the procedure. Their health status and behaviors were monitored until weaning at 21 days of age. Sham-operated rats underwent isolation of the left common carotid artery, without ligation or hypoxia.
Intracerebral transplantation
Three days after HIBD surgery, 2 μL of buffer was stereotactically injected into the left sensorimotor cortex of rats in the PBS, NSCs and transgene NSCs groups, with or without 1 × 105 normal or transfected NSCs. The time points and sites for cell transplantation were according to our previous study and were based on the optimal response of the animals (Zhang et al., 2008). In brief, rats were fixed in a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA), and the transplantation site was the left sensorimotor cortex (anteroposterior: −0.3 mm; mediolateral: −2 mm; dorsoventral: −1.5 mm; 0.3 mm posterior to and 2 mm left of the bregma; depth of 1.5 mm). Buffer or NSC suspensions (2 μL) were delivered using a microsyringe inserted perpendicularly to the location. The whole procedure was very gentle, and each insertion, injection and removal of the needle was performed over a period of at least 5 minutes. After the scalp was sutured, the rats were returned to their mother. Their health status and behaviors were monitored closely until weaning at 21 days.
VEGF mRNA detection
Brain VEGF mRNA levels were measured 7 days after transplantation (at 17 days of age), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The primers used are shown in Table 1. Total RNA was extracted from brain tissue using Trizol reagent. RNA concentration and quality were determined using the A260 nm/A280 nm ratio, and RNA integrity was assayed by agarose gel electrophoresis. RT-PCR amplification was performed using 2 μg total RNA and the RT-PCR one-step method (Promega, Fitchburg, WI, USA), according to the kit instructions. Twenty-five amplification cycles were performed, and PCR products were electrophoresed on a 1% agarose gel and scanned with a gel-imaging system. The relative expression of the VEGF gene was determined using the ratio of the VEGF signal to that of GAPDH.
Table 1.
Primers used for amplification
Immunohistochemical detection of VEGF
Immunohistochemical detection of VEGF protein was performed 7 days after transplantation. Rats were anesthetized with chloral hydrate, and fixed with 4% paraformaldehyde. After embedding in paraffin, serial coronal sections were prepared from the left brain tissue, between the bregma and 3 mm posterior to the bregma. Sections of 3-μm thickness were used for VEGF immunohistochemistry. Immunostaining was performed using a VEGF immunohistochemistry kit (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). Sections were incubated with polyclonal rabbit anti-mouse VEGF and CD34 monoclonal antibody (1:100, 1:50; Santa Cruz Biotechnology, Dallas, TX, USA) at 4°C overnight, and then with biotin-labeled goat anti-rabbit antibody (1:200, Wuhan Boster Biological Technology Co., Ltd., Wuhan, China) at 37°C for 15 minutes. The sections were subsequently incubated with horseradish peroxidase-labeled streptavidin at 37°C for 15 minutes. The slices were visualized with freshly prepared diaminobenzidine (Wuhan Boster Biological Technology Co., Ltd.), and counterstained with hematoxylin. Negative controls were incubated with 0.01 M PBS, instead of primary antibody. The density of VEGF-positive cells was counted in five fields under a microscope (Leica, Solms, Germany) at 40× magnification.
Histological observation
Twenty-eight days after hypoxia-ischemia surgery (at 35 days of age, after functional assessments), the rats were anesthetized with 10% chloral hydrate and perfused with 0.9% saline followed by 4% paraformaldehyde in PBS, as described previously (Zheng et al., 2010). After dewaxing, brain sections (5 μm) were cut from the cortex and hippocampal regions, and were used for Nissl staining to evaluate neuronal cell loss and morphology. Ten sections from each hemisphere at intervals of about 400 μm apart were evaluated. Neuronal cells were positively identified by Nissl stain, and the numbers of positively-stained nuclei were counted in the cortex and CA1 region using confocal scanning microscopy software (LSM 510; Chongqing Optical Instrument Factory, Chongqing, China). A systematic, random counting procedure was followed, as described previously (Williams et al., 1988; Wang et al., 2008). An average positive cell density for the cortex and hippocampal CA1 region was obtained (cells/mm2). Ten sections from each hemisphere were assessed per animal, and 12 animals were analyzed for each group.
Behavioral tests
Cellular and tissue changes may lead to changes in brain functions. Therefore, we evaluated brain function using two behavioral tests. Radial arm maze test assesses memory and spatial learning ability, while attitudinal reflex test evaluates motor function. Behavioral testing was performed in a double-blind manner when the rats were 30 days old. The people performing the tests were not informed of the animal grouping information.
Radial arm maze test
The radial arm maze test evaluates hippocampal functions that are associated with spatial learning and memory (Floresco et al., 1997). The test was performed according to the method described by Balduini et al. (2001) 27 days after hypoxic-ischemic injury. The radial arm maze consists of a central platform 30 cm in diameter, and eight radially symmetrical arms 50 cm long and 12 cm wide, with a hole at the end of each arm. Animals were deprived of water for 48 hours before the test, with water only available for 30 minutes every evening. Each hole in the maze was filled with 50 μL water for the first 2 days, and the animals were free to seek the water in the maze. For the space test, only three arms were filled with water, at adjacent angles of 135°, 90° and 135° respectively. To complete the maze successfully, the rat must go down each arm only once, using short-term memory and spatial cues to remember which arms have already been visited. If a rat goes down any arm twice, this counts as an error. Animals were tested five times daily, at intervals of 1 minute, for 3 days. At the beginning of each test, rats were placed in the center of the maze, facing the third arm that had been filled with water. The rats were required to find all three arms. The time taken to find all three arms, the number of times the animal entered an arm that had already been entered, and the number of times an animal entered an arm not filled with water were recorded. Twelve animals were tested for each of the treatment groups.
Attitudinal reflex test
The attitudinal reflex experiments were performed at 34 days of age (27 days after hypoxic-ischemic injury) for evaluating sensorimotor function, which reflects the status of the sensorimotor area in the cortex, according to a previously described protocol (Zheng et al., 2010). Briefly, when lifted 50 cm above the surface with its tail grasped by the researcher, a rat would normally stretch its two forelimbs toward the surface (score: 0). An injured brain hemisphere may prevent it from extending the contralateral limb (score: 1). Afterwards, rats were placed on a table, and the shoulder was pressed to one side until the forelimb extended. This procedure was repeated several times. An impairment was noted if the response was weakened (contralateral to the injured brain hemisphere) (score: 2). Twelve animals were tested for each of the treatment groups.
Statistical analysis
Parametric data are presented as the mean ± SD. All statistical analyses were performed with SPSS 17.0 software (SPSS, Chicago, IL, USA). Data for the various groups were compared using one-way analysis of variance, followed by Tukey's honestly significant difference test when an overall significance was detected (P < 0.05). The maze test data were analyzed using two-way analysis of variance followed by Tukey's honestly significant difference test to evaluate differences at each time point. Ranked data were compared using the Mann-Whitney U test. A probability value of less than 5% was considered statistically significant.
Results
Effects of hypoxic-ischemic injury and NSC transplantation on brain VEGF mRNA and protein levels
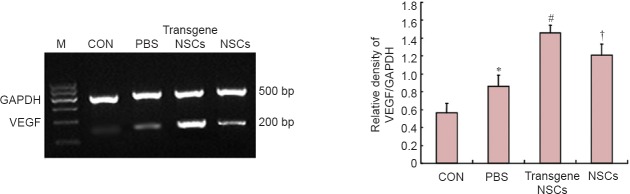
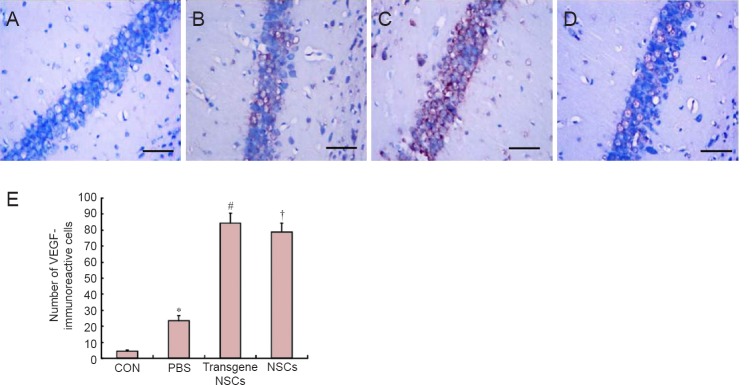
VEGF mRNA was analyzed by RT-PCR in the ischemic ipsilateral hemisphere of the brain, and VEGF protein was examined by immunohistochemistry in the hippocampal region of the same ipsilateral hemisphere. Interestingly, RT-PCR (Figure 2) and immunohistochemistry (Figure 3) showed that both VEGF mRNA and protein levels were increased significantly by the hypoxia-ischemia procedure itself (P < 0.05). Transplantation of NSCs into HIBD animals increased VEGF mRNA and protein levels (P < 0.05), and transplantation of transgenic NSCs increased VEGF mRNA and protein levels to a greater extent (P < 0.05).
Figure 2.
VEGF gene expression assessed by RT-PCR in the affected brain region post HIBD in the different groups.
VEGF mRNA expression levels were quantified by scanning the optical density of the bands and normalizing against GAPDH. Data are expressed as the mean ± SD (5 rats per group). The data for the different groups were compared using one-way analysis of variance followed by Tukey's honestly significant difference test when an overall significance was detected. *P < 0.05, vs. CON group; #P < 0.05, vs. NSCs group or PBS group; †P < 0.05, vs. PBS group. NSCs: Neural stem/progenitor cells; CON: sham-operated control; PBS: phosphate-buffered saline; HIBD: hypoxic-ischemic brain damage; VEGF: vascular endothelial growth factor; M: marker; RT-PCR: reverse transcription-polymerase chain reaction; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Figure 3.
VEGF immunoreactivity in the affected brain region post HIBD in the different groups.
(A–D) VEGF immunoreactivity in CON, PBS, transgene NSCs and NSCs groups. Scale bars: 20 µm. (E) The quantification of VEGF-immunoreactive cells was performed by immunohistochemical staining of the hippocampal region in hypoxic-ischemic ipsilateral hemispheres. Data are expressed as the mean ± SD (12 rats in each group). The data for the different groups were compared using one-way analysis of variance followed by Tukey's honestly significant difference test when an overall significance was detected. *P < 0.05, vs. CON group; #P < 0.05, vs. NSCs group or PBS group; †P < 0.05, vs. PBS group. NSCs: Neural stem/progenitor cells; CON: sham-operated control; PBS: phosphate-buffered saline; HIBD: hypoxic-ischemic brain damage; VEGF: vascular endothelial growth factor.
Effect of hypoxic-ischemic injury and NSC transplantation on brain histology
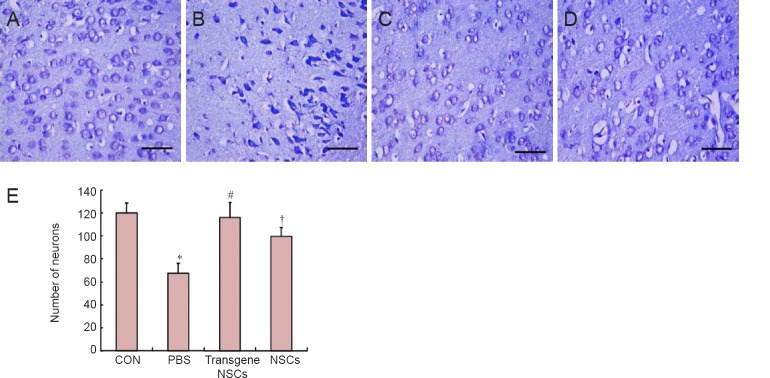
As shown in Figure 4, the HIBD (PBS) group exhibited marked neurodegeneration in the hemisphere ipsilateral to the occluded carotid artery, and a severe loss of pyramidal cells in the cortical region. In the hypoxic-ischemic rats, the cells displayed a condensed, degenerative morphology. These changes were partially attenuated by NSC transplantation. For example, the loss of pyramidal cells in the cortical region was largely prevented in the transgene NSCs group. To quantify the number of pyramidal cells in the tissue, we counted Nissl-stained cells in the cortex and hippocampal CA1 region. In addition to the changes in cell morphology (condensed nucleus and cytoplasm), the cell density (cells/mm2) was significantly reduced by 45% in HIBD rats (P < 0.01). The transplantation of NSCs almost completely prevented the neuronal loss. The protective effect was even more significant in rats that received VEGF-transfected NSCs (P < 0.01).
Figure 4.
Number of neurons in the affected cortex post HIBD in the different groups.
(A–D) Changes in the cortex of rats in the CON, PBS, transgene NSCs and NSCs groups (scale bar: 20 µm). (E) Neuron densities were quantified by counting Nisslstained cells. Data are expressed as the mean ± SD (12 rats in each group). The data for the different groups were compared using one-way analysis of variance followed by Tukey's honestly significant difference test when an overall significance was detected. *P < 0.05, vs. CON group; #P < 0.05, vs. NSCs group or PBS group; †P < 0.05, vs. PBS group. NSCs: Neural stem/progenitor cells; CON: sham-operated control; PBS: phosphate-buffered saline; HIBD: hypoxic-ischemic brain damage.
Effects of ischemia and NSC transplantation on brain functions
Radial arm maze test
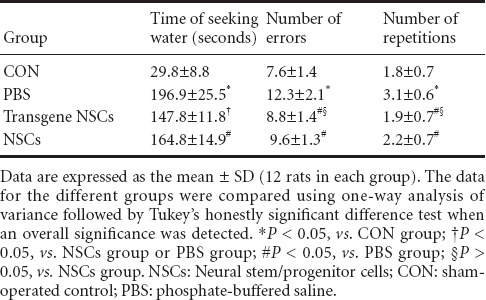
Rats in the PBS group took a significantly longer time to find water, had more errors, and more repetitions compared with sham-operated rats (P < 0.01; Table 2). This indicates that ischemia had a significant negative effect on memory and spatial learning abilities. Transplantation of NSCs, either with or without genetic modification, partially prevented these negative changes (P < 0.05). The improvements produced by transgenic NSCs were not of the same degree for all parameters, with some being more dramatic than others. For example, the time to find water in the maze was significantly improved by transgenic NSCs compared with NSCs alone (P < 0.05). There were no significant differences in the numbers of errors and repetitions made by the two groups (P > 0.05).
Table 2.
Radial arm maze test results for neonatal rats given different treatments after hypoxic-ischemic brain damage
Attitudinal reflex test
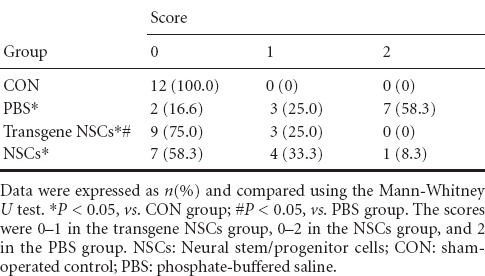
The scores in the attitudinal reflex test are shown in Table 3. The higher the score, the worse the sensorimotor function. The score for all control rats was 0, indicating intact motor functions. Brief ischemia significantly increased the average scores from 0 to mostly 2 (P < 0.05), while NSC transplantation partially prevented these increases (P < 0.05). There was no significant difference between rats given transgenic NSCs and those given non-transgenic NSCs.
Table 3.
Attitudinal reflex scores for neonatal rats given different treatments after hypoxic-ischemic brain damage
Discussion
HIBD is a diffuse brain injury resulting in changes in the cerebral cortex, striatum and hippocampus (Balduini et al., 2000; Pimentel et al., 2011). Cortical injury can induce sensorimotor impairment, while striatal damage leads to changes in spontaneous movement, and hippocampal damage triggers declines in spatial memory and learning abilities (Bona et al., 1997; Balduini et al., 2001).
In the present study, hypoxic-ischemic lesions in the neonatal period (day 7) resulted in the impairment of sensorimotor function and working memory and learning abilities 4 weeks later (on day 35). These deficits were partially prevented by transplantation with exogenous NSCs (Wang et al., 2014). Moreover, rats transplanted with genetically modified VEGF-expressing NSCs appeared to perform even better than those given unmodified NSCs. Although the underlying neuroprotective mechanisms are unclear, maintenance of neuronal numbers by preventing cell death and/or promoting the production of new cells might contribute to the therapeutic effects of the NSCs. Indeed, cell loss in the cortex and CA1 area of the hippocampus in ischemic rats was partially prevented by NSC transplantation, consistent with the brain functional improvements.
The nervous system undergoes precisely timed and spatially regulated changes during development. Developmental disorders at the cellular and tissue levels can have neurological consequences in later life. The nervous system is more sensitive than other tissues to hypoxic-ischemic damage (Verklan et al., 2009). Rats subjected to hypoxic-ischemic injury on postnatal day 7 showed increased spontaneous activities at weaning, behavioral asymmetry, and marked spatial learning deficits in their adult life. Because of individual variation, some HIBD rats may show little abnormality in one test, but perform much worse in another (Bona et al., 1997). Therefore, in our study, we used two different tests to assess behavioral dysfunction for a more reliable assessment. Although the tests revealed consistent differences between the PBS and the two NSCs groups, the differences between the transgenic NSCs and regular NSCs groups were not fully consistent. For example, the time to find the water in the maze test was significantly improved in animals given transgenic NSCs compared with rats given non-transgenic NSCs; however, there were no significant differences in the numbers of errors or repetitions made by the two groups. While this inconsistency may in part be caused by limitations of the testing method itself, these results might indicate a real difference in neural responses to these treatments. If the latter is true, future studies should clarify the underlying mechanisms.
NSCs have the ability for self-renewal, the capacity to differentiate into cells of every glial and neuronal lineage, and the ability to populate developing or degenerating regions of the central nervous system. In addition to serving as precursors of new cells, NSCs may also serve as vehicles for gene therapy (Kim et al., 2007; Lee et al., 2010). Transplanted NSCs can migrate and reintegrate into the host tissue and stably express foreign genes. These properties permit combining cellular therapy and gene therapy. In this study, we used this approach, and the results are promising. We found that genetically modified NSCs expressing VEGF had better therapeutic effects than unmodified NSCs in most tests in rats with HIBD.
VEGF is a specific mitogen for endothelial cells. VEGF acts directly on vascular endothelial cells to stimulate their proliferation and migration, and the formation of new blood vessels (Holmes et al., 2005). In addition, VEGF may also have direct neuroprotective effects, even before new blood vessels have formed, thereby prolonging cell survival and reducing neuronal damage under stress (Cao et al., 2004). For example, VEGF can protect both HN33 and cortical neurons against cell death induced by hypoxia (Jin et al., 2001; Li et al., 2005). Also, VEGF can directly stimulate the proliferation of neuronal progenitors and adult hippocampal cells (Fournier et al., 2012). VEGF expression is significantly up-regulated by ischemia in various neural tissues and cells (Damert et al., 1997; Marti et al., 2000; Xie et al., 2013; Yokogami et al., 2013). This hypoxia-dependent increase in VEGF is considered an important adaptive response of the tissue to the stressor. The increase in VEGF expression is an important step in the subsequent repair and regenerative processes, in which angiogenesis plays a central role. In our present study, we confirmed these previous findings that ischemia increases both VEGF mRNA and protein levels in the brain, even without transplantation of NSCs.
Because of its neurotrophic and neuroprotective effects in a variety of neurological disorders, VEGF is considered a therapeutic candidate for preventing ischemic brain damage (Lambrechts et al., 2006; Sköld et al., 2008; Ruiz et al., 2009). Sun et al. (2003) injected exogenous VEGF into rats with acute cerebral infarction and showed that cerebral angiogenesis in VEGF-treated rats was four-fold higher than in the control, and that infarct size was significantly reduced. These observations support the conclusion that VEGF can function as a neurotrophic factor and reduce ischemic brain injury. However, VEGF protein is expensive and unstable in vivo, and requires repeated administration of large volumes, which limits the feasibility of application. VEGF gene therapy can overcome these limitations. Gene transfer may be achieved by the delivery of genetic material into the host's nervous tissue by different vectors, such as retroviral vectors, adeno-associated virus-based vectors, adeno-virus-based vectors and liposomes. In addition to safety concerns, direct delivery of these currently available vectors into host tissues is challenging because of the difficulty in targeting the vectors to a specific neural cell type in situ and the difficulty in controlling gene expression (Park et al., 2002). In this study, we transfected NSCs with a recombinant lentiviral vector in vitro prior to transplantation into the host. Our results suggest that this strategy is more therapeutically effective than the transplantation of unmodified NSCs.
In summary, transplantation of NSCs into neonatal rats partially rescues ischemia-induced neural dysfunction. NSCs that express VEGF produce an even better outcome. The mechanisms by which these treatments promote the recovery of neurological function after ischemia is still unknown, but they may reduce neuronal cell death and/or increase neuronal cell production. Moreover, an increase in local VEGF levels may also be involved. Further studies are required to clarify the underlying repair mechanisms.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81070523 and 81270728.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Patel B, Robens J, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Balduini W, De Angelis V, Mazzoni E, Cimino M. Simvastatin protects against long-lasting behavioral and morphological consequences of neonatal hypoxic/ischemic brain injury. Stroke. 2001;32:2185–2191. doi: 10.1161/hs0901.094287. [DOI] [PubMed] [Google Scholar]
- Balduini W, De Angelis V, Mazzoni E, Cimino M. Long-lasting behavioral alterations following a hypoxic/ischemic brain injury in neonatal rats. Brain Res. 2000;859:318–325. doi: 10.1016/s0006-8993(00)01997-1. [DOI] [PubMed] [Google Scholar]
- Bona E, Johansson BB, Hagberg H. Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in seven-day-old rats. Pediatr Res. 1997;42:678–683. doi: 10.1203/00006450-199711000-00021. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R, Jiang K, Sun G, Wu JC, Steinberg GK. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516–523. doi: 10.1161/STROKEAHA.109.573691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damert A, Machein M, Breier G, Fujita MQ, Hanahan D, Risau W, Plate KH. Up-regulation of vascular endothelial growth factor expression in a rat glioma is conferred by two distinct hypoxia-driven mechanisms. Cancer Res Sep. 1997;57:3860–3864. [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier NM, Lee B, Banasr M, Elsayed M, Duman RS. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology. 2012;63:642–652. doi: 10.1016/j.neuropharm.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6:209. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Batteur SP, McEachron E, Leahy A, Greenberg DA. Caspase-3 and the regulation of hypoxic neuronal death by vascular endothelial growth factor. Neuroscience. 2001;108:351–358. doi: 10.1016/s0306-4522(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Kim HT, Kim IS, Lim SE, Lee IS, Park KI. Gene and cell replacement via neural stem cells. Yonsei Med J. 2004;45(Suppl):32–40. doi: 10.3349/ymj.2004.45.Suppl.32. [DOI] [PubMed] [Google Scholar]
- Kim SU. Genetically engineered human neural stem cells for brain repair in neurological diseases. Brain Dev. 2007;29:193–201. doi: 10.1016/j.braindev.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Carmeliet P. VEGF at the neurovascular interface: therapeutic implications for motor neuron disease. Biochim Biophys Acta. 2006;1762:1109–1121. doi: 10.1016/j.bbadis.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Lee IS, Jung K, Kim M, Park KI. Neural stem cells: properties and therapeutic potentials for hypoxic-ischemic brain injury in newborn infants. Pediatr Int. 2010;52:855–865. doi: 10.1111/j.1442-200X.2010.03266.x. [DOI] [PubMed] [Google Scholar]
- Li D, Marks JD, Schumacker PT, Young RM, Brorson JR. Physiological hypoxia promotes survival of cultured cortical neurons. Eur J Neurosci. 2005;22:1319–1326. doi: 10.1111/j.1460-9568.2005.04335.x. [DOI] [PubMed] [Google Scholar]
- Li YB, Wang Y, Tang JP, Chen D, Wang SL. Neuroprotective effects of ginsenoside Rg1-induced neural stem cell transplantation on hypoxic-ischemic encephalopathy. Neural Regen Res. 2015;10:753–759. doi: 10.4103/1673-5374.156971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CJ, Yin F, Zheng XR, Zhang SS, Tan JL. The isolating culture of neural stem cells from rat's fetus brain and transfection by electroporation. Zhonghua Yi Xue Za Zhi. 2009;89:3007–3009. [PubMed] [Google Scholar]
- Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg A, Broch H. Etiology of cerebral palsy. J Perinat Med. 2004;32:434–439. doi: 10.1515/JPM.2004.143. [DOI] [PubMed] [Google Scholar]
- Park KI, Ourednik J, Ourednik V, Taylor RM, Aboody KS, Auguste KI, Lachyankar MB, Redmond DE, Snyder EY. Global gene and cell replacement strategies via stem cells. Gene Ther. 2002;9:613–624. doi: 10.1038/sj.gt.3301721. [DOI] [PubMed] [Google Scholar]
- Pimentel VC, Pinheiro FV, De Bona KS, Maldonado PA, da Silva CR, de Oliveira SM, Ferreira J, Bertoncheli CM, Schetinger MR, Da Luz SC, Moretto MB. Hypoxic-ischemic brain injury stimulates inflammatory response and enzymatic activities in the hippocampus of neonatal rats. Brain Res. 2011;1388:134–140. doi: 10.1016/j.brainres.2011.01.108. [DOI] [PubMed] [Google Scholar]
- Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- Sköld MK, Kanje M. Vascular endothelial growth factor in central nervous system injuries-a vascular growth factor getting nervous. Curr Neurovasc Res. 2008;5:246–259. doi: 10.2174/156720208786413451. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Zheng X, Zhang S, Yang Y, Wang X, Yu X, Zhong L. Response of the sensorimotor cortex of cerebral palsy rats receiving transplantation of vascular endothelial growth factor 165-transfected neural stem cells. Neural Regen Res. 2014;9:1763–1769. doi: 10.4103/1673-5374.141785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verklan MT. The chilling details: hypoxic-ischemic encephalopathy. J Perinat Neonatal Nurs. 2009;23:59–68. doi: 10.1097/01.JPN.0000346221.48202.7e. [DOI] [PubMed] [Google Scholar]
- Wang L, Jiang F, Li Q, He X, Ma J. Mild hypothermia combined with neural stem cell transplantation for hypoxic-ischemic encephalopathy: neuroprotective effects of combined therapy. Neural Regen Res. 2014;9:1745–1752. doi: 10.4103/1673-5374.143417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Zhao YS, Yang YJ, Xie M, Yu XH. Therapeutic window of hyperbaric oxygen therapy for hypoxic-ischemic brain damage in newborn rats. Brain Res. 2008;1222:87–94. doi: 10.1016/j.brainres.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Xie L, Mao X, Jin K, Greenberg DA. Vascular endothelial growth factor-B expression in postischemic rat brain. Vasc Cell. 2013;5:8. doi: 10.1186/2045-824X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogami K, Yamashita S, Takeshima H. Hypoxia-induced decreases in SOCS3 increase STAT3 activation and upregulate VEGF gene expression. Brain Tumor Pathol. 2013;30:135–143. doi: 10.1007/s10014-012-0122-0. [DOI] [PubMed] [Google Scholar]
- Zhang SS, Zheng XR, Yang YJ. Adenovirus-mediated VEGF165 gene transfer has neuroprotective effects in neonatal rats following hypoxic-ischemic brain damage. Zhongguo Dang Dai Er Ke Za Zhi. 2008;10:737–742. [PubMed] [Google Scholar]
- Zhang SS, Zheng XR, Yin F, Tan JL, Yang YJ. Lentiviral-mediated vascular endothelial growth factor 165 gene transfer into neural stem cells promotes proliferation. Neural Regen Res. 2011;6:1–5. [Google Scholar]
- Zheng XR, Zhang SS, Yang YJ, Yin F, Wang X, Zhong L, Yu XH. Adenoviral vector-mediated transfection of VEGF improves neural functional recovery after hypoxia-ischemic brain damage in neonatal rats. Brain Res Bull. 2010;81:372–377. doi: 10.1016/j.brainresbull.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Zhu JM, Zhao YY, Chen SD, Zhang WH, Lou L, Jin X. Functional recovery after transplantation of neural stem cells modified by brain-derived neurotrophic factor in rats with cerebral ischaemia. J Int Med Res. 2011;39:488–498. doi: 10.1177/147323001103900216. [DOI] [PubMed] [Google Scholar]
- Zhu W, Mao Y, Zhou LF. Reduction of neural and vascular damage by transplantation of VEGF-secreting neural stem cells after cerebral ischemia. Acta Neurochir. 2005;95:393–397. doi: 10.1007/3-211-32318-x_80. [DOI] [PubMed] [Google Scholar]